Abstract
Salt sensitivity is one of the crucial risk factors of hypertension. The aim of the present prospective cohort study was to assess the clinical impact of alcohol drinking on an association between salt intake and blood pressure. The present study included 451 employees at a pharmaceutical company in Japan who underwent annual health checkups in both 2017 and 2018. The main exposure of interest was self‐reported drinking frequency at their first checkups: rarely, occasionally, and daily. To assess the association between the change of salt intake estimated from single‐spot urine specimens and that of blood pressure, the differences in systolic/diastolic blood pressure and salt intake between 2017 and 2018 were calculated for each subject. Multivariable‐adjusted linear regression models adjusting for clinically relevant factors clarified a drinking frequency‐dependent association between Δsalt intake and Δsystolic blood pressure (per 1 g/d of Δsalt intake adjusted β [95% confidence interval] 0.19 [−0.73, 1.12], 0.84 [0.14, 1.53], and 1.78 [0.86, 2.69] in rare, occasional, and daily drinkers). A similar association between Δsalt intake and Δdiastolic blood pressure was also observed (−0.24 [−1.02, 0.54], 0.67 (0.18, 1.16), 0.95 [0.38, 1.51], in rare, occasional, and daily drinkers). The interactions between drinking frequency and Δsalt intake were found to be statistically significant (P for interaction = .028 and .006 for ∆systolic blood pressure and ∆diastolic blood pressure, respectively). The present study identified enhanced salt sensitivity in the subjects who drink at a higher frequency, suggesting that the reduction in alcohol consumption may improve salt sensitivity in higher frequency drinkers.
Keywords: alcohol drinking, cohort study, salt sensitivity
1. INTRODUCTION
The salt sensitivity of blood pressure is characterized by the blood pressure (BP) response to salt intake. The salt‐sensitive subjects will sustain an increase in BP with salt loading and a decrease in BP with salt depletion, whereas the salt‐resistant subjects will not. 1 In the United States, 26% of normotensive subjects were salt‐sensitive. 2 Compared with the Caucasian population, salt sensitivity may be more common in the Japanese population. 3 A prospective cohort study reported that the incidence of hypertension is higher in salt‐sensitive subjects than in salt‐resistant subjects during approximately 15 years of the follow‐up period. 4 Several observational studies identified salt sensitivity as a risk factor of cardiovascular mortality and morbidity, 5 , 6 independent of blood pressure. Modifiable lifestyle factors affecting salt sensitivity should be identified, because high BP and high salt intake are the leading causes of the global burden of disease, especially in east Asia, including Japan. 7
Besides physiological, 8 , 9 genetic, 10 , 11 , 12 demographic, 13 , 14 and environmental factors, 15 , 16 dietary factors play a pivotal role in salt sensitivity. 17 , 18 Interestingly, an Italian study reported that salt sensitivity was more common in heavy alcoholics than in non‐drinkers, 19 suggesting that chronic heavy drinkers were more prone to enhanced salt sensitivity. In the general population, the effect of drinking alcohol on salt sensitivity remains unclear, although drinking alcohol is one of the major risk factors of hypertension. 20 , 21
The aim of the present prospective cohort study was to assess the clinical impact of drinking frequency on the association between salt intake and blood pressure in 451 employees of a pharmaceutical company in Japan. The present study provides a deep insight into alcohol drinking as a potential enhancer of salt sensitivity in the general population.
2. METHODS
2.1. Participants
Eligible participants in the present prospective cohort study were 507 employees of a pharmaceutical company, Shionogi & Co., Ltd., who underwent annual health checkups in both 2017 and 2018, and gave informed consent to their participation in the present study. After excluding 55 (10.8%) employees with self‐reported hypertension, who had a positive answer to the question “Do you take antihypertensive medications now?” and 1 (0.2%) pregnant female, the present study finally included 451 (89.0%) employees without current use of antihypertensive drugs. Because of the prospective nature of the present study, the sample size was dependent on the number of the company's employees. The study protocol was approved by the ethics committees of Shionogi Pharmaceutical Research Center, the Health and Counseling Center, Osaka University, and Osaka University Hospital.
