Abstract
Hypertension guidelines recommend that blood pressure (BP) should be measured using a monitor that has passed validation testing for accuracy. BP monitors that have not undergone rigorous validation testing can still be cleared by regulatory authorities for marketing and sale. This is the situation for most BP monitors worldwide. Thus, consumers (patients, health professionals, procurement officers, and general public) may unwittingly purchase BP monitors that are non‐validated and more likely to be inaccurate. Without prior knowledge of these issues, it is extremely difficult for consumers to distinguish validated from non‐validated BP monitors. For the above reasons, the aim of this paper is to provide consumers guidance on how to check whether a BP monitor has been properly validated for accuracy. The process involves making an online search of listings of BP monitors that have been assessed for validation status. Only those monitors that have been properly validated are recommended for BP measurement. There are numerous different online listings of BP monitors, several are country‐specific and two are general (international) listings. Because monitors can be marketed using alternative model names in different countries, if a monitor is not found on one listing, it may be worthwhile cross‐checking with a different listing. This information is widely relevant to anyone seeking to purchase a home, clinic, or ambulatory BP monitor, including individual consumers for use personally or policy makers and those procuring monitors for use in healthcare systems, and retailers looking to stock only validated BP monitors.
Keywords: ambulatory blood pressure/home blood pressure monitor, blood pressure determination, device, validation
1. INTRODUCTION
Raised blood pressure (BP) is the leading risk factor for morbidity and mortality and is responsible for >10 million deaths globally each year. 1 Accurate identification of hypertension and initiation of BP‐lowering strategies (eg, lifestyle modification, antihypertensive medication) considerably lowers the risk of heart disease, stroke, and other adverse clinical events. 2 Thus, accurate measurement of BP has been described as one of the most important tests in clinical medicine. 3
Worldwide, hypertension guidelines state that it is essential for automated BP monitors to be validated for accuracy. 4 , 5 , 6 , 7 Validation testing of BP monitors should be conducted by investigators independent of the manufacturer with strict adherence to a standardized international validation protocol, 4 , 5 , 8 , 9 , 10 and the results should be published by peer‐review journals. The validation protocol involves comparisons with a reference standard BP measurement (mercury manometers and auscultatory method) in a population of people of different sex, body size, and BP level. A monitor is regarded as validated for accuracy if the BP measurements meet or surpass the stipulated accuracy requirements of the designated protocol.
Since regulatory authorities mainly focus on ensuring that BP monitors are safe, they are often cleared for sale without undergoing rigorous, independent validation testing for accuracy. Consequently, of the BP monitors available on the market, only between 6% and 15% are validated. 11 , 12 , 13 This is quite problematic and a major cause for concern because non‐validated BP monitors are more likely to be inaccurate compared with validated BP monitors, 14 , 15 and if these non‐validated BP monitors are used for clinical decisions there is a higher likelihood for incorrect medical management.
These problems are relevant to all consumers (ie, patients, health professionals, procurement officers, and general public) 4 as they are faced with many hundreds of choices when it comes to purchasing a BP monitor, particularly online. Policy makers also need to consider actions that can be taken to address these problems. The situation may be even worse in low‐middle income countries where the regulatory environment is often weaker. 16 Critically, there is a lack of reliable information on validation status at the point of purchase (in‐store or online), and thus, buyers may unwittingly purchase non‐validated monitors that are not recommended for use. There is also little guidance for consumers on how to check whether a monitor has been validated for accuracy. 17 Thus, this paper aims to provide a guide for consumers on how to check whether a BP monitor has been properly tested for accuracy (validation status). The paper is also relevant to policy makers seeking to ensure clinical use of validated BP devices and retailers seeking to stock only validated BP devices. This work is aligned with ongoing efforts to advocate for and improve knowledge on the importance of accurate BP monitoring, and has been written for the Accuracy in Measurement of BP (AIM‐BP) collaborative. 18 This effort also coincides with the recent publication of the Lancet Commission on Hypertension Group position statement on improvement of accuracy standards for BP devices 19 and the World Health Organisation on technical specifications for automated non‐invasive cuff BP devices. 20
2. HOW TO CHECK THAT A BLOOD PRESSURE MONITOR HAS BEEN PROPERLY VALIDATED FOR ACCURACY
The easiest way to check whether a BP monitor is validated for accuracy is to search an online registry (Figure 1). However, users need to know the characteristics of such registries and how to choose the most appropriate one. Indeed, there are several registries managed by reputable country‐specific and international organizations that track the validation status of BP monitors (Table 1 and Figure 1). Each country‐specific registry is focused on BP monitors available in that particular country or region, whereas the general (international) registries aim to provide more global coverage. There are differences in the processes for determining validation status between the registries. For example, the American Medical Association registry does not list monitors validated with the European Society of Hypertension protocols due to their smaller sample size compared to other protocols (eg, n = 33 vs n = 85). 21 The way the registries define equivalence to previously validated monitors also varies. Some registries (eg, British or Canadian) accept a monitor as valid if it is a derivative of a previously validated one and the vital BP measurement componentry remains identical. Other registries (eg, Medaval) require that derivative devices are in accordance with European Union Medical Device Regulation 2017/745. Taken altogether, these differences explain potential variation in recommendations of validated BP monitors between each registry.
