Abstract
Accurate office blood pressure measurement remains crucial in the diagnosis and management of hypertension worldwide, including Latin America (LA). Office blood pressure (OBP) measurement is still the leading technique in LA for screening and diagnosis of hypertension, monitoring of treatment, and long‐term follow‐up. Despite this, due to the increasing awareness of the limitations affecting OBP and to the accumulating evidence on the importance of ambulatory BP monitoring (ABPM), as a complement of OBP in the clinical approach to the hypertensive patient, a progressively greater attention has been paid worldwide to the information on daytime and nighttime BP patterns offered by 24‐h ABPM in the diagnostic, prognostic, and therapeutic management of hypertension. In LA countries, most of the Scientific Societies of Hypertension and/or Cardiology have issued guidelines for hypertension care, and most of them include a special section on ABPM. Also, full guidelines on ABPM are available. However, despite the available evidence on the advantages of ABPM for the diagnosis and management of hypertension in LA, availability of ABPM is often restricted to cities with large population, and access to this technology by lower‐income patients is sometimes limited by its excessive cost. The authors hope that this document might stimulate health authorities in each LA Country, as well as in other countries in the world, to regulate ABPM access and to widen the range of patients able to access the benefits of this technique.
Keywords: ambulatory blood pressure monitoring, arterial hypertension, hypertension diagnosis and management, Latin America
1. INTRODUCTION
Accurate office blood pressure measurement remains crucial in the diagnosis and management of hypertension worldwide, including Latin America (LA).1, 2 Office blood pressure (OBP) measurement is still the leading technique in LA for screening and diagnosis of hypertension, monitoring of treatment, and long‐term follow‐up. Despite this, due to the increasing awareness of the limitations affecting OBP and to the accumulating evidence on the importance of ambulatory BP monitoring (ABPM), as a complement of OBP in the clinical approach to the hypertensive patient, a progressively greater attention has been paid worldwide to the information on daytime and nighttime BP patterns offered by 24‐h ABPM in the diagnostic, prognostic, and therapeutic management of hypertension.
In LA countries, most of the Scientific Societies of Hypertension and/or Cardiology have issued guidelines for hypertension care,3, 4, 5, 6, 7 and most of them include a special section on ABPM. Also, full guidelines on ABPM are available.5
However, in LA, the availability of ABPM is often restricted to cities with large population, and access to this technology by lower‐income patients is sometimes limited by its excessive cost.
In the following sections, we will address in detail the available evidence on the advantages of ABPM for the diagnosis and management of hypertension also in LA.
We hope that this document might stimulate health authorities in each LA Country, as well as in other countries in the world, to regulate ABPM access and to widen the range of patients able to access the benefits of this technique.
2. HISTORICAL PERSPECTIVE
The ABPM technique was first described in 1960s by Kain and colleagues, and was initially based on semi‐automated BP measurements.8, 9
First devices were cumbersome8; but progress in technology has made currently available ambulatory monitors smaller, lighter, and minimally noisy, with most of them fully automated and using the oscillometric technique. The use of modern validated ABPM devices thus allows BP to be reliably monitored for 24 hours or longer while patients attend their usual daily activities.10 Nowadays, the large amount of evidence supporting ABPM advantages over the conventional OBP technique for the diagnosis and management of hypertension has highlighted the superiority of the former over the latter approach, which is now acknowledged by all main international guidelines for hypertension management.2, 11, 12, 13 Most of the advantages of 24‐h ABPM come from its ability to provide a large number of measurements over the 24 hours and from the possibility to obtain BP measurements in subjects' daily life, and both during wakefulness and during sleep. This implies, from a practical perspective, that whenever ABPM is not available or is difficult to access, at least part of its advantages can nevertheless be obtained either through the use of home BP self‐monitoring (recently further improved by the current availability of devices with nocturnal BP monitoring function) or through an increase in the number of automated readings which can be obtained by repeating BP measurements both within visits and over repeated visits, and then averaged.14, 15 However, even in developed countries the use of ABPM is at present still recommended in selected cases only, although indications for ABPM are becoming progressively wider, based on the evidence that a larger use of ABPM could contribute to reduce health care cost, prevent cardiovascular events, and be life‐saving.16, 17
3. GENERAL CONSIDERATIONS
3.1. Why is ABPM superior to conventional BP measurement?
OBP measurements are widely available and have an acceptable performance for clinical practice, mainly when obtained through automated and validated oscillometric devices. However, we should be aware that OBP values are characterized by great variability, due both to a random error that affects casual readings and a systematic error in relation to the patient alert reaction, known as “white coat phenomenon.” Furthermore, OBP readings do not provide information about BP during a subject's usual activities, including nighttime sleep, or over prolonged observation periods. ABPM overcomes all these limitations of OBP and has a stronger association with target organ damage18 and cardiovascular prognosis.19
3.2. Recommendations for ABPM use in clinical practice
3.2.1. Frequency and timing
Outcome‐based approach
Two recent publications analyzed the reliability of the number of ABPM recordings to determine the Ambulatory Arterial Stiffness Index (AASI), the pulse pressure (PP), and the reading‐to‐reading BP variability based on outcome.20, 21 Excluding up to 16 readings from recordings reduced the discordance of AASI, but not PP variability over repeated recordings. Taking readings at fixed 1‐ or 2‐hour intervals along the recording significantly affected the concordance of both AASI and PP variability estimates. In terms of outcome (cardiovascular mortality), AASI lost its prognostic significance when the number of randomly excluded readings increased from 8 to 16 or when the interval between readings was 1 hour or longer. While PP variability did not predict cardiovascular mortality, the predictive accuracy of AASI for cardiovascular mortality was affected when the number of readings was lower than 35. Using the Average Real Variability index (ARV) to determine the reading‐to‐reading BP variability, a minimum of 48 readings allowed an accurate assessment of cardiovascular risk.21 There is, however, no published evidence supporting a minimum number of readings based on outcome to determine the blood pressure mean and variability levels derived from ABPM.
Clinical approach
There is no new evidence in this field. The evidence from the pioneering works of Di Rienzo22 and Thijs23, 24 is still the main references supporting the frequency and timing of ABPM. Some evidence on the number of ambulatory BP readings required for reliable assessment of ambulatory BP levels was also provided by the IDACO group.25
3.2.2. Recommended schedule for ABPM
For the reasons exposed above, ABPM must be performed over 24 hours and should include both daytime and nighttime periods.26, 27 One simple and popular method for identifying daytime and nighttime subperiods is to assess the time of awakening and sleeping from diary card entries. Another method is to use a fixed‐narrow time interval approach. According to this approach, nighttime and daytime are defined by removing blood pressure measurement performed during the transitional phases between day and night in the evening and between night and day in the morning. The interval between readings should not be longer than 60 minutes and ideally shorter than 30 minutes.27, 28, 29 It is recommended to identify the recording times without measurements, and when their duration is 2 hours or longer, this should be reported as major limitation of an ABPM tracing, requiring repetition of the examination. To avoid losing prognostic accuracy when reporting AASI and ARV or other BPV indices, it is recommended to have at least 35 and 48 readings, respectively.
Currently available ABPM guidelines by the European Society of Hypertension (ESH) Working Group on BP monitoring and cardiovascular variability27, 28, 29, 30 recommend to consider as acceptable a minimum number of 20 valid daytime (awake) measurements and 7 measurements at night (asleep), based on the requirement to have at least 70% of measurements being obtained at least every 30 min, or more frequently, throughout the entire 24‐h period (Box 1).27
Box 1. Evaluation of ABPM data. modified from Parati et al27 by permission.
|
Definition of daytime and nighttime
Editing and requirements
|
3.2.3. Diagnostic and therapeutic thresholds
We did not find new evidence proposing different cut‐off thresholds for ABPM since the last ESH ABPM position paper was published in 2013.28 We support that it is necessary to move from thresholds based on statistical evidence to outcome‐driven thresholds. Based on a 10‐year cardiovascular risk equivalent to the risk obtained with office blood pressure measurement, suggested thresholds for ABPM were 131/79 mmHg for 24‐hour, 138/86 mmHg for daytime, and 120/71 mmHg for nighttime blood pressure31 substantially supporting the current indications provided by ESH guidelines, that is, 130/80 mmHg for 24 hours, 135/85 mmHg for daytime, and 120/70 mmHg for nighttime32, 33 (Table 1).
Table 1.
ABPM‐based hypertension diagnostic thresholds. Modified by permission from Parati et al (27)
| Blood pressure | Systolic (mmHg) | Diastolic (mmHg) |
|---|---|---|
| 24‐hour average | ≥130 | ≥ 80 |
| Daytime average | ≥ 135 | ≥85 |
| Nighttime average | ≥ 120 | ≥ 70 |
3.3. Cost‐effectiveness of ABPM
As reported in the 2013 ESH Working Group ABPM guidelines, ABPM may allow health care costs reduction by identifying individuals with white coat hypertension who, when at low cardiovascular risk, may not require pharmacological treatment despite an OBP elevation. ABPM is indeed the most effective technique for identifying white coat hypertension, which may be present in as many as 20% of people who appear to have isolated office BP elevation, and these patients may avoid years of unnecessary and expensive drug treatment, which is often not free from side effects. They may also avoid being penalized unnecessarily because of health insurance or employment‐related issues when erroneously diagnosed as having “hypertension”.34 The cost‐effectiveness of identifying white coat hypertension through ABPM is related to the fact that the cost of care for hypertension is largely dominated by the cost of cardiovascular complications occurring in the long term, and by the cost for drug treatment, rather than by the cost of doctors' visits and of diagnostic investigations. This is of importance in LA for the limited resources available for health care, which require a long‐term acceptable cost‐effectiveness ratio for any approach proposed for hypertension management.33, 34, 35 When assessing the cost‐effectiveness of ABPM,33, 35, 36 in particular in LA where its use is only allowed in large cities and in some countries, the potential of this technique not only to improve the diagnosis and management of hypertension, but also as a means of ensuring implementation of a more effective control of hypertension at community level should be taken into consideration (Box 2).