2.2. Measurements
The baseline variables measured in 2017 included age, sex, drinking frequency, smoking status, current treatment for hypertension, dyslipidemia, diabetes, body mass index (=body weight [kg]/height [m]2), systolic and diastolic blood pressure (SBP and DBP), hemoglobin A1c, serum concentration of total cholesterol, triglyceride, and creatinine, and urine concentration of sodium and creatinine. BP was measured using an oscillometric device (Omron HBP 1300), after participants were relaxed a few minutes and seated with legs uncrossed, and back and arm supported. The middle of the cuff on the upper arm was set at the level of the heart. The BP measurement methods in 2017 and 2018 were identical. Urinary sodium and creatinine were measured using single‐spot urine specimens. To estimate 24‐hour sodium excretion, Tanaka's equation 22 was used; estimated 24‐hour sodium excretion [mEq/d] = 21.98 × (urinary sodium [mEq/L]/[urinary creatinine [mg/dL] × 10] × 14.89 × body weight [kg] + 16.14 × height [cm] − 2.04 × age [year] − 2244.45)0.392. Salt intake (g/d) was calculated by multiplying 24‐hour sodium excretion (mEq/d) by 0.0585. Estimated glomerular filtration rate (eGFR) was calculated using a three‐variable equation modified for Japanese patients; eGFR (mL/min/1.73 m2) = 194 × age (year)−0.287 × serum creatinine (mg/dL)−1.094 × 0.739 (if female). 23
Drinking frequency, smoking status, and current treatment for dyslipidemia and diabetes were obtained self‐reported standard questionnaires. Drinking frequency was classified by the question “How often do you drink alcoholic beverages?” with responses of rarely, occasionally, or daily. Smoking status was categorized into non‐, past, and current smokers, according to the question: “Do you smoke?” with possible answers “I do not smoke,” “I quit smoking,” or “I smoke.” Current treatment for dyslipidemia and diabetes was determined based on positive answers to the question, “Do you take a lipid‐lowering drug now?” and “Do you take an antidiabetic drug now?”
The outcome measures of the present study were the change of SBP and DBP between 2017 and 2018 (ΔSBP = SBP in 2018 − SBP in 2017; ΔDBP = DBP in 2018 − DBP in 2017). To assess an association between the change of salt intake and that of blood pressure, we calculated the difference in salt intake between 2017 and 2018: Δsalt intake = salt intake in 2018 (g/d) − salt intake in 2017 (g/d). Drinking frequency was also collected in 2018 to evaluate how the baseline drinking frequency reflected the drinking frequency during the follow‐up period.
2.3. Statistical analysis
Baseline characteristics stratified on drinking frequency (rarely, occasionally, daily) were compared using ANOVA, the Kruskal‐Wallis test, or chi‐square test, as appropriate. Reproducibility of the drinking frequency at the baseline visit and 1 year after at the baseline visit was assessed using the weighted Cohen's kappa statistics. Reproducibility with <0.40, 0.41‐0.60, 0.61‐0.80, and 0.81‐1.00 of kappa statistics was regarded as fair, moderate, substantial, and almost perfect, respectively. 24
To describe the distributions of ∆salt intake, ∆SBP, and ∆DBP, kernel density plots were used. The associations of drinking frequency with ∆salt intake, ΔSBP, and ΔDBP were compared using ANOVA. The associations of Δsalt intake with ΔSBP and ΔDBP were assessed using simple linear regression models and multivariable linear regression models adjusting for the baseline variables, including age, sex, smoking status, drinking frequency, current treatment for dyslipidemia and diabetes, body mass index, systolic blood pressure (if ΔSBP), diastolic blood pressure (if ΔDBP), total cholesterol, triglyceride, hemoglobin A1c, eGFR, and salt intake. Effect modification between Δsalt intake and the baseline drinking frequency was assessed by incorporating their interaction term into the multivariable‐adjusted model. To clarify their interaction, the associations of Δsalt intake with ΔSBP and ΔDBP were assessed in three subgroups stratified on drinking frequency.
