Abstract
Home blood pressure monitoring (HBPM) is increasingly being promoted in hypertension guidelines to improve hypertension management. Possessing a HBPM device could improve blood pressure (BP) control and prognostic impact. The aims of this study were to estimate the possession rate of HBPM devices in the French population and in hypertensive adults, and to investigate the determinants of possessing such devices at home. Cross‐sectional analyses were performed using data from the Esteban survey, which comprised a representative sample of the French population. Among the 2,054 study participants, 673 had hypertension. Of these, 385 were aware they had it. Weighted logistic regressions were performed to investigate the factors (socioeconomic, clinical, drug treatment, and healthcare visits) associated with possessing a HBPM device. 20.9% of the study sample, 42.1% of those with hypertension, and 54% of those aware of their hypertension, possessed a HBPM device. Female gender (OR = 2.03, 95%CI [1.46; 2.60]), smoking (OR = 2.33, 95%CI [1.51; 3.15]), antihypertensive drugs (OR = 1.75, 95%CI [1.06; 2.44]), general practitioner (GP) visits (OR = 3.28, 95%CI [1.84; 4.68]), and diabetes (OR = 0.41 95% CI [0.14; 0.68]) were associated with possessing a HBPM device among those aware of their hypertension. Over 20% of the study population possessed a HBPM device at home. This proportion rose to one in two in those aware or their hypertension. Among the latter, possessing a device was positively associated with female gender, GP visits, and antihypertensive drug use. Increasing possession of HBPM devices in the hypertensive population could foster better management of the condition.
Keywords: drug treatment, healthcare, home blood pressure monitoring, hypertension, socioeconomic status
1. INTRODUCTION
Hypertension is one of the main risk factors for mortality and morbidity from cardiovascular (CV) diseases, renal failure, and cognitive decline. 1 Epidemiological studies have shown that more than one‐third of adults in France and other developed countries are hypertensive. 2 Reducing blood pressure (BP) levels by modifying lifestyle behaviors and using antihypertensive drugs should improve BP control and decrease‐related morbidity and mortality. 3 European guidelines recommend a BP target under 140/90 mm Hg for most patients with uncomplicated hypertension. 4 Nevertheless, more than 50% of hypertensive people do not have their BP controlled. 2 In addition, a large proportion of adults are unaware of their hypertension, especially in France. 2
Hypertension diagnosis in France is based on clinical measures of BP, even though confirmation using ambulatory measurements is recommended, especially before starting antihypertensive therapy. 5 Many previous studies have highlighted that Home Blood Pressure Monitoring (HBPM) is useful in improving BP control, treatment adherence, the detection of white coat and masked hypertension, and prognosis. 6 , 7 , 8 , 9 , 10 Consequently, European and French guidelines suggest the use of HBPM in the diagnosis and therapeutic management of hypertension. 4 , 5
In a study in the United Kingdom, over 30% of aware hypertensive patients reported HBPM and 60% performed it at least monthly. 11 In a Canadian study, these proportions were higher, with approximately 70% of aware hypertensive patients reporting they had measured their BP at home during the previous 12 months. 12
The use of self‐measuring devices has increased significantly over the past several years and physicians increasingly recommend their use for monitoring hypertension in their patients. While several studies have described the frequency of HBPM possession in treated hypertensive patients, few have evaluated this proportion in untreated hypertensive patients and none have evaluated this proportion in the general population. In addition, very few studies have identified the determinants of HBPM possession in a representative sample of the French population.
The aims of the present study were to estimate the proportion of HBPM device possession in the French general population, especially in hypertensive adults, and to investigate the determinants of possessing such a device in adults aware of their hypertension. To ensure comparability with previous studies, we have chosen to focus on people who are aware of their hypertension for the study of determinants, since people who discovered their hypertension status at the time of the health examination in our study have a different profile and probably make little use of these devices.
2. METHODS
2.1. Study design
The probability of study inclusion for the 3021 participants initially included in ESTEBAN was calculated in order to ensure that the study sample was representative of the French population.