Box 2. Advantages of ABPM. adapted from Parati et al 27 by permission.
Provides much larger number of readings
Provides highly reproducible average 24‐h, daytime, and nighttime values
Identifies white coat and masked hypertension phenomena in untreated and treated individuals
Provides a profile of BP behavior in the individual's usual daily environment
Demonstrated nocturnal hypertension and dipping patterns
Assesses BP variability over the 24‐h period
Assesses the 24‐h efficacy of antihypertensive medication
Detect excessive BP‐lowering during 24h
Is a much stronger predictor of cardiovascular morbidity and mortality.
ABPM is indeed superior to other BP measurement techniques in demonstrating the efficacy of antihypertensive drugs and the achievement of 24‐h coverage.28, 37
Adjustment of antihypertensive therapy according to ABPM rather than office BP measurements has been shown to result in less antihypertensive medications being prescribed without compromising target organ involvement. It has also been shown that, in patients on treatment with BP‐lowering drugs, ABPM was a better predictor of cardiovascular outcome than office BP.27, 28, 37 Reduction in office BP over time due to a progressive reduction in the white coat effect, especially in elderly patients, who are characterized by a greater BP variability, may be, in fact, erroneously attributed to a BP‐lowering effect of antihypertensive treatment when ABPM is not used to assess treatment efficacy.38 There is a high rate of uncontrolled hypertension in LA due to poor adherence to prescribed treatment.39 It is motivated by social and economic factors in most of these countries. In such a context, it should be acknowledged that ABPM and home blood pressure monitoring (HBPM)40 may help to evaluate the effectiveness of hypertension treatment and may help achieving a better hypertension control.41, 42
It is accepted that ABPM can also identify individuals with normal BP in the office but elevated BP levels in daily life (“masked hypertension”), a condition that has been shown to carry the same adverse prognosis as sustained arterial hypertension, that is, a condition characterized by a BP elevation both in the clinic and in daily life (Figure 1).16, 27, 28
Figure 1.
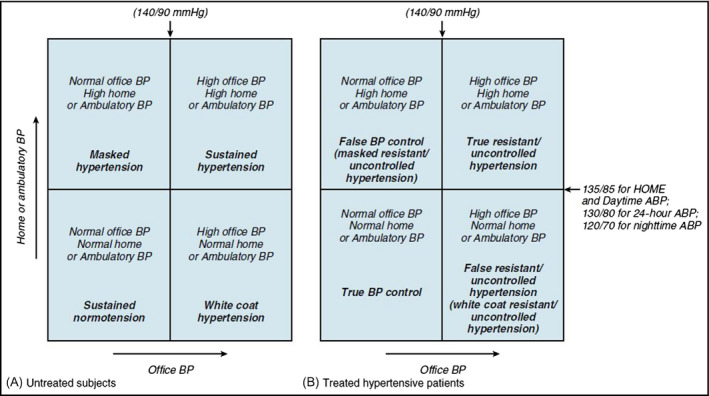
Different blood pressure phenotypes in treated and untreated hypertensives, respectively, defined by comparing office and out‐of‐office BP measurements. Reproduced from Parati et al 96 by permission
A number of studies have analyzed the cost‐benefit aspects of ABPM. Krakoff has shown potential savings of 3‐14% for cost of care for hypertension and 10‐23% reduction in treatment days when ABPM was incorporated into the diagnostic process.43 On an annual basis, the cost of ABPM would be less than 10% of treatment costs. Other cost‐benefit analyses have shown that ABPM is most cost‐effective for the diagnosis and management of newly diagnosed hypertension.
As the cost of ABPM and that of hypertension management differ greatly from country to country and is dependent on the method of health care delivery, the cost‐effectiveness of ABPM may need to be evaluated at a national level.
3.4. Advantages and Limitations of ABPM
As it was described earlier, ABPM has many advantages over other available BP measurement techniques (Table 2).
Table 2.
Comparison of features of main methods for blood pressure measurement
| OBP | AOBP | ABPM | HBPM | |
|---|---|---|---|---|
| Approx. no. of readings | 2‐3 | 3‐6 | 50‐100 | 10‐30a |
| Operator dependency | Yes | No | No | No |
| Reproducibility | Poor | Better than OBP | Good | Good |
| White coat effect | Yes | No | No | No |
| Patient training | No | No | Limited | Required |
| Patients' acceptance | Good | Good | Sometimes poor | Usually good |
| Improvement in patient involvement and adherence | No | No | No | Yes |
| Nighttime blood pressure | No | No | Yes | Nob |
| Outcome evidence | +++ | + | +++ | ++ |
| Hypertension diagnosis thresholds (mmHg) | 140/90 | 130‐135/85 |
24 h: 130/80 Day: 135/85 Night: 120/70 |
135/85 |
| Monitoring of treatment | During visits | During visits | Good for 24‐h effects | Good for long‐term monitoring, some insight in 24‐h effects |
| Cost | Low | Low | High | Low |
ABPM, ambulatory blood pressure monitoring; AOBP, automated office blood pressure; HBPM, home blood pressure monitoring; OBP, office blood pressure. Modified from Parati G et al 40 by permission.
With a standard protocol of few days' monitoring.
Nighttime measurements implemented in some models.
Despite these advantages, also ABPM faces a number of possible difficulties in its application (Box 3).
Box 3. Limitations of ABPM. modified from 27 by permission.
Limited availability
The provision of a number of intermittent measurements during which the patient is sedentary rather than "ambulatory," whenever subjects' behavior is not standardized
The possibility of inaccurate readings during activity and the inability to detect possibly artifactual measurements
May cause discomfort, particularly during the night, interfering with sleep patterns and resulting in resistance from some patients to having ABPM repetition during follow‐up
They include its limited availability, the fact that a number of measurements are taken when the patient is “sedentary” rather than “ambulatory,” the possibility of inaccurate readings during activity and the inability to easily identify possibly artifactual measurements, and, finally, the fact that ABPM may cause discomfort, particularly during the night, interfering with sleep patterns and resulting in resistance from some patients to having ABPM repetition during follow‐up.27
4. DEVICES AND SOFTWARE
An accurate device is a fundamental requirement for all BP measurements; if the device used to measure BP is inaccurate, then any discussion on methodological details becomes irrelevant. It is acknowledged that the accuracy of BP‐measuring devices should not be based solely on claims from manufacturers, which can at times be somewhat misleading, but should rather rely on independent validation using an established protocol with the results published in peer‐reviewed journals. The most popular validation protocol is the European Society of Hypertension International Protocol (ESH‐IP),29 and a recent review44 showed that from the publication of the first version of the ESH‐IP in 2002 until June 2010, 48 studies of device accuracy have been reported using the British Hypertension Society (BHS) protocol,45 38 using the AAMI standard, and 104 using the ESH‐IP 2010.30 Thus, it seems that the ESH‐IP succeeded in expanding by 3‐fold to 4‐fold the use of validation procedures worldwide compared with the period before its publication. The availability of the ESH‐IP30, 44 in an online version will further facilitate the validation of ABPM devices.
Since the time when the ESH‐IP29 was first published, there has been an improvement in the performance of the oscillometric devices for BP measurement, but protocol violations and misreporting have been particularly common, suggesting that there is a need for stricter standardization for conducting and reporting a validation study. The revised version of the ESH‐IP30 protocol applied tighter validation criteria for the pass level, and the application of these more stringent criteria is expected to double the validation failure rate allowing more accurate devices to enter the market.
The AAMI protocol requires additional testing for ABPM devices in 85 patients in the supine, seated, and standing positions, and stipulates that three devices should be assessed in ambulatory conditions, all of which would be very difficult and costly to perform. The BHS protocol also requires an in‐use (field) assessment of ambulatory monitors45 The International Organization for Standardization (ISO) protocol also requires additional clinical validation in 35 patients in standardized conditions of physical activity (after exercise on a bicycle ergometer or treadmill to increase heart rate by 10‐20%).46 In the attempt to standardize the approach to validation of BP‐measuring devices, an international protocol jointly prepared and approved by ISO, AAMI, and ESH is currently being developed.47
4.1. Choosing an ABPM device
One important issue in LA is that each country has national regulatory agencies for validation of medical devices, a situation which may more easily allow non‐validated devices to be available in the market. A uniform policy across LA countries, incorporating the same international protocol, may contribute to reduce the presence in the market of inaccurate BP monitors, thus improving the precision of BP measurements.