Continuous variables were expressed as mean ± SD or median (interquartile range), as appropriate, and categorical variables as number (proportion). Statistical significance was set at P < .05. Statistical analyses were performed using Stata, version 15.1 (StataCorp, www.stata.com).
3. RESULTS
The baseline characteristics of 451 participants, stratified on three categories of drinking frequency, are shown in Table 1. Daily drinkers were likely to be older and have higher levels of systolic and diastolic blood pressure, whereas rare drinkers had lower body mass index. Compared with rare drinkers, those who drank more frequently were likely to have a higher prevalence of current smokers. Reproducibility of the drinking frequency in 2017 and 2018 was 0.88 of weighted kappa statistics, suggesting that the baseline drinking frequency in 2017 reflected the drinking frequency during the follow‐up period.
TABLE 1.
Clinical characteristics of 451 participants stratified on drinking frequency
| Clinical characteristics | Drinking frequency at baseline visit | P | ||||
|---|---|---|---|---|---|---|
| Rare | Occasional | Daily | ||||
| Number | 91 | 237 | 123 | |||
| Baseline characteristics | ||||||
| Age (y)* | 44 (38‐51) | 43 (39‐49) | 50 (41‐54) | <.001 | ||
| Male (n [%]) | 57 (62.6) | 166 (70.0) | 93 (75.6) | .123 | ||
| Smoking status (n [%])* | ||||||
| Non‐smoker | 81 (89.0) | 185 (78.1) | 68 (55.3) | <.001 | ||
| Past smoker | 7 (7.7) | 33 (13.9) | 39 (31.7) | |||
| Current smoker | 3 (3.3) | 19 (8.0) | 16 (13.0) | |||
| Current treatment for | ||||||
| Dyslipidemia (n [%]) | 2 (2.2) | 6 (2.5) | 3 (2.4) | .985 | ||
| Diabetes mellitus (n [%]) | 0 (0.0) | 2 (0.8) | 0 (0.0) | .404 | ||
| Body mass index (kg/m2)* | 21.5 ± 2.5 | 22.3 ± 2.9 | 22.2 ± 2.7 | .042 | ||
| Systolic blood pressure (mm Hg)* | 116 ± 11 | 117 ± 11 | 121 ± 12 | .008 | ||
| Diastolic blood pressure (mm Hg)* | 73 ± 9 | 74 ± 9 | 78 ± 10 | <.001 | ||
| Total cholesterol (mg/dL) | 202 ± 30 | 203 ± 31 | 204 ± 35 | .933 | ||
| Triglyceride (mg/dL) | 69 (48‐94) | 69 (53‐100) | 70 (50‐104) | .494 | ||
| Hemoglobin A1c (%) | 5.3 ± 0.2 | 5.3 ± 0.3 | 5.3 ± 0.3 | .088 | ||
| eGFR (mL/min/1.73 m2) | 79 ± 12 | 79 ± 11 | 77 ± 11 | .351 | ||
| Salt intake (g/d) | 8.1 ± 1.7 | 8.3 ± 1.9 | 8.7 ± 1.7 | .088 | ||
| Drinking frequency 1 y after the baseline visit a | ||||||
| Rare (n [%]) | 83 (91.2) | 6 (2.5) | 1 (0.8) | |||
| Occasional | 8 (8.8) | 222 (93.7) | 14 (11.4) | |||
| Daily | 0 (0.0) | 9 (3.8) | 108 (87.8) | |||
Mean ± SD; median (25%–75%).
Abbreviation: eGFR, estimated glomerular filtration rate.
Reproducibility of drinking frequency at the baseline visit and 1 y after the baseline visit was 0.88 of the weighted kappa statistics.