The protocol of the Esteban survey has been previously published. 13 , 14 The design of the Esteban survey was multistage stratified random sample at three degrees. The first stage of sampling involved random selection of urban units stratified on eight large regions and on the degree of urbanization. At the second level, households were randomly selected by telephone sampling. The concerned households composed of at least one adult aged 18 to 74 years. At the third level, a single individual was selected among the eligible household members according to Kish's method. 15
This complex survey design was taken into account in the estimation of the initial weighting applied to each person who participated in the first visit. This weighting corresponded to the number of eligible persons in the household, multiplied by the inverse of the probability of drawing from the household and by the inverse of the probability of drawing from the primary unit. To account for adults who withdrew the study between the first visit and the health examination, a non‐response correction was performed using the score method. Finally, a recalibration was made using the margin calibration method. Calibration was performed using the SAS macro program CALMAR (CALibration on MARgins). The margins used in the calibration were taken from the 2012 Census of Population and were based on the following data: age, sex, diploma of the household reference person and whether the reference person lived in a couple or not and period of data collection. The study was registered with the French National Agency for Medicines and Health Products Safety (No. 2012‐A00456‐34) and was approved by the French Advisory Committee for Protection of Persons in Biomedical Research.
2.2. Data sources
Esteban was a cross‐sectional national health study, carried out in France between 2014 and 2016, on a representative sample of the French adult population. The study protocol has been published elsewhere. 13 , 16 , 17 One of Esteban's objectives was to investigate chronic diseases and vascular risk factors. The study design used three‐stage stratified random sampling. Data were collected during a home visit using a face‐to‐face questionnaire. In a subsequent step, a health examination was performed in a medical center or at home, and participants completed a self‐administered questionnaire. Finally, all individual data were matched with data from the SNDS (Système national de données de santé) database which contains comprehensive individual data on all outpatient services and treatments reimbursed by the French universal health insurance system. 18
2.3. Study population
The number of adult participants initially included in Esteban between April 2014 and March 2016 was 3,021. However, after participant withdrawals, only 2,054 had a clinical examination with valid BP values and responded to a question (see below) about possessing a HBPM device at home. Of the latter, 673 participants were hypertensive and among them, 385 were aware they had hypertension (ie, answered “yes” to at least one of the two following questions: “Has a doctor ever told you that your blood pressure is too high?” and as “Have you ever had hypertension?” (Figure 1).
Figure 1.
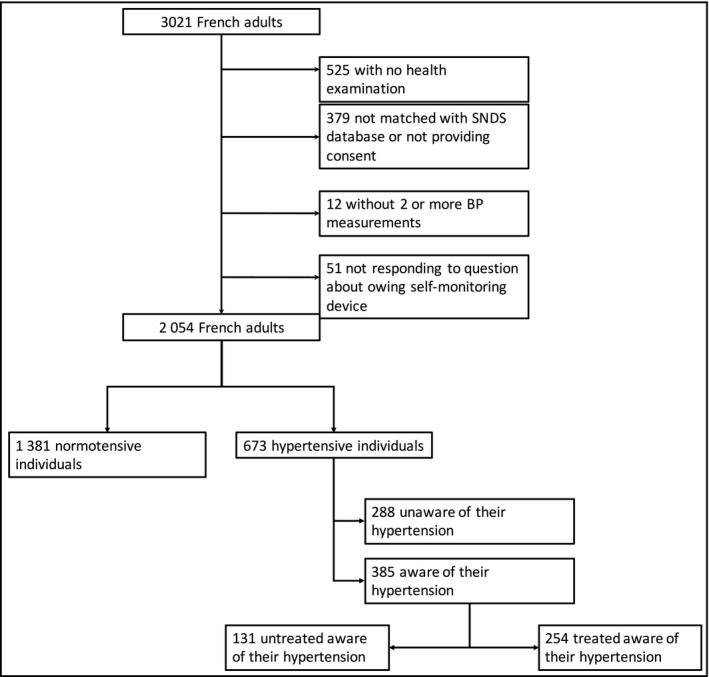
Flowchart of Esteban study 2014‐2016
2.4. Blood pressure and hypertension
During the health examination, BP was measured with an Omron 705‐IT BP monitor on the right arm using a cuff adapted to the arm circumference. Measurements were taken 30 minutes after a blood sample was taken and again after 5 minutes of rest, without changing position. Each time, three measurements were made, 1 minute apart. The systolic BP (SBP) and diastolic BP (DBP) for each person equaled the average of the final two measurements. People who did not have at least two BP measurements were excluded from the analysis. Prevalent hypertension was defined as systolic BP ≥ 140 mm Hg and/or mean diastolic BP ≥ 90 mm Hg and/or taking antihypertension treatment. 4
2.5. Home blood pressure monitoring
The question about HBPM device possession was as follows: “Do you have a device for measuring blood pressure at home?”