4.1.1. Selecting an accurate device
In LA, several types of ABPM devices are in use. However, some of them are not validated according to the international protocols. A list of validated devices should be available on the Web site of National LA Hypertension Societies, to facilitate a proper choice by clinicians.
4.1.2. Validation requirements for ABPM devices in special populations
Separate validation is required in specific populations, such as children and adolescents, pregnant women, and the elderly, and in certain diseases, such as obesity and arrhythmias.48 Patients with arrhythmias, children, the elderly, and pregnant women present special challenges for the validation of oscillometric ABPM devices. However, although ABPM devices are used frequently in these patient populations, specific validation studies have often not been performed. This limitation applies also to other methods of BP measurement, such as home and office measurements. The validation status of ABPM devices in different populations can be checked on dedicated Web sites (eg, the Web site of the British and Irish Hypertension Society and the recently created STRIDE‐BP.org Web site: https://www.stridebp.org/bp-monitors). Although specific validation studies of ABPM devices in these special populations are desirable in the future, at present devices that have been validated in the general hypertensive population can be used so that high‐risk patients are not denied the benefit of ABPM.
4.2. Software for ABPM data analysis
Recommendations on procedures for ABPM use tend to focus on the accuracy of BP measurements provided by any given device, with little attention being paid to presentation and analysis of ABPM data, which may make translating the results of an ABPM into clinical practice decisions a more difficult task for physicians due to the considerable amount of data provided by ABPM reports currently made available from companies proprietary software. Therefore, in daily practice, only a one‐page report, including basic data such as average 24‐h, daytime, and nighttime values, the individual readings for a direct accuracy check and a graphic plot showing the 24‐h BP values distribution might be enough, whereas for research purposes more details on the 24‐h profile and other indices may be required. An additional problem can arise from the calculation of 24‐h average BP values that are not weighted for the different number of hourly measurements during daytime and nighttime; weighted hourly BP values have indeed been shown to be more reliable than calculations based on the average of all individual readings.49
The use of ABPM in clinical practice can be facilitated by standardizing the graphic presentation of ABPM data, so that the presentation of data is independent of the type of ABPM monitor used and the user is not required to become familiar with a variety of different programs. Standardization also facilitates the interchange of ABPM recordings between databases, such as hospitals and primary care practices. Moreover, if ABPM software programs provide a printed report of the standardized ABPM data with a validated interpretative report, doctors and nurses unfamiliar with the technique are assisted in learning the variety of patterns generated by ABPM, and because an individualized physician report is not a requirement in most cases, the cost of ABPM performance in daily practice could be reduced.50
4.2.1. Software requirements
A standardized single‐page report should be provided by all types of monitors. This request was originally raised by the ESH and later supported by other scientific societies in LA.27, 51
The software should display the raw BP and heart rate (HR) data and summary statistics for these parameters during 24 h, daytime, and nighttime separately. A plot of all BP and HR readings taken during the 24‐h period is also mandatory. The plot should indicate individual's awake and asleep time intervals and normality bands for diurnal and nocturnal periods. Finally, a short interpretative report automatically generated, with the possibility of manual editing, would be desirable.
4.2.2. Indices derived from ABPM recordings
Main parameters for clinical decisions are the 24‐h, daytime, and nighttime BP and HR averages. Those parameters should be always included and highlighted in all ABPM r eports.52
Other indices derived from ABPM are still restricted for research and cannot yet be recommended to derive clinical decisions in individual patients. These additional indices include short‐term BP variability indices (24h, daytime, and nighttime SD, weighted‐24‐h SD, and ARV); circadian BP variability indices (night‐to‐day BP ratio, nighttime % BP dipping, and morning surge BP parameters); ambulatory arterial stiffness index (AASI); area under the curve calculations; BP load parameters; rate‐pressure product; cusum statistics, trough and peak levels; and smoothness index.27, 28
4.2.3. New Devices for Ambulatory blood pressure monitoring and central blood pressure estimation
A few 24‐h oscillometric ABPM monitors use specific algorithms and software for pulse wave analysis. They offer the conventional 24‐h ABPM data and, in addition, a number of parameters derived from pulse waveform analysis, including pulse wave velocity and central BP estimates. Further studies are needed, however, to demonstrate the validity and clinical usefulness of these parameters.
The interest in the use of devices with the availability of central/aortic blood pressure measurement assumes that the evaluation of these hemodynamic parameters non‐invasively may improve patients' diagnostic and prognostic assessment.
An increasing number of these devices are being available, using different methodologies for central blood pressure estimation. Their validation, however, was in most cases obtained only in laboratory conditions, so that their actual accuracy in an ambulatory setting still remains a pending issue. For these reasons, and for lack of clinical data on the actual diagnostic and prognostic value ambulatory central BP and PWV, these parameters should be considered for the present time only for research applications, and not used for making clinical decisions.
A recent meta‐analysis of validation studies of commercial devices for non‐invasive estimation of aortic BP has provided the following conclusions:
There is a major discrepancy in the methodologies used by these devices to estimate central BP; thus, a consensus is needed for their standardization.
The estimation of aortic SBP by the currently available devices seems to be accurate, but only at rest, when the devices were calibrated using invasive intra‐arterial BP measurements and when comparing invasive and non‐invasive waveforms, by considering the first 6 to 10 harmonics of the pulse waveforms recorded.
For the estimation of pulse wave velocity, which is considered a more solid index of organ damage with some prognostic implications, more information about the algorithms used is required. The state of the art methodology for PWV assessment is still currently the approach based on tonometric detection of pulse waveforms in standardized conditions at rest.
Information on central BP could be useful for the evaluation of systolic hypertension in young, although most of the current evidence in this regard has been obtained with the tonometric approach in a laboratory setting. More studies are still needed to further validate the ambulatory central BP assessment provided by novel ABPM devices equipped with this function.
A consensus paper has recently been published on how such validation should be performed.53
Recent evidence emphasizes the limitations of the algorithmic approach used by these ABPM devices in measuring central BP and PWV, because in some devices the algorithmic estimate of PWV is only based on squared age values and arm cuff systolic BP and is not really measured, thus being unable to pick‐up alterations in aortic wall properties in specific types of patients, for example, in Marfan's syndrome patients.54, 55
5. ABPM PROCEDURES
5.1. Training requirements
Before prescribing an ABPM, we should assess whether the patient will be capable of understanding the procedure, thus contributing to the accuracy of readings. Before starting the recording, patients should receive specific instructions, as detailed in paragraph 5.3.
5.2. Fitting an ABP monitor
Preferably, ABPM should be performed on a working day to assess the patient's out‐of‐office blood pressure in usual daily conditions.
ABP monitor programming includes entering patient details, programming frequency of measurement (every 15‐30 min) separately for daytime and nighttime, and inactivating display of measurements during the recording to avoid excessive anxiety. Avoidance of sounds alerting the beginning of measurements is recommended at least during nighttime.
The sequence of fitting the ABP monitor includes selecting an appropriate cuff according to standard recommendations (the cuff should encircle 80‐100% of arm circumference), and placing the cuff on the non‐dominant arm, with the tube passing upwards around patient's neck to be connected to the monitor on the waist.
Finally, a trial measurement should be performed before the patient leaves the clinic, to check whether the device is properly working and to familiarize the patient with the monitor.27, 28
5.3. Instructions to patient
These instructions have been detailed in the 2014 ESH Practice Guidelines paper.27 In particular, patients should be instructed to deal with what they are expected to do during a 24‐h ABP recording. Patients should keep a diary card, reporting major events occurring during the recording time, and writing down the name, dose, and time of administration of each prescribed drug, the sleeping and awakening times, time of main meals, and also a short description of any unusual activity and of any symptoms that might occur.
Patient should be also instructed to follow their usual daily activities, but to remain still and to keep the arm hanging down along the body each time the cuff starts inflation during the measurements. Patients should also be warned to avoid taking a shower and immersion baths during the recording time while wearing the device, which should not be removed either for such purposes. Instructions to the patient on how to reposition the cuff in case of cuff displacement should also be provided. Finally, the patient should be instructed to turn the device off in case of malfunction.
5.4. Identification of daytime and nighttime periods and editing ABPM data
As mentioned above, the identification of daytime and nighttime periods should be based on the patient's diary card. Whenever this is not possible, adoption of criteria based on narrow‐fixed time intervals, which exclude the measurements taken in the transition phase between sleep and wakefulness, may also provide reliable data. Editing ABPM data should be avoided as much as possible. However, clearly artifactual readings should be identified, as suggested in the ESH guidelines.27, 28
5.5. Conditions in which ABPM may be difficult to perform
These conditions include obesity, arm amputations, skin injuries or burns, patients under hemodialysis with previous arteriovenous fistulae, patients with severe parkinsonism or neurologic lesion, marked arterial stiffness and calcification, and women with arm lymphedema, resulting from breast cancer surgery or other causes.
6. CLINICAL INDICATIONS FOR ABPM
6.1. Role of ABPM in the diagnosis of hypertension
The most well‐established indication for using ABPM is to identify white coat and masked hypertension (see definition below) and to describe alterations in 24‐h BP profiles (Table 3).
Table 3.