P < .05
Because of the similar distribution of salt intake between 2017 and 2018, ∆salt intake was −0.1 ± 2.0 g/d (Figure 1). The association between drinking frequency and ∆salt intake was not statistically significant (0.1 ± 1.8, 0.0 ± 2.0, and −0.3 ± 2.0 g/d, in rare, occasional, and daily drinkers, respectively; P = .202). Regarding the change of blood pressure over the 1 year, ΔSBP and ∆DBP were 0 ± 9 mm Hg and 0 ± 6 mm Hg, respectively (Figure 1). Drinking frequency was not associated with either ΔSBP (−1 ± 7, 1 ± 10, and 0 ± 8 mm Hg, in rare, occasional, and daily drinkers, respectively; P = .164) or ΔDBP (0 ± 6, 1 ± 6, and 0 ± 5 mm Hg, in rare, occasional, and daily drinkers, respectively; P = .095).
FIGURE 1.
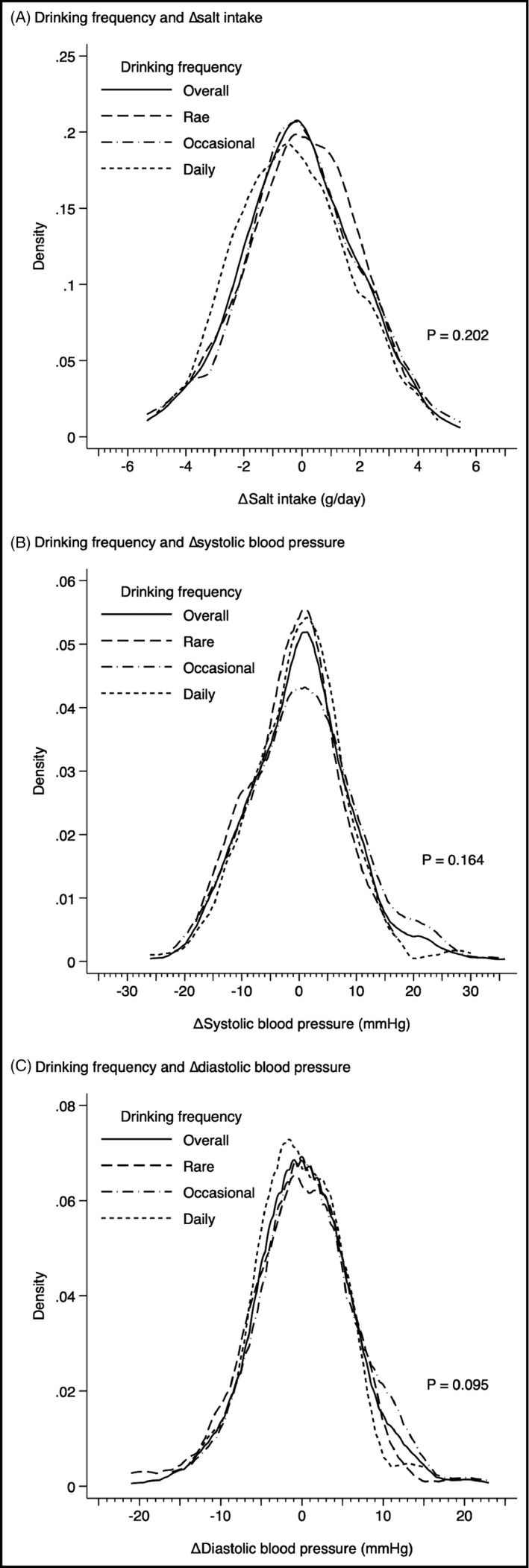
Distributions of the change of salt intake (∆salt intake), systolic blood pressure (∆SBP), and diastolic blood pressure (∆DBP) in total participants (n = 451), rare drinkers (n = 91), occasional drinkers (n = 237), and daily drinkers (n = 123)
Unadjusted linear regression models showed significant associations of Δsalt intake with both ΔSBP and ΔDBP (∆salt intake [per 1 g/d], β 0.75 [95% confidence interval 0.34, 1.16], P < .001 for ∆SBP; 0.44 [0.16, 0.72], P = .002 for ∆DBP) (Table 2). Even after adjusting for clinically relevant factors, Δsalt intake was significantly associated with ΔSBP and ΔDBP (Δsalt intake [per 1 g/d], adjusted β 0.92 [0.46, 1.39], P < .001 for ∆SBP; 0.50 [0.17, 0.83], P = .003 for ∆DBP), indicating that 1 g/d of an increase in salt intake resulted in 0.92 and 0.50 mm Hg of increases of SBP and DBP, respectively.