Figure 1.

How to check whether a blood pressure (BP) device has been validated for accuracy. An overview of regional and general registries of validated BP devices is included. This figure with live links can be downloaded at https://www.menzies.utas.edu.au/documents/pdfs/Blood‐pressure‐devices.pdf
Table 1.
Online registries of blood pressure monitors that have been tested for accuracy according to best practice scientific protocols
| Society, organization or company | Weblink |
|---|---|
| Regional registries | |
| American Medical Association | https://www.validatebp.org/ |
| British and Irish Hypertension Society | https://bihsoc.org/bp‐monitors/ |
| Hypertension Canada | https://hypertension.ca/bpdevices |
| German Hypertension Society a | https://www.hochdruckliga.de/messgeraete‐mit‐pruefsiegel.html |
| Japanese Society of Hypertension | http://www.jpnsh.jp/com_ac_wg1.html |
| General registries | |
| STRIDE BP (European based) | https://stridebp.org/ |
| Medaval b | https://medaval.ie/blood‐pressure‐monitors/ |
Only lists monitors that pass the German Hypertension League Quality Seal Protocol.24,25
Medaval is not affiliated with a specific scientific organization. As well as listing validated monitors, the Medaval registry details those monitors available for purchase that are not validated, with a warning that these monitors are not recommended.
There are two general (international) registries, STRIDE BP and Medaval, that can be used to check whether a BP monitor is validated. To search either registry, the manufacturer name and model of the monitor are needed. This information should be marked on the box or casing of the BP monitor. If a BP monitor is missing this fundamental information, it is unlikely to have been validated. 13 Locating manufacturer name and model information for BP monitors may be more difficult when shopping online compared with in‐store.
The STRIDE‐BP registry (https://www.stridebp.org/bp‐monitors ) can be searched by typing the precise model of the BP monitor into the search box (Figure 2). The manufacturer name can also be entered, and the results can be scanned to check whether the BP monitor of interest is listed. If the monitor is listed, then it has been validated and can be used for BP measurement. STRIDE BP also uses a series of rules to label “Preferred Devices” with a ribbon icon. Preferred devices are those that are upper‐arm cuff monitors, have had a validation study published in the last 10 years and, for home BP monitors, have the capacity for automatic data storage or data transfer to smartphones/computers. 21 However, any properly validated monitor will be listed on STRIDE BP as validated. If there are “No Results Found” from the search, then it is possible that the monitor is not validated and thus not recommended for use. Occasionally, manufacturers will market a monitor under different model names or numbers in different regions or countries. If a monitor is not found on the STRIDE BP site, it may be worthwhile to cross‐check the monitor for sale in one particular region on alternative sites to determine whether this is the case.
Figure 2.