Clinical indications for ABPM. Modified from Parati et al 27 by permission
| Compelling Indications |
|---|
| Identifying White Coat Hypertension Phenomena |
|
| Identifying Masked Hypertension Phenomena |
|
| Identifying Abnormal 24‐h BP Patterns |
|
| Assessment of Treatment |
|
| Additional Indications |
|---|
|
6.1.1. White coat phenomena
White coat hypertension
White coat hypertension (WCH), also referred as isolated clinic hypertension, is defined in untreated patients by an elevated BP at doctor's office (SBP ≥ 140/DBP ≥ 90 mmHg) associated with normal BP during usual daily activities (daytime SBP < 135/DBP < 85 mmHg). During the last years, in order to incorporate the prognostic value of nighttime BP, the 24‐h BP threshold (SBP < 130/DBP < 80 mmHg) was also accepted for this diagnosis.27, 28 Indeed, considering normal ambulatory BP during all interval periods (ie, daytime, nighttime, and 24 h) as reference value improves cardiovascular risk prediction in white coat hypertension.56 The magnitude of the cardiovascular risk associated with WCH is small, and its clinical significance has been long debated. Results from the PAMELA study indicates in WCH a higher prevalence of metabolic alterations and a higher risk of developing sustained hypertension during follow‐up.57 On the other hand, a recent publication found that CVD risk in most persons with WCH is comparable to age‐ and risk‐adjusted normotensive control subjects.58 Recent 2018 ESH Hypertension Guidelines have emphasized that the occurrence of an increased risk of cardiovascular complications in white coat hypertension is not negligible.59
White coat effect
The transient BP rise associated with the presence of a doctor in the office was defined as “white coat effect”,60 and could lead to an overestimation of BP in many subjects.60, 61 The best assessment of a WCE should be obtained by directly assessing beat by beat BP changes during as compared to before a doctor's visit.60 Conventionally, however, a surrogate definition of WCE is employed, based on the difference between office and out‐of‐office (usually awake) BP levels. Patients with an office BP of at least 20mmHg systolic and/or 10mmHg diastolic higher than the awake ambulatory BP have been designated as having a “clinically important” white coat effect according to this indirect approach.62 WCH is commonly considered as a consequence of the white coat effect. The most common finding is the observation of patients with mild office hypertension, slightly higher than a normal ambulatory blood pressure (ie, with a small WCE).63 However, there are situations in which the white coat effect does not configure WCH. As an example, patients with sustained hypertension may have a marked “white coat effect” with high clinic BP also associated with higher than normal ambulatory BP levels.61, 63 Finally, hypertensive subjects under drug treatment presenting marked white coat effect and normal ambulatory BP should be considered as having “uncontrolled white coat hypertension”.64, 65
6.1.2. Masked phenomena
Masked hypertension
Masked hypertension (MHT) characterizes untreated individuals with elevated ambulatory BP but normal office BP.66 The classic definition of MHT combines office BP under the thresholds for hypertension (SBP < 140/DBP < 90 mmHg) and ambulatory daytime BP over the thresholds for hypertension (SBP ≥ 135/DBP ≥ 85 mmHg). However, for the reasons exposed for WCH the definition was extended to ambulatory 24‐h thresholds (SBP ≥ 130/DBP ≥ 80 mmHg), in order to include also nocturnal BP.67 However, a recent publication shows that considering additional interval periods than daytime (ie, nighttime and/or 24 h) does not improve cardiovascular risk prediction of masked hypertension.26 The prevalence of masked hypertension ranges from 10% to 30% of individuals, and this proportion is higher in special populations such as adolescents, overweight and obese individuals, and patients with chronic kidney disease. A recent study in healthy employees highlighted ambulatory BP exceeding office BP by more than 10 mmHg much more frequently than office BP exceeded ambulatory BP.16 Furthermore, the cardiovascular risk attained with masked hypertension is equivalent to the risk associated with sustained hypertension.33, 68
Masked uncontrolled hypertension
In a patient receiving BP‐lowering drugs, the hypertensive status is evident; therefore, this patient cannot be defined as “masked hypertensive.” Masked uncontrolled hypertension is the situation defined by the presence of controlled office BP (SBP < 140/DBP < 90mmHg) and uncontrolled ambulatory BP (daytime SBP ≥ 135/DBP ≥ 85 mmHg or 24‐h SBP ≥ 130/DBP ≥ 80 mmHg) in treated patients. In patients with masked uncontrolled HT, antihypertensive treatment should be optimized to reach BP control throughout the 24‐h period, aimed at preventing the cardiovascular consequences of uncontrolled hypertension.16, 59, 64 This recommendation, however, is only based on observational studies and experts' opinion, in the absence of intervention controlled trials specifically addressing this issue.59 The ongoing master study might help clarifying this issue.69
6.1.3. Abnormal 24‐h blood pressure patterns
Abnormal BP patterns include deviations from the normal circadian pattern of BP, including daytime hypertension; altered siesta dipping/post‐prandial hypotension; nocturnal hypertension; reduced dipping, non‐dipping; extreme dipping and rising; morning hypertension and enhanced morning BP surge; and an increase in short‐term BP variability.70 Main characteristics of these patterns are summarized in Box 4.
Box 4. Abnormal BP patterns over 24 hours identified by using ABPM. modified from O'Brien et al28 by permission.
Daytime hypertension
Siesta dipping/post‐prandial hypotension
Nocturnal hypertension
Dipping status
Morning hypertension and morning blood pressure surge
Obstructive sleep apnea
Increased blood pressure variability
Obstructive sleep apnea
The prevalence of hypertension ranges from 35% to 80% in OSA patients and appears to be influenced by the severity of OSA. Patients with respiratory disease tend to be hypertensive, and up to 40% of hypertensive patients are diagnosed with OSA, with this prevalence being as high as 85% in patients with resistant hypertension.71, 72 There are several reasons why ABPM is useful in this population: Many patients have multiple risk factors and, therefore, require a particularly accurate diagnosis of hypertension and evaluation of BP control; the prevalence of drug‐resistant hypertension is high, often requiring complex treatment regimens to achieve adequate 24‐h BP control; and the prevalence of a nocturnal non‐dipper or riser profile is common and it may be possible to improve nocturnal reduction in BP and achieve normalization of circadian BP profile with antihypertensive treatment combined with effective continuous positive airway pressure (CPAP) ventilation.73
6.2. Assessment of treatment
ABPM has been used to assess the effects of therapeutic strategies on reducing nocturnal BP, and there is some evidence suggesting that administration of antihypertensive drugs in the evening rather than in the morning may restore normal nocturnal dipping, thus contributing to a reduction in cardiovascular risk, but these results need to be confirmed by further trials. Treatment effects on short‐term BP variability may also be assessed, through the calculation of various ABPM indices, aimed at quantifying the persistence and homogeneity of BP reduction by antihypertensive drugs.74, 75 Visit‐to‐visit office and ambulatory BP variability, which predict cardiovascular outcomes, may also provide a means to assess drug treatment efficacy.
6.2.1. Assessing efficacy of blood pressure control
ABPM may be useful in the management of hypertensive patients receiving drug therapy. Latin America has participated in several antihypertensive treatment trials.76, 77 In a well‐controlled study, the adjustment of antihypertensive treatment based on either ABPM or clinic BP measurement resulted in less‐intensive drug treatment in patients managed with ABPM despite comparable BP control in both groups. Furthermore, left ventricular mass was not increased in patients managed through ABPM, even though they received less antihypertensive medications.78 ABPM also provides a better assessment of the response to treatment than clinic BP does; the efficacy of treatment can be ascertained without interference by the white coat effect, and the duration of BP control over the 24‐h period and the consequences of missed doses on BP can be demonstrated. Furthermore, an excessive drug effect can be identified and linked with the occurrence of corresponding symptoms.
6.2.2. Resistant hypertension
The recommendation to use out‐of‐office BP measurements in resistant hypertension is based on the evidence that ABPM gives better prognostic information than office measurement.64, 79, 80 Thus, the observation of persistently elevated office BP values in treated hypertensives taking 3 drugs at full doses including a diuretic is not enough to conclude for the occurrence of resistant hypertension, in the absence of the accompanying elevation of home or ambulatory BP levels. One study showed that in patients with resistant hypertension, with or without a previous cardiovascular event, ABPM was an independent marker of risk for new cardiovascular events, suggesting that ABPM was useful in stratifying the risk in patients with resistant hypertension.81 Two studies79, 80 have confirmed these results and reinforced the superiority of ABPM over office BP for stratifying risk. Also, home BP monitoring may be useful in identifying patients with “true‐resistant” and “false‐resistant” hypertension, although this approach at present cannot provide information on nighttime BP.80, 82
6.3. Hypertension in the elderly
In the elderly patient, ABPM is particularly useful, given that in the elderly BP is characterized by a greater variability. In fact, periods of elevated BP levels can be followed by periods of low BP levels, which may represent a manifestation of autonomic neural system dysfunction, characterized by a reduced sensitivity of the arterial baroreflex, often associated with increased arterial stiffness. Systolic blood pressure (SBP) tends to increase in the course of life, and toward 60‐70 years of age, the increase in SBP is often accompanied by a reduction in diastolic blood pressure (DBP), with the resulting increase in pulse pressure. In the elderly, an increased 24‐hour SBP is associated with increased cardiovascular risk, in particular with stroke and less with coronary disease.83
The nocturnal fall decreases with increasing age so it is more common to find non‐dippers or risers among elderly individuals, which has been associated with cognitive as well as autonomic dysfunction. It should be considered, however, that in the elderly sleep quality may be affected by concomitant conditions such as sleep apnea, prostatic hypertrophy, or sleep fragmentation, which makes the clinical interpretation of a non‐dipping pattern more difficult.2
6.4. Hypertension in children and adolescents
It is increasingly recognized that the ABPM is essential for the diagnosis of hypertension in this age group, since BP variability is greater in these young individuals. ABPM can be particularly useful to identify white coat hypertension, which has been reported to have a highly variable prevalence, from 0.6% to 32%. In this age range, masked hypertension has been reported to be particularly frequent, its prevalence ranging from 7.6% to 15%, being more frequent in young obese individuals and being suggested to carry the same cardiovascular risk as sustained hypertension.84 Masked hypertension may be characterized by nocturnal hypertension in young subjects with type 1 diabetes mellitus, an association which has been reported to be frequently accompanied by the development of microalbuminuria. ABPM in children and adolescents should be performed by using an appropriate cuff size and devices validated for this age range.85 Reference threshold values to diagnose hypertension with ABPM in children and adolescents are reported in Table 4, as a function of age and height.84, 86
Table 4.