TABLE 2.
Changes of salt intake (Δsalt intake [per 1 g/d]) and changes of systolic and diastolic blood pressure (ΔSBP and ΔDBP [mm Hg])
| Drinking frequency | N | Unadjusted model | Adjusted model a | P for interaction † | |||
|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | ||||
| ΔSBP (mm Hg) | Overall | 451 | 0.75 (0.34, 1.16) | <.001 | 0.92 (0.46, 1.39) | <.001 | .028 |
| Rare | 91 | 0.00 (−0.84, 0.84) | .998 | 0.19 (−0.73, 1.12) | .677 | ||
| Occasional | 237 | 0.76 (0.17, 1.36) | .012 | 0.84 (0.14, 1.53) | .018 | ||
| Daily | 123 | 1.23 (0.50, 1.96) | .001 | 1.78 (0.86, 2.69) | <.001 | ||
| ΔDBP (mm Hg) | Overall | 451 | 0.44 (0.16, 0.72) | .002 | 0.50 (0.17, 0.83) | .003 | .006 |
| Rare | 91 | −0.40 (−1.12, 0.32) | .276 | −0.24 (−1.02, 0.54) | .544 | ||
| Occasional | 237 | 0.47 (0.07, 0.86) | .021 | 0.67 (0.18, 1.16) | .007 | ||
| Daily | 123 | 0.89 (0.44, 1.35) | <.001 | 0.95 (0.38, 1.51) | .001 | ||
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Adjusted for age (y), sex, smoking status (non‐, past, vs current smoking), drinking frequency (rare, occasional, vs daily), current treatment for dyslipidemia and diabetes, body mass index (kg/m2), SBP (mm Hg) (if ΔSBP), DBP (mm Hg) (if ΔDBP), total cholesterol (mg/dL), triglyceride (log mg/dL), hemoglobin A1c (%), estimated glomerular filtration rate (mL/min/1.73 m2), and salt intake (g/d) at the baseline visit
P for interaction between Δsalt intake and drinking frequency in adjusted models with ΔSBP and ΔDBP as a dependent variable, respectively.
Because of a significant interaction between drinking frequency and Δsalt intake in an adjusted model including ΔSBP as a dependent variable (P for interaction = 0.028 in Table 2), we assessed the association between Δsalt intake and ΔSBP in subjects within each category of drinking frequency, separately. In rare drinkers, no significant association between Δsalt intake and ΔSBP was observed, whereas Δsalt intake was significantly associated with ΔSBP in occasional drinkers and their association was much stronger in daily drinkers (Δsalt intake [per 1 g/d], adjusted β 0.19 [−0.73, 1.12], P = .677 in rare drinkers; 0.84 [0.14, 1.53], P = .018 in occasional drinkers; 1.78 [0.86, 2.69], P < .001 in daily drinkers) (Figure 2). Similar drinking frequency‐dependent associations were also observed in ∆DBP (Δsalt intake [per 1 g/d], adjusted β −0.24 [−1.02, 0.54], P = .544 in rare drinkers; 0.67 (0.18, 1.16), P = .007 in occasional drinkers; 0.95 [0.38, 1.51], P = .001 in daily drinkers). These results suggested that blood pressure was more sensitive to salt intake in drinkers in a dose‐dependent manner.
FIGURE 2.