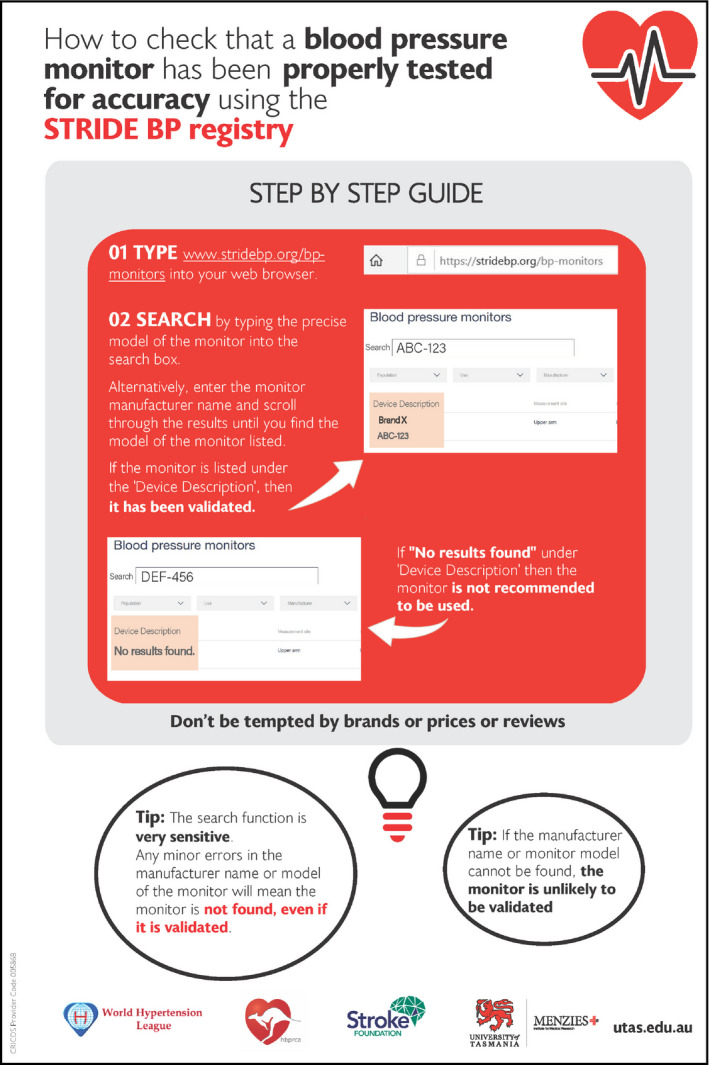
How to check whether a blood pressure (BP) device has been validated for accuracy using the STRIDE BP registry. This figure with live links can be downloaded at https://www.menzies.utas.edu.au/documents/pdfs/Blood‐pressure‐devices.pdf
The Medaval registry (https://medaval.ie/blood‐pressure‐monitors/) is a listing that is not affiliated with a specific scientific organization. Nevertheless, Medaval currently has the largest repository of information on both validated and non‐validated monitors and provides recommendations as to whether the BP monitor should be used. The registry also references to BP monitor assessments made by other listings, where relevant. Due to sensitivities in the Medaval search engine, the easiest way to access the Medaval registry is to use a general search engine (eg, Google) and type in the manufacturer name and model of the monitor, as well as the term “medaval” (Figure 3). In the Internet search results click the link to the Medaval registry for the BP monitor of interest. Find the “Assessment” section, and if the monitor is recommended, then it is validated. Medaval also assigns a five star rating scale to monitors (https://medaval.ie/medaval‐star‐rating‐criteria/), but importantly, any properly validated monitor will be recommended, irrespective of the number of stars. If the monitor is “Not recommended” in the assessment section, the BP monitor is not validated and therefore is not recommended for use.
Figure 3.

How to check whether a blood pressure (BP) device has been validated for accuracy using the Medaval registry. This figure with live links can be downloaded at https://www.menzies.utas.edu.au/documents/pdfs/Blood‐pressure‐devices.pdf
There are some caveats to the above registries which mean that a monitor may be searched and incorrectly deemed non‐validated. First, on registries where users need to type the monitor name or model number, input errors may lead to the incorrect assumption that a monitor is non‐validated. Second, new monitors may be validated but have not been added to a registry. Third, as mentioned above, some manufacturers use different names or monitor model numbers in different countries. This could mean people that search for a monitor on a registry find that it appears non‐validated when in fact an identical monitor with a different monitor model number has been validated. Last, a monitor may have been validated following a proper protocol, but the data remain unpublished, despite being recognized by specific expert groups.