Reference ambulatory blood pressure values for children and adolescents. Modified from O'Brien et al 28 by permission
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Day | Night | Day | Night | |||||
| Height (cm) | 90th | 95th | 90th | 95th | 90th | 95th | 90th | 95th |
| 120 | 122/80 | 125/82 | 103/61 | 106/63 | 118/80 | 120/82 | 103/63 | 106/65 |
| 125 | 122/80 | 125/82 | 105/61 | 108/63 | 119/80 | 121/82 | 104/63 | 107/66 |
| 130 | 122/80 | 126/82 | 106/62 | 110/64 | 120/80 | 122/82 | 106/63 | 108/66 |
| 135 | 123/80 | 126/82 | 108/63 | 111/65 | 120/80 | 123/82 | 107/63 | 109/66 |
| 140 | 123/80 | 126/82 | 109/63 | 113/65 | 121/80 | 124/82 | 108/63 | 110/66 |
| 145 | 124/79 | 127/81 | 111/64 | 114/66 | 123/80 | 125/82 | 109/63 | 112/66 |
| 150 | 125/79 | 128/81 | 112/64 | 116/66 | 124/80 | 126/80 | 110/63 | 113/66 |
| 155 | 127/79 | 130/81 | 113/64 | 117/66 | 125/80 | 128/82 | 111/63 | 114/66 |
| 160 | 129/79 | 133/81 | 114/64 | 118/66 | 126/80 | 129/82 | 111/63 | 114/66 |
| 165 | 132/80 | 135/82 | 116/64 | 119/66 | 127/80 | 130/82 | 112/63 | 114/66 |
| 170 | 134/80 | 138/82 | 117/64 | 121/66 | 128/80 | 131/82 | 112/67 | 115/71 |
| 175 | 136/81 | 140/83 | 119/64 | 122/66 | 129/81 | 131/82 | 113/63 | 115/66 |
| 180 | 138/81 | 142/83 | 120/64 | 124/66 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| 185 | 140/81 | 144/84 | 122/66 | 125/66 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
Height in cm; 90th and 95th refer to percentiles; blood pressure values are in mmHg. Day and night refer to daytime periods.
6.5. Hypertension in pregnancy
The use of ABPM in pregnancy is especially useful to detect white coat hypertension (which may occur in up to one third of these patients). In 40% of cases, pregnant women can develop gestational hypertension, and ABPM has been reported to better predict the risk of developing proteinuric hypertension. In 60% of pregnancies, nocturnal hypertension could be observed, and its prevalence is higher in patients with preeclampsia than in those with gestational hypertension.87 Among patients with chronic hypertension who become pregnant, nearly 50% have no nocturnal decline in BP. Reference BP values for 24‐h ABPM according to gestational age can be seen in Table 5.
Table 5.
Ambulatory blood pressure monitoring values according to gestational age (blood pressure range with upper normal value in parentheses). Modified from O'Brien et al 28 by permission
| 24h | Sleep | |||||||
|---|---|---|---|---|---|---|---|---|
| Day | Night | Day | Night | |||||
| Gestational age | 9‐17 | 18‐22 | 26‐30 | 31‐40 | 9‐17 | 18‐22 | 26‐30 | 31‐40 |
| SBP Upper normal value | 101‐118 (121) | 96‐127 (126) | 97‐133 (128) | 103‐136 (131) | 93‐109 (110) | 88‐120 (114) | 87‐125 (117) | 85‐131 (123) |
| DBP Upper normal value | 60‐71 (73) | 56‐78 (76) | 56‐84 (78) | 57‐85 (82) | 50‐64 (64) | 46‐68 (66) | 46‐76 (68) | 47‐77 (72) |
6.6. Hypertension in high‐risk patients
6.6.1. Diabetes
The use of ABPM in diabetic patients is particularly important since the phenomenon of non‐dipping or nocturnal hypertension is more common in this condition. A non‐dipping pattern may reflect autonomic dysfunction, but its presence may be due to other pathogenetic factors such as obstructive sleep apnea, also frequent in the obese diabetic patient.27 Masked hypertension is common in diabetic patients, with a prevalence up to 50% during both day and night periods. In diabetes, BP variability over 24 hours is increased and may be a marker of autonomic neuropathy, which can also cause post‐prandial hypotension (a BP decrease after a meal, which can be even greater than the nocturnal BP fall). For the same reason, the variability of heart rate is reduced. Pulse pressure (an indicator of arterial stiffness) is frequently increased. Because of the enhanced BP variability, it is particularly important in diabetes to evaluate the effectiveness of antihypertensive treatment all over the 24 hours and in particular during night time. Oscillometric devices for ABPM would need to be separately validated in diabetic patients, due to the increased arterial stiffness. The possible need of considering different thresholds for diagnosing hypertension with ABPM as well as different treatment goals in this condition has not been definitely clarified.27, 88, 89
6.6.2. Stroke
ABPM can be useful both in monitoring the acute hemodynamic effects of ischemic and hemorrhagic stroke and in predicting outcome in stroke survivors. A frequent finding in stroke patients is the loss of nocturnal BP dipping, which may lead to worse target organ damage and facilitate recurrent stroke. Moreover, BP recorded during sleep or in the early morning is more predictive of first or recurring stroke events than daytime SBP, especially in the elderly.90 Conventional BP measurements, therefore, should be coupled with out‐of‐office BP monitoring, in particular with ABPM to precisely identify the above changes in BP over 24h. There are limited data on alterations of the circadian BP profile in acute stroke patients. Both hypertensive and normotensive patients have been shown to display similarly increased prevalence of abnormal circadian rhythm of SBP, with more frequent non‐dipping and reverse dipping patterns when assessed with repeated ABPM.91 The possible occurrence of differences in the ABPM profile in acute stroke subtypes of different etiology, which might have implications for the optimal management of post‐stroke hypertension, has also been reported.92 Moreover, there is evidence of a close association between stroke and increased 24‐h BP variability. An increase in short‐term BP variability and in morning BP surge may indeed increase the risk of stroke and cardiovascular events.93 In summary, both hypertensive and normotensive survivors of stroke frequently develop a chronic disruption of circadian BP patterns, which can vary with the stroke type. Preservation of nocturnal BP dipping and of a physiological circadian pattern of BP may have a protective effect on cerebral circulation in patients with ischemic stroke. Also, the modulation of short‐term BP variability by treatment may be beneficial in stroke patients, but the actual value of pharmacologic interventions aimed at maintaining the physiological dipping phenomena and in modulating short‐term BP variability after acute stroke has yet to be explored in large randomized controlled trials.94
6.6.3. Coronary heart disease
Only a few studies have addressed the usefulness of ABPM in patients with coronary heart disease. In most cases, a significant relationship was found between coronary heart disease prevalence and either non‐dipping or increased ambulatory pulse pressure. ABPM can also be useful in predicting prognosis in patients with coronary heart disease and hypertension.95
6.6.4. Chronic kidney disease
There is a direct correlation between the level of BP and cardiovascular risk (CVR), mortality, and progression of kidney damage in patients with renal failure, so a strict BP control over 24 hours is required. Indeed, these patients have frequent disturbances of circadian BP profile: About 50% have no nocturnal dipping in BP, a prevalence which can increase up to 80% in those with stage 5, chronic kidney disease. In 28%‐35% of renal patients, white coat hypertension has been reported to occur, while the prevalence of masked hypertension is around 26%‐34%. In patients on a dialysis program, the intravascular volume at the end of dialysis is significantly reduced, while it undergoes a progressive increase in the inter‐dialysis period, leading to significant fluctuations in BP, both in adults and in children with end‐stage renal disease. In chronic kidney disease, nocturnal BP has been shown to be a better predictor of fatal and nonfatal cardiovascular events than daytime BP, the pattern of non‐dipping being associated with the development of microalbuminuria, increased proteinuria, decreased renal function, worse renal prognosis, and left ventricular hypertrophy. Given its ability to evaluate these phenomena, ABPM has been reported to be superior to conventional clinic BP measurements in assessing the increased risk associated with altered 24‐h BP patterns.96, 97, 98, 99 Even more, the addition of eGFR to cardiovascular risk prediction models adds very little beyond 24‐hour ABPM.100 In patients under dialysis, out‐of‐office blood pressure measurement is recommended for diagnosis and risk stratification.101 Diagnostic thresholds are an average blood pressure equal or superior to 130/80 mmHg over 24‐h monitoring during a mid‐week day free of hemodialysis. Whenever feasible, ABPM should be extended to 44 h, that is covering a whole mid‐week dialysis interval. In patients under peritoneal dialysis, the same diagnostic thresholds (130/80 mmHg) are considered over 24 h.101