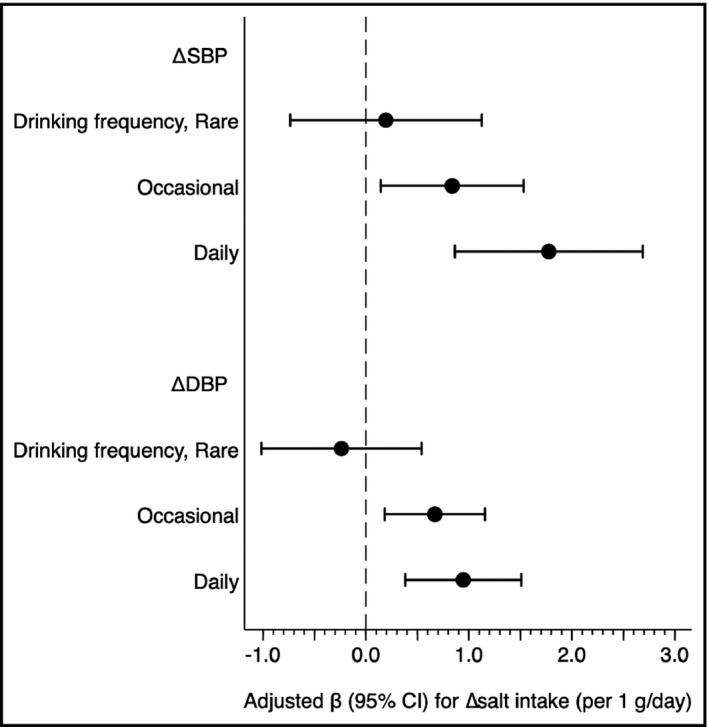
Drinking frequency modifies an association of the change of salt intake (Δ salt intake) with the change of systolic and diastolic blood pressure (∆SBP and ∆DBP). Adjusted β value were calculated using a linear regression model adjusting for age (y), sex, smoking status (non‐, past, vs current smoking), current treatment for dyslipidemia and diabetes, body mass index (kg/m2), SBP (if ΔSBP) (mm Hg), DBP (if ΔDBP) (mm Hg), total cholesterol (mg/dL), triglyceride (log mg/dL), hemoglobin A1c (%), estimated glomerular filtration rate (mL/min/1.73 m2), and salt intake (g/d) at the baseline visit
4. DISCUSSION
The present study revealed that drinking frequency modified salt sensitivity, which was the association between the change of salt intake and that of blood pressure. These results suggested that alcohol reduction might be effective in improving salt sensitivity. One of the advantages of the present study was the inclusion of Japanese subjects, who are at a high risk of salt sensitivity. 3 The results of the present study might provide clinically useful evidence to identify the subjects with enhanced salt sensitivity, which is one of the risk factors of hypertension 4 and cardiovascular disease. 5 , 6
Although large cohort studies clarified that alcohol consumption is one of the most critical factors for hypertension, 20 , 21 few studies assessed a clinical impact of alcohol on salt sensitivity. Di Gennaro et al 19 examined salt sensitivity in 30 non‐drinkers and 30 heavy alcoholics at 6‐12 months after the treatment of in‐hospital detoxification. Heavy alcoholics exhibited significant BP changes in response to salt intake. Furthermore, salt sensitivity seemed more prevalent in heavy alcoholics than in non‐drinkers. Similar to these results in the heavy drinkers, the present study showed that drinking frequency modified the association of the change of salt intake and the change of SBP and DBP in 451 employees in a pharmaceutical company, suggesting that drinking alcohol enhanced salt sensitivity in normal drinkers.
The mechanism of salt sensitivity enhanced by alcohol remains to be elucidated. As recent studies have reported that alcohol suppressed the expression of endothelial nitric oxide synthase (eNOS), 25 one of the main mechanisms of alcohol‐induced hypertension is the impairment of NO‐dependent vascular relaxation by decreasing nitric oxide (NO) in the vascular endothelium due to the suppression of eNOS or oxidative injury to the endothelium. 26 Regarding salt sensitivity, Kurtz et al 9 suggested that the similar vasodysfunction, a failure to reduce peripheral resistance to accommodate the increased volume, is the major pathophysiology of salt sensitivity. The disturbances in the NO activity are highlighted as one of the causes of vasodysfunction. Thus, alcohol‐induced vasodysfunction mediated by the suppression of NO activity may contribute to enhanced salt sensitivity. Further studies are essential to clarify the association between alcohol consumption and salt sensitivity.