3. PRACTICAL GUIDE FOR CONSUMERS ON HOW TO CHECK WHETHER A BP MONITOR HAS BEEN PROPERLY TESTED FOR ACCURACY (VALIDATION STATUS)
A practical document has been developed to guide consumers (patients, health professionals, procurement officers, and general public), policy makers and retailers on how to search online registries of validated BP monitors (Figures 1, 2, 3). The document has also been translated to several languages other than English to facilitate global dissemination. Links to the translated document are available in Table 2.
Table 2.
Practical guides on how to check whether a BP monitor has been properly tested for accuracy in different languages
4. CONSIDERATIONS BEYOND MONITOR VALIDATION FOR PROPER BLOOD PRESSURE MEASUREMENT
Accuracy of BP measurement is also contingent on other factors beyond validation of the BP monitor. 3 These include a properly prepared patient in a quiet, comfortable location, using a correctly sized BP cuff and following a proper BP measurement protocol. Without following these steps, there is a greater likelihood of inaccurate BP measurement. 7 , 23 , 24 There are many practical resources available on self‐BP monitoring available from hypertension societies and public health organizations. A free online certification course on measuring BP has also recently been developed. 25
In conclusion, hypertension remains a leading risk factor for death and disability, and accurate measurement of BP is crucial for optimal management. Most BP monitors are not validated for accuracy, which makes them unsuitable for clinical or home use. The current paper provides a guide to using online validated BP monitor registries and is relevant to anyone seeking to buy a BP monitor.
CONFLICT OF INTEREST
Aletta E Schutte: Her University has received equipment and funding from manufacturers of BP devices including IEM and Omron. Azra Mahmud has received equipment and research funding from manufacturers of BP devices including AtCor Medical and IEM but has no personal commercial interests in these or any other BP companies. Raj Padwal: Canadian representative to the ISO Sphygmomanometer committee and sits on the AAMI Sphygmomanometer committee. Co‐Founder and CEO of a digital health company (mmHg Inc), based at the University of Alberta. James E Sharman: His university has received equipment and research funding from manufacturers of BP devices including AtCor Medical, IEM and Pulsecor (Uscom). He has no personal commercial interests related to BP companies. Cintia Lombardi and Pedro Ordunez are staff members of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization. Tammy Brady: is Co‐Chair of the Association for the Advancement of Medical Instrumentation (AAMI) Sphygmomanometer Committee and is a nominated expert on the IEC (International Electrotechnical Commission)/ISO (International Organization for Standardization) joint working group on non‐invasive blood pressure monitoring devices. The remaining authors have no disclosures.
AUTHOR CONTRIBUTIONS
Each of the listed authors (DSP, RP, NRCC, PB, TMB, MHO, CD, CL, AM, YM, GGM, PO, HTP, GP, AES, KS, XZ, and JES) meet the criteria for “Authorship” in accordance with the ICMJE recommendations as outlined below: (1) Substantial contributions to the conception or design of the work; (2) Drafting the work or revising it critically for important intellectual content; (3) Final approval of the version to be published; (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
This manuscript was produced as part of the Accuracy in Measurement of Blood Pressure (AIM‐BP) Collaborative, an initiative advocating for best measurement practices globally. AIM‐BP members and their affiliations can be found on the World Hypertension League website. The contents of this paper reflect the opinions of its authors and not necessarily all members of AIM‐BP. This publication was supported by Grant Cooperative Agreement number NU2GGH001873 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, the Department of Health and Human Services, The Task Force for Global Health, Inc. or TEPHINET.
Picone DS, Padwal R, Campbell NRC, et al. How to check whether a blood pressure monitor has been properly validated for accuracy. J. Clin. Hypertens. 2020;22:2167–2174. 10.1111/jch.14065
Funding information
CD is supported by a British Heart Foundation Centre of Research excellence Award (RE/18/6/34217). TMB is supported by Resolve to Save Lives, an initiative of Vital Strategies.