6.6.5. Obesity
Obesity is associated with higher levels of BP. This is often due to the high prevalence of obstructive sleep apnea, that may be present since childhood and adolescence in case of obesity,27, 69 and which may be largely responsible for an increased prevalence of nocturnal BP non‐dipping or rising, with the possibility of isolated nocturnal hypertension.27, 69 Increased 24‐h ABP is also associated with markers of vascular aging such as arterial stiffness, central hypertension, and carotid intima‐media thickness,102 and represents a risk factor for the occurrence of resistant hypertension and a worsen patients' quality of life.79
6.6.6. Arrhythmias
There is poor information on the use of ABPM in patients with arrhythmias, especially atrial fibrillation. However, when heart rate is controlled by treatment, ABPM seems to be useful and reliable, given that the proportion of erroneous readings, the degree of BP variability, and repeatability of the ABPM‐derived parameters do not appear to be different from those obtained in patients with sinus rhythm. However, it has been found that 24‐h DBP may be slightly higher than DBP measured with conventional measurement and even than ambulatory DBP obtained when ABPM is repeated with the patient in sinus rhythm.27
7. ABPM REGISTRIES
7.1. ARTEMIS registry
The ARTEMIS (International Ambulatory blood pressure Registry: Tele Monitoring of hypertension and cardiovascular rISk project) is a worldwide registry endorsed by the ESH and is the first international registry of ABPM aimed at assessing the actual degree of BP and cardiovascular risk control of hypertensive patients followed by doctors in many countries (www.artemisnet.org). Objectives are the creation of a worldwide network of centers performing ABPM recordings, and the collection of a large amount of clinical data in patients from different countries and continents, in whom basic clinical information is available as well as at least one ABPM fulfilling predefined criteria. Creation of this registry has favored the design and initiation of a series of research studies focusing on ABPM and based on the collected dataset; the promotion of the application of ABPM in clinical practice; and the dissemination of knowledge on its correct use and interpretation. Finally, the ARTEMIS registry has favored an active collaboration among international scientific societies in their activities related to ABPM, in particular in the preparation of recommendations for ABPM use in clinical and research settings. The registry was launched in 2010, with a two‐step approach. A first phase has collected data from approximately 10.000 patients from 38 countries in five continents (Europe, America, Asia, Oceania, and Africa). Preliminary results provide interesting comparative information on hypertension phenotypes in treated and untreated patients attending hypertension clinics worldwide. In particular, white coat hypertension was found more common in untreated patients, whereas masked uncontrolled hypertension was more common in treated patients. In addition, significantly higher prevalence of sustained hypertension was observed in clinics in Europe and South America than in Asia and Oceania. A second phase using a dedicated Web‐based multilingual telemedicine system will allow collection of data from patients having consecutive ABPMs. An important observation recently published showed a high prevalence of masked hypertension in Latin America.103, 104 The ARTEMIS registry has recently evolved into the MASTER study, a multinational randomized intervention trial aimed at investigating whether there is any difference in the impact on outcome in patients with masked uncontrolled hypertension of a management based on office BP rather than on ambulatory BP monitoring.69
7.2. IDACO registry
In 2007, an international consortium of investigators started constructing the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) including prospective population‐based studies reporting fatal as well as nonfatal outcomes.105 The database currently includes 12.854 participants recruited in 12 countries from three continents. Latin America made an important participation.21, 106, 107 Articles published so far have contributed to determine ABPM thresholds based on 10 years of cardiovascular risk,108 reported the prognostic superiority of daytime ambulatory over conventional blood pressure,68 and demonstrated that although the absolute cardiovascular risk is lower in women than it is in men, the relationship between the risk of a cardiovascular event and the ambulatory BP is stronger in women than men.108 In population‐based cohorts, both diabetes mellitus and the ambulatory BP contributed equally to the risk of cardiovascular complications without evidence for a synergistic effect.109, 110
CONFLICT OF INTEREST
The authors declare no conflicts of interest in relation to this paper.
Sánchez RA, Boggia J, Peñaherrera E, et al. Ambulatory blood pressure monitoring over 24 h: A Latin American Society of Hypertension position paper—accessibility, clinical use and cost effectiveness of ABPM in Latin America in year 2020. J Clin Hypertens. 2020;22:527–543. 10.1111/jch.13816
REFERENCES
- 1. Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60:449‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH / ESC Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension ( ESH ) and of the European Society of Cardiology ( ESC ). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 3. Consenso Argentino de Hipertensión Arterial. Rev Arg Cardiol. 2018;86(Suppl 2):1‐53. [Google Scholar]
- 4. Romero C, Ventura JE, Schwedt E, Ambrosoni P, Berenguer D, Alvaro García‐Austt J, del Fontáns MC, et al. 3er Consenso Uruguayo de Hipertensión Arterial. 2005. http://www.cardiosalud.org/files/documents/chscv-consenso-2005-3-hta.pdf
- 5. Brazilian guidelines on ambulatory blood pressure measurement and III Brazilian guidelines on home blood pressure measurement. Arq Bras Cardiol. 2011;97(3 Supl 3):1‐24. [Google Scholar]
- 6. Prat MH, Valdés SG, Román AO, Zarate MLH. Update of consensus recommendations of the Chilean Hypertension Society about ambulatory blood pressure monitoring. Rev Med Chil. 2009;137:1235‐1247. [PubMed] [Google Scholar]
- 7. Task Force of the Latin American Society of Hypertension: Guidelines on the management of arterial hypertension and related comorbidities in Latin America. J Hypertens. 2017;35:1529‐1545. [DOI] [PubMed] [Google Scholar]
- 8. Kain HK, Hinman AT, Sokolow M. Arterial blood pressure measurements with a portable recorder in hypertensive Patients. I. Variability and correlation with “Casual” Pressures. Circulation. 1964;30:882‐892. [DOI] [PubMed] [Google Scholar]
- 9. Sokolow M, Werdegar D, Kain HK, Hinman AT. Relationship between level of blood pressure measured casually and by portable recorders and severity of complications in essential hypertension. Circulation. 1966;34:279‐298. [DOI] [PubMed] [Google Scholar]
- 10. Pickering TG, Shimbo D, Haas D. Ambulatory blood‐pressure monitoring. N Engl J Med. 2006;354:2368‐2374. [DOI] [PubMed] [Google Scholar]
- 11. Hypertension in adults: diagnosis and management NICE guideline [NG136]. Published date: August 2019. https://www.nice.org.uk/guidance/ng136. Accessed January 28, 2020.