The present study has several limitations. First, the salt sensitivity was not strictly measured in each subject using an interventional method of salt load and depletion. Because the present study assessed the association between the change of salt intake and that of blood pressure in a certain group of subjects, not individually, the results of the present study might be biased. The effect of modifying drinking frequency on salt sensitivity should be assessed in details after the salt sensitivity of each subject is measured using the interventional method of salt load and depletion. Second, the generalizability of the results of the present study should be examined in different cohorts. In this study, the mean estimated salt intake at baseline visit was 8.4 g/d, which was lower than in the previous studies, 27 , 28 including the National Health and Nutrition Survey Japan in 2012 (the average salt intake of 10.4 g/d). 29 Another characteristic of the participants of the present study was the low prevalence of diabetes (0.4%) and dyslipidemia (2.4%). The results of the present study should be confirmed in the general population with higher salt intake and the patients with cardiometabolic disease, including diabetes, and dyslipidemia. Third, information of drinking frequency was based on a simple self‐reported questionnaire, in which never drinkers and past drinkers were categorized into rare drinkers. Given that past drinkers, including sick quitters, were more salt‐sensitive than never drinkers, the association between ∆salt intake and ∆SBP (or ∆DBP) in rare drinkers overestimated that in never drinkers, suggesting that the difference in salt sensitivity among rare, occasional, and daily drinkers in this study were underestimated, compared with the difference in salt sensitivity among never occasional, and daily drinkers. Fourth, self‐reported drinking frequency might be biased. Several studies reported that alcohol drinking was likely to be underreported. 30 , 31
In conclusion, the present study identified enhanced salt sensitivity in the subjects with higher frequency of drinking. These results strongly suggest that the reduction in alcohol consumption may be more effective for improvement of salt sensitivity in higher frequency drinkers. Its efficacy should be evaluated in well‐designed randomized controlled trials.
CONFLICT OF INTEREST
TM and YI are employed as occupational physicians by Shionogi & Co., Ltd.; YM is an employee of Shionogi & Co., Ltd. as a managing director of Shionogi Health Insurance Association; other authors declare no conflict of interest.
AUTHORS' CONTRIBUTION
Moriyama T and Moriguchi Y contributed to research idea, study design, data acquisition, and organization of the study; Yoshimura R contributed to data management and statistical analysis; Yamamoto R, Shinzawa M, Tomi R, Ozaki S, and Fujii Y contributed to interpretation; Isaka Y, Tanabe K, and Ito T contributed to supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Yoshimura R, Yamamoto R, Shinzawa M, et al. Drinking frequency modifies an association between salt intake and blood pressure: A cohort study. J Clin Hypertens. 2020;22:649–655. 10.1111/jch.13844
Funding information
The present study was funded by Shionogi & Co., Ltd.