REFERENCES
- 1. GBD Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387(10022):957‐967. [DOI] [PubMed] [Google Scholar]
- 3. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142‐161. [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 6. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334‐1357. [DOI] [PubMed] [Google Scholar]
- 7. Sharman JE, Howes FS, Head GA, et al. Home blood pressure monitoring: Australian Expert Consensus Statement. J Hypertens. 2015;33(9):1721‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malachias MVB, Gomes MAM, Nobre F, Alessi A, Feitosa AD, Coelho EB. 7th Brazilian Guideline of Arterial Hypertension: Chapter 2 ‐ Diagnosis and Classification. Arq Bras Cardiol. 2016;107(3 Suppl 3):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 10. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada's 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2018;34(5):506‐525. [DOI] [PubMed] [Google Scholar]
- 11. Medaval Ltd . Blood Pressure Monitors. 2020; https://medaval.ie/device‐category/blood‐pressure‐monitors/. Accessed 14 January, 2020
- 12. STRIDE BP . Only 6% of blood pressure device on the market approved by the STRIDE BP. 2020; https://www.stridebp.org/. Accessed 22 May, 2020.
- 13. Picone DS, Deshpande RA, Schultz MG, et al. Nonvalidated Home Blood Pressure Devices Dominate the Online Marketplace in Australia: Major Implications for Cardiovascular Risk Management. Hypertension. 2020;75(6):1593‐1599. [DOI] [PubMed] [Google Scholar]
- 14. Akpolat T, Dilek M, Aydogdu T, Adibelli Z, Erdem DG, Erdem E. Home sphygmomanometers: validation versus accuracy. Blood Press Monit. 2009;14(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 15. Jung MH, Kim GH, Kim JH, et al. Reliability of home blood pressure monitoring: in the context of validation and accuracy. Blood Press Monit. 2015;20(4):215‐220. [DOI] [PubMed] [Google Scholar]
- 16. Lombardi C, Sharman J, Padwal R, et al. Weak and fragmented regulatory frameworks on the accuracy of blood pressure measuring devices pose a major impediment for the implementation of HEARTS in the Americas. Journal of Clinical Hypertension. 2020;22:2184–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Logan AG, Dunai A, McIsaac WJ, Irvine MJ, Tisler A. Attitudes of primary care physicians and their patients about home blood pressure monitoring in Ontario. J Hypertens. 2008;26(3):446‐452. [DOI] [PubMed] [Google Scholar]
- 18. Padwal R, Campbell NRC, Weber MA, et al. The Accuracy in Measurement of Blood Pressure (AIM‐BP) collaborative: Background and rationale. J Clin Hypertens (Greenwich). 2019;21(12):1780‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharman JE, O’Brien E, Alpert B, et al. Lancet Commission on Hypertension group position statement on the global improvement of accuracy standards for devices that measure blood pressure. J Hypertens. 2020;38(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . WHO technical specifications for automated non‐invasive blood pressure measuring devices with cuff. Geneva, Switzerland. World Health Organization;2020; https://www.who.int/docs/default‐source/searo/indonesia/who‐tech‐spec‐for‐automated‐non‐invasive‐blood‐pressure‐measuring‐devices‐with‐cuff.pdf?sfvrsn=b112be47_2
- 21. Cohen JB, Padwal RS, Gutkin M, et al. History and Justification of a National Blood Pressure Measurement Validated Device Listing. Hypertension. 2019;73(2):258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stergiou GS, O'Brien E, Myers M, Palatini P, Parati G, Board SBSA. STRIDE BP: an international initiative for accurate blood pressure measurement. J Hypertens. 2020;38(3):395‐399. [DOI] [PubMed] [Google Scholar]
- 23. Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padwal R, Campbell NRC, Schutte AE, et al. Optimizing observer performance of clinic blood pressure measurement: a position statement from the Lancet Commission on Hypertension Group. J Hypertens. 2019;37(9):1737‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell NRC, Khalsa T, Ordunez P, et al. Brief online certification course for measuring blood pressure with an automated blood pressure device. A free new resource to support World Hypertension Day Oct 17, 2020. J Clin Hypertens (Greenwich). 2020. 10.1111/jch.14017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