- 12. Shimamoto K, Ando K, Fujita T, Hasebe N. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014; 37(4): 253‐390. [DOI] [PubMed] [Google Scholar]
- 13. Head GA, McGrath BP, Mihailidou AS, et al. National heart foundation and high blood pressure research council of Australia ambulatory blood pressure monitoring consensus committee: Ambulatory blood pressure monitoring. Aust Fam Physician. 2011;40(11):877‐880. [PubMed] [Google Scholar]
- 14. Jardim TV, Gaziano TA, Nascente FM, et al. Office blood pressure measurements with oscillometric devices in adolescents: a comparison with home blood pressure. Blood Press. 2017;26(5):272‐278. [DOI] [PubMed] [Google Scholar]
- 15. Vieira da Silva MA, Mendes da Silva AP, Artigas Giorgi DM, Ganem F. Successive blood pressure measurements to evaluate suspected and treated hypertension. Blood Pressure Monitoring. 2016;21(2):69‐74. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz JE, Burg MM, Shimbo D, et al. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer‐based US population clinical perspective. Circulation. 2016;134:1794‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grezzana GB, Moraes DW, Stein AT, Pellanda LC. Impact of different normality thresholds for 24‐hour ABPM at the primary health care level. Arq Bras Cardiol. 2017;108(2):143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devereux RB, Pickering TG, Harshfield GA, et al. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation. 1983;68:470‐476. [DOI] [PubMed] [Google Scholar]
- 19. Melgarejo JD, Thijs L, Zhang ZY, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322(5):409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kikuya M, Staessen JA, Ohkubo T, et al. How many measurements are needed to provide reliable information in terms of the ambulatory arterial stiffness index? The Ohasama study. Hypertens Res. 2011;34240:314‐318. [DOI] [PubMed] [Google Scholar]
- 21. Mena LJ, Maestre GE, Hansen TW, et al. How many measurements are needed to estimate blood pressure variability without loss of prognostic information? Am J Hypertens. 2014;27:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24‐ hour average blood pressure. Hypertension. 1983;5:264‐269. [DOI] [PubMed] [Google Scholar]
- 23. Thijs L, Staessen J, Fagard R, Zachariah P, Amery A. Number of measurements required for the analysis of diurnal blood pressure profile. J Hum Hypertens. 1994;8:239‐244. [PubMed] [Google Scholar]
- 24. Thijs L, Staessen J, O'Brien E, et al. The ambulatory blood pressure in normotensive and hypertensive subjects: results from an international database. Neth J Med. 1995;46:106‐114. [DOI] [PubMed] [Google Scholar]
- 25. Yang WY, Thijs L, Zhang ZY, et al. Evidence‐based proposal for the number of ambulatory readings required for assessing blood pressure level in research settings: an analysis of the IDACO database. Blood Press. 2018;27(6):341‐350. [DOI] [PubMed] [Google Scholar]
- 26. Pickering TG. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A Statement for professionals from the subcommittee of professional and public education of the American Heart Association Cou. Circulation. 2005;111:697‐716. [DOI] [PubMed] [Google Scholar]
- 27. Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 28. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 29. O'Brien E, Pickering T, Asmar R, et al. Working group on blood pressure monitoring of the European society of hypertension international protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3‐17. [DOI] [PubMed] [Google Scholar]
- 30. Stergiou GS, Karpettas N, Atkins N, OʼBrien E. European Society of Hypertension International Protocol for the validation of blood pressure monitors: a critical review of its application and rationale for revision. Blood Press Monit. 2010;15:39‐48. [DOI] [PubMed] [Google Scholar]
- 31. Kikuya M, Hansen TW, Thijs L, et al. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10‐year cardiovascular risk. Circulation. 2007;115:2145‐2152. [DOI] [PubMed] [Google Scholar]
- 32. Nobre F, Mion D Jr, Gomes MAM, et al. 6a Diretrizes de monitorização ambulatorial da pressão arterial e 4a diretrizes de monitorização residencial da pressão arterial. Arq Bras Cardiol. 2018;110(5 Suppl 1):1‐29.29538519 [Google Scholar]
- 33. Forestiero D, Lustoza Mauad J, Forestiero CV, et al. Impact on hypertension reclassification by ambulatory blood pressure monitoring (ABPM) According to the V Brazilian Guidelines on ABPM. Arq Bras Cardiol. 2013;100(2):175‐179. [DOI] [PubMed] [Google Scholar]
- 34. Abdalla M. Ambulatory blood pressure monitoring: A complementary strategy for hypertension diagnosis and management in low‐income and middle‐income countries. Cardiol Clin. 2017;35:117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruilope LM, Chagas ACP, Brandão AA, et al. Hypertension in Latin America: Current perspectives on trends and characteristics. Hipertens y Riesgo Vasc. 2017;34:50‐56. [DOI] [PubMed] [Google Scholar]
- 36. Boubouchairopoulou N, Karpettas N, Athanasakis K, et al. Cost estimation of hypertension management based on home blood pressure monitoring alone or combined office and ambulatory blood pressure measurements. J Am Soc Hypertens. 2014;8:732‐738. [DOI] [PubMed] [Google Scholar]
- 37. Islam MS. Ambulatory blood pressure monitoring in the diagnosis and treatment of hypertension. Adv Exp Med Biol. 2017;956:109‐116. [DOI] [PubMed] [Google Scholar]
- 38. O'Brien E, Dolan E. Ambulatory blood pressure monitoring for the effective management of antihypertensive drug treatment. Clin Ther. 2016;38:2142‐2151. [DOI] [PubMed] [Google Scholar]
- 39. Rubinstein AL, Irazola VE, Calandrelli M, et al. Multiple cardiometabolic risk factors in the Southern Cone of Latin America: A population‐based study in Argentina, Chile, and Uruguay. Int J Cardiol. 2015;183:82‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26(8):1505‐1526. [DOI] [PubMed] [Google Scholar]
- 41. Grezzana GB, Stein AT, Pellanda LC, International database on ambulatory blood pressure in relation to cardiovascular outcomes (IDACO) Investigators . Twenty‐four‐hour ambulatory blood pressure monitoring for clinical evaluation of hypertensive patients in primary care: which groups would most benefit? Blood Press Monit. 2017;22(2):72‐78. [DOI] [PubMed] [Google Scholar]
- 42. Melgarejo JD, Maestre GE, Thijs L, et al. Prevalence, treatment, and control rates of conventional and ambulatory hypertension across 10 populations in 3 continents. Hypertension. 2017;70(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krakoff LR. Ambulatory blood pressure improves prediction of cardiovascular risk: implications for better antihypertensive management. Curr Atheroscler Rep. 2013;15:317. 10.1007/s11883-013-0317-9 [DOI] [PubMed] [Google Scholar]
- 44. Stergiou GS, Asmar R, Myers M, et al. Improving the accuracy of blood pressure measurement: the influence of the European Society of Hypertension International Protocol (ESH‐IP) for the validation of blood pressure measuring devices and future perspectives. J Hypertens. 2018;36(3):479‐487. [DOI] [PubMed] [Google Scholar]
- 45. O'Brien E, Petrie J, Littler W, et al. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993; 11: 43‐62. [DOI] [PubMed] [Google Scholar]
- 46. International Organization for Standardization (ISO‐81060‐2; 2018) . Non‐invasive sphygmomanometers: Clinical investigation of intermittent automated measurement type (ISO 81060–2: 2018). https://www.iso.org/standard/73339.html. Accessed January 28, 2020.
- 47. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices. Association for the advancement of medical instrumentation/European society of hypertension/International organization for standardization (AAMI/ESH/ISO) collaboration statement hypertension. Hypertension. 2018;71:368‐374. [DOI] [PubMed] [Google Scholar]
- 48. Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation. J Hypertens. 2012;30:2074‐2082. [DOI] [PubMed] [Google Scholar]
- 49. Octavio JA, Contreras J, Amair P, et al. Time‐weighted vs. conventional quantification of 24‐h average systolic and diastolic ambulatory blood pressures. J Hypertens. 2010;28:459‐464. [DOI] [PubMed] [Google Scholar]
- 50. Omboni S, Palatini P, Parati G, on behalf of the Working Group on Blood Pressure Monitoring of the Italian Society of Hypertension . Standards for ambulatory blood pressure monitoring clinical reporting in daily practice: recommendations from the Italian Society of Hypertension. Blood Pressure Monitoring. 2015;20(5):241‐244. [DOI] [PubMed] [Google Scholar]
- 51. Sociedad Argentina de Hipertension Arterial (Panel de Expertos) . Toma de posición ‐ Medición de la Presión Arterial Fuera del Consultorio ‐ MAPA‐MDPA. 2015. http://www.saha.org.ar/1/pdf/TomaDePosicion-Libro2015.pdf. Accessed January 28, 2020.