REFERENCES
- 1. Elijovich F, Weinberger MH, Anderson CAM, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7‐e46. [DOI] [PubMed] [Google Scholar]
- 2. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:127‐134. [DOI] [PubMed] [Google Scholar]
- 3. Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521‐525. [DOI] [PubMed] [Google Scholar]
- 4. Barba G, Galletti F, Cappuccio FP, et al. Incidence of hypertension in individuals with different blood pressure salt‐sensitivity: results of a 15‐year follow‐up study. J Hypertens. 2007;25:1465‐1471. [DOI] [PubMed] [Google Scholar]
- 5. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429‐432. [DOI] [PubMed] [Google Scholar]
- 6. Morimoto A, Uzu T, Fujii T, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734‐1737. [DOI] [PubMed] [Google Scholar]
- 7. Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt‐induced hypertension. Circulation. 2016;133:894‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. Changing views on the common physiologic abnormality that mediates salt sensitivity and initiation of salt‐induced hypertension: Japanese research underpinning the vasodysfunction theory of salt sensitivity. Hypertens Res. 2019;42:6‐18. [DOI] [PubMed] [Google Scholar]
- 10. Kelly TN, He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens. 2012;30:861‐873. [DOI] [PubMed] [Google Scholar]
- 11. Imaizumi T, Ando M, Nakatochi M, et al. Association of interactions between dietary salt consumption and hypertension‐susceptibility genetic polymorphisms with blood pressure among Japanese male workers. Clin Exp Nephrol. 2017;21:457‐464. [DOI] [PubMed] [Google Scholar]
- 12. Liu Z, Qi H, Liu B, et al. Genetic susceptibility to salt‐sensitive hypertension in a Han Chinese population: a validation study of candidate genes. Hypertens Res. 2017;40:876‐884. [DOI] [PubMed] [Google Scholar]
- 13. He J, Gu D, Chen J, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Ann Intern Med. 2001;135:1019‐1028. [DOI] [PubMed] [Google Scholar]
- 15. Rebholz CM, Gu D, Chen J, et al. Physical activity reduces salt sensitivity of blood pressure: the Genetic Epidemiology Network of Salt Sensitivity Study. Am J Epidemiol. 2012;176(suppl 7):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart DL, Harshfield GA, Zhu H, Hanevold CD. Stress and salt sensitivity in primary hypertension. Curr Hypertens Rep. 2015;17:2. [DOI] [PubMed] [Google Scholar]
- 17. Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in dahl salt‐sensitive rats. Clin Exp Hypertens. 2009;31:451‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabral PD, Hong NJ, Abdul Hye Khan M, et al. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension. 2014;63:e68‐e73. [DOI] [PubMed] [Google Scholar]
- 19. Di Gennaro C, Barilli A, Giuffredi C, Gatti C, Montanari A, Vescovi PP. Sodium sensitivity of blood pressure in long‐term detoxified alcoholics. Hypertension. 2000;35(4):869‐874. [DOI] [PubMed] [Google Scholar]
- 20. Sesso HD, Cook NR, Buring JE, Manson JE,Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51:1080‐1087. [DOI] [PubMed] [Google Scholar]
- 21. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. J Clin Hypertens. 2012;14:792‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97‐103. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 24. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303‐308. [DOI] [PubMed] [Google Scholar]
- 25. Husain K, Ferder L, Ansari RA, Lalla J. Chronic ethanol ingestion induces aortic inflammation/oxidative endothelial injury and hypertension in rats. Hum Exp Toxicol. 2011;30:930‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Husain K, Ansari RA, Ferder L. Alcohol‐induced hypertension: Mechanism and prevention. World J Cardiol. 2014;6:245‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi N, Tanabe K, Adachi T, et al. Awareness of salt restriction is not reflected in the actual salt intake in Japanese hypertensive patients. Clin Exp Hypertens. 2015;37:388‐392. [DOI] [PubMed] [Google Scholar]
- 28. Uechi K, Asakura K, Masayasu S, Sasaki S. Within‐country variation of salt intake assessed via urinary excretion in Japan: a multilevel analysis in all 47 prefectures. Hypertens Res. 2017;40:598‐605. [DOI] [PubMed] [Google Scholar]
- 29. Takimoto H, Saito A, Htun NC, Abe K. Food items contributing to high dietary salt intake among Japanese adults in the 2012 National Health and Nutrition Survey. Hypertens Res. 2018;41:209‐212. [DOI] [PubMed] [Google Scholar]
- 30. Stockwell T, Zhao J, Sherk A, Rehm J, Shield K, Naimi T. Underestimation of alcohol consumption in cohort studies and implications for alcohol's contribution to the global burden of disease. Addiction. 2018;113:2245‐2249. [DOI] [PubMed] [Google Scholar]
- 31. Garnett C, Crane D, West R, Michie S, Brown J, Winstock A. Normative misperceptions about alcohol use in the general population of drinkers: a cross‐sectional survey. Addict Behav. 2015;42:203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]