- 52. Yang WY, Melgarejo JD, Thijs L, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322(5):409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sharman JE, Avolio AP, Baulmann J, et al. Validation of non‐invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38(37):2805‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salvi P, Furlanis G, Grillo A, et al. Unreliable estimation of aortic pulse wave velocity provided by the mobil‐O‐graph algorithm‐based system in marfan syndrome. J Am Heart Association. 2019;8(9):e04028. 10.1161/JAHA.118.011440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salvi P, Scalise F, Rovina M, et al. Noninvasive estimation of aortic stiffness through different approaches. Hypertension. 2019;74(1):117‐129. [DOI] [PubMed] [Google Scholar]
- 56. Verdecchia P, Schillaci G, Borgioni C, et al. White coat hypertension and white coat effect similarities and differences. Am J Hypertens. 1995;8:790‐798. [DOI] [PubMed] [Google Scholar]
- 57. Mancia G, Bombelli M, Facchetti R, et al. Long‐term risk of sustained hypertension in white‐coat or masked hypertension. Hypertension. 2009;54(2):226‐232. [DOI] [PubMed] [Google Scholar]
- 58. Franklin SS, Thijs L, Asayama K, et al. The cardiovascular risk of white‐coat hypertension. J Am Coll Cardiol. 2016;68:2033‐2043. [DOI] [PubMed] [Google Scholar]
- 59. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension : The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 60. Parati G, Ulian L, Santucciu C, Omboni S, Mancia G. Difference between clinic and day‐time blood pressure is not a measure of the white coat effect. Hypertension. 1998;31(5):1185‐1189. [DOI] [PubMed] [Google Scholar]
- 61. Myers MG. Pseudoresistant hypertension attributed to White‐coat effect. Hypertension. 2012;59:532‐533. [DOI] [PubMed] [Google Scholar]
- 62. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white‐coat, masked and sustained hypertension versus true normotension: a meta‐analysis. J Hypertens. 2007;25:2193‐2198. [DOI] [PubMed] [Google Scholar]
- 63. Ugajin T, Hozawa A, Ohkubo T, et al. White‐coat hypertension as a risk factor for the development of home hypertension. Arch Intern Med. 2005;165:1541‐1546. [DOI] [PubMed] [Google Scholar]
- 64. Departamento de Hipertensão Arterial da Sociedade Brasileira de Cardiologia pelos autores: I Brazilian Position Paper on Prehypertension, White Coat Hypertension and Masked Hypertension: Diagnosis and Management. Arq Bras Cardiol. 2014;102(2):110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Myers MG, Reeves RA. White coat phenomenon in patients receiving antihypertensive therapy. Am J Hypertens. 1991;4:844‐849. [DOI] [PubMed] [Google Scholar]
- 66. Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40:795‐796. [DOI] [PubMed] [Google Scholar]
- 67. Bobrie G, Clerson P, Ménard J, Postel‐Vinay N, Chatellier G, Plouin P‐F. Masked hypertension: a systematic review. J Hypertens. 2008;26:1715‐1725. [DOI] [PubMed] [Google Scholar]
- 68. Hansen TW, Kikuya M, Thijs L, et al. IDACO Investigators: Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta‐analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554‐1564. [DOI] [PubMed] [Google Scholar]
- 69. Parati G, Agabiti‐Rosei E, Bakris GL, et al. MASked‐unconTrolled hypERtension management based on office BP or on ambulatory blood pressure measurement (MASTER) Study: a randomised controlled trial protocol. BMJ Open. 2018;8:e021038. 10.1136/bmjopen-2017-021038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amodeo C, Guimarães GG, Picotti JC, et al. Morning blood pressure surge is associated with death in hypertensive patients. Blood Pressure Monitoring. 2014;19:199‐202. [DOI] [PubMed] [Google Scholar]
- 71. Calhoun DA. Obstructive sleep apnea and hypertension. Curr Hypertens Rep. 2010;12:189‐195. [DOI] [PubMed] [Google Scholar]
- 72. Correia CM, Gismondi RA, Cunha AR, Neves MF, Oigman W. Twenty‐four hour blood pressure in obese patients with moderate‐to‐ severe obstructive Sleep Apnea. Arq Bras Cardiol. 2017;109(4):313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension. J Hypertens. 2012;30:633‐646. [DOI] [PubMed] [Google Scholar]
- 74. Parati G, Omboni S, Rizzoni D, Agabiti‐Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens. 1998;16:1685‐1691. [DOI] [PubMed] [Google Scholar]
- 75. Parati G, Schumacher H, Bilo G, Mancia G. Evaluating 24‐h antihypertensive efficacy by the smoothness index: a meta‐analysis of an ambulatory blood pressure monitoring database. J Hypertens. 2010;28:2177‐2183. [DOI] [PubMed] [Google Scholar]
- 76. Agarwal R, Bills JE, Hecht TJW, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: A Systematic review and meta‐analysis. Hypertension. 2011;57:29‐38. [DOI] [PubMed] [Google Scholar]
- 77. Mancia G, Parati G, Bilo G, et al. Ambulatory blood pressure values in the ongoing telmisartan alone and in combination with ramipril global endpoint trial (ONTARGET). Hypertension. 2012;60:1400‐1406. [DOI] [PubMed] [Google Scholar]
- 78. Staessen JA, Byttebier G, Buntinx F, Celis H, O'Brien ET, Fagard R. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators. JAMA. 1997;278:1065‐1072. [PubMed] [Google Scholar]
- 79. Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension. 1998;31:712‐718. [DOI] [PubMed] [Google Scholar]
- 80. Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422‐1428. [DOI] [PubMed] [Google Scholar]
- 81. Salles GF, Cardoso CRL, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340‐2346. [DOI] [PubMed] [Google Scholar]
- 82. Corrêa NB, de Faria AP, Ritter AM, et al. A practical approach for measurement of antihypertensive medication adherence in patients with resistant hypertension. J Am Soc Hypertens. 2016;10(6):510‐516. [DOI] [PubMed] [Google Scholar]
- 83. Staessen JA, Thijs L, O'Brien ET, et al. Ambulatory pulse pressure as predictor of outcome in older patients with systolic hypertension for the syst‐eur trial investigators. Am J Hypertens. 2002;15(10 Pt 1):835‐843. [DOI] [PubMed] [Google Scholar]
- 84. Flynn JT, Daniels SR, Hayman LL, et al. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the american heart association. Hypertension. 2014;63:1116‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stergiou GS, Boubouchairopoulou N, Kollias A. Accuracy of automated blood pressure measurement in children evidence, issues, and perspectives. Hypertension. 2017;69:1000‐1006. [DOI] [PubMed] [Google Scholar]
- 86. Lurbe E, Agabiti‐Rosei E, Cruickshankd JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887‐1920. [DOI] [PubMed] [Google Scholar]
- 87. Rojo‐Contreras W, Salazar‐Páramo M, Parra‐Carrillo JZ, et al. Circadian rhythm of blood pressure in patients with chronic hypertension and pregnancy. Ginecol Obstet Mex. 2014;82:604‐612. [PubMed] [Google Scholar]
- 88. Rubinstein AL, Irazola VE, Calandrelli M, et al. Prevalence, awareness, treatment, and control of hypertension in the Southern Cone of Latin America. Am J Hypertens. 2016;29:1343‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. López‐Jaramillo P, Sánchez RA, Diaz M, et al. Latin America Expert Group: Latin American consensus on hypertension in patients with diabetes type 2 and metabolic syndrome. J Hypertens. 2013;31(2):223‐238. [DOI] [PubMed] [Google Scholar]
- 90. Verdecchia P, Schillaci G, Reboldi G, Franklin SS, Porcellati C. Different prognostic impact of 24‐hour mean blood pressure and pulse pressure on stroke and coronary artery disease in essential hypertension. Circulation. 2001;103(21):2579‐2584. [DOI] [PubMed] [Google Scholar]
- 91. Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night–day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645‐653. [DOI] [PubMed] [Google Scholar]
- 92. Kario K, Matsuo T, Kobayashi H, et al. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27(1):130‐135. [DOI] [PubMed] [Google Scholar]
- 93. Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689‐1712. [DOI] [PubMed] [Google Scholar]
- 94. Yano Y, Inokuchi T, Hoshide S, Kanemaru Y, Shimada K, Kario K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am J Hypertens. 2011;24:285‐291. [DOI] [PubMed] [Google Scholar]
- 95. Fagard RH, Thijs L, Staessen JA, et al. Prognostic significance of ambulatory blood pressure in hypertensive patients with history of cardiovascular disease. Blood Press Monit. 2008;13(6):325‐332. [DOI] [PubMed] [Google Scholar]
- 96. Parati G, Ochoa JE, Bilo G, Agarwal R. Hypertension in chronic kidney disease part 1: Out‐of‐office blood pressure monitoring: methods, thresholds, and patterns. Hypertension. 2016;67(6):1093‐1101. [DOI] [PubMed] [Google Scholar]
- 97. Parati G, Ochoa JE, Bilo G, et al. Hypertension in chronic kidney disease part 2. Role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension. 2016;67:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 98. Park J, Rhee CM, Sim JJ, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013;84:795‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ruiz‐Hurtado G, Gorostidi M, Waeber B, Ruilope LM. Ambulatory and home blood pressure monitoring in people with chronic kidney disease. Time to abandon clinic blood pressure measurements? Curr Opin Nephrol Hypertens. 2015;24:488‐491. [DOI] [PubMed] [Google Scholar]
- 100. Boggia J, Thijs L, Li Y, et al. Risk stratification by 24‐hour ambulatory blood pressure and estimated glomerular filtration rate in 5322 subjects from 11 populations. Hypertension. 2013;61:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sarafidis PA, Persu A, Agarwal R, et al. Hypertension in dialysis patients. J Hypertens. 2017;35:657‐676. [DOI] [PubMed] [Google Scholar]
- 102. Olszanecka‐Glinianowicz M, Zygmuntowicz M, Owczarek A, Elibol A, Chudek J. The impact of overweight and obesity on health‐related quality of life and blood pressure control in hypertensive patients. J Hypertens. 2014;32:397‐407. [DOI] [PubMed] [Google Scholar]
- 103. International Ambulatory Blood Pressure Monitoring Registry ‐ Artemis . http://www.artemisnet.org/study_information.aspx. Accessed January 28, 2020.
- 104. Omboni S, Aristizabal D, De la Sierra A, et al. Hypertension types defined by clinic and ambulatory blood pressure in 14143 patients referred to hypertension clinics worldwide. Data from the ARTEMIS study. J Hypertens. 2016;34:2187‐2198. [DOI] [PubMed] [Google Scholar]
- 105. Thijs L, Hansen TW, Kikuya M, et al. The international database of ambulatory blood pressure in relation to cardiovascular outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255‐262. [DOI] [PubMed] [Google Scholar]
- 106. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 107. Melgarejo JD, Maestre GE, Thijs L, et al. Prevalence, treatment, and control rates of conventional and ambulatory hypertension across 10 populations in 3 continents novelty and significance. Hypertension. 2017;70:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Boggia J, Thijs L, Hansen TW, et al. Ambulatory blood pressure monitoring in 9357 subjects From 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57:397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Franklin SS, Thijs L, Li Y, et al. International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators: Response to Masked hypertension in untreated and treated patients with diabetes mellitus: attractive but questionable interpretations and response to Is masked hypertension related to diabetes mellitus? Hypertension. 2013;62(4):e23‐e25. [DOI] [PubMed] [Google Scholar]
- 110. Sehestedt T, Hansen TW, Li Y, et al. Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. 2011;34:714‐721. [DOI] [PubMed] [Google Scholar]