2.6. Treatment with antihypertensive drugs
Antihypertensive drugs were identified according to the Anatomical Therapeutic Chemical (ATC) codes of the medications delivered to each participant as follows: beta blockers (ATC codes C07, excluding C07AA07, C07AA12, and C07AG02); agents acting on the renin‐angiotensin system (ATC codes C09); thiazide diuretics (ATC codes C03, excluding C03BA08 and C03CA01); calcium channel antagonists (ATC codes C08); and miscellaneous antihypertensive drugs (ATC codes C02, excluding C02KX01).
In participants with self‐reported hypertension, treatment with medications having the above codes was attributed to hypertension. For those who did not report hypertension but who had medications with these codes, medical doctors searched for another indication for their delivery (eg, heart failure and migraine).
Hypertensive individuals were considered treated if they had at least one delivery of antihypertensive drugs in the 6 months preceding study inclusion.
2.7. Adherence to antihypertensive therapy
Adherence to treatment was defined as the proportion of days covered (PDC) by antihypertensive treatment between the first treatment delivery in the 12 months preceding the clinical examination and the date of examination itself. In order to calculate the PDC, we first multiplied the number of deliveries of hypertensive medication by the number of pills delivered on each occasion, then divided the result by the number of pills taken each day, and then divided this new value by the number of days between the first treatment delivery and the date of examination. For participants prescribed dual therapy, only one tablet was counted per day. A patient was considered adherent if the PDC was greater than 80%. 19
2.8. GP and cardiologist visits
The notion of GP and cardiologist visits was defined as at least one visit during the year preceding the clinical examination. The number of GP and cardiologist visits for each participant was recorded using healthcare service reimbursement data from the SNDS database, for the 12 months preceding the clinical examination to the date of the examination itself.
2.9. Socioeconomic status
Sociodemographic and socioeconomic data were collected by a questionnaire administered face to face during the home visit. Education level was recorded according to the International Standard Classification of Education (ISCED) 20 and then classified into three levels: low: high school diploma or less (≤ 13 years of education); moderate: university undergraduate degree (14‐16 years of education); and high: postgraduate degree (≥17 years of education). From the baseline (ie, home visit) questionnaire, we used the following sociodemographic variables: marital status (ie, single (single/separated/widowed or divorced) and in a couple (whether married or not)); household income (ie, <1900; 1900‐3100; >3100 euros per month).
2.10. Covariates
Weight and height were measured during the health examination (using a stadiometer fixed to a wall and a Tanita scale with digital read‐out, respectively). BMI—calculated as body weight (kg) divided by the square of height (m)—was categorized as normal (<25), overweight (25‐30), and obese (>30). In terms of tobacco use, participants were classified as current smokers or non‐smokers (ie, previously or never smoked).
The DASH‐style diet, developed by Fung et al, was used to measure dietary patterns. It includes 8 dietary components which may be increased (fruits, vegetables, legumes, whole grains, and low‐fat dairy) or decreased (salt, sweetened beverages, red and processed meats). 21 The final DASH score ranged from 8 to 40 in our study population, classified into high (DASH > 25), moderate (between 20 and 25), and low (<20).
Physical activity was assessed using the French version of Recent Physical Activity Questionnaire (RPAQ). 22 Physical activity level was classified as low (0‐2), moderate (3‐4), or high (5‐6).
Hypercholesterolemia was considered if a participant had at least one delivery of cholesterol‐lowering treatment in the 6 months preceding study inclusion or if the LDL‐cholesterol level ≥ 1.6g/L. Diabetes was also considered by taking into account medical diagnosis, treatment, or a fasting glucose ≥ 7 mmol/L.
Chronic kidney disease (CKD) was defined as known proteinuria or decreased renal function (creatinine clearance < 60 mL/min, calculated using the Cockcroft‐Gault equation) for more than 3 months, 23 or chronic kidney disease diagnosed by biopsy or renal ultrasound and confirmed by a nephrologist. Having had a stroke, heart failure, peripheral artery disease, myocardial infarction, and/or angina pectoris were all defined as previous CV events.
2.11. Statistical analysis
Descriptive analysis was performed for the entire study sample and for each gender using weighted counts and percentages or mean ± standard deviation (SD) for quantitative variables, and frequency and percentages for categorical variables. For categorical variables, we used the weighted Pearson's chi‐square test or Fisher's exact test where applicable. Continuous quantitative variables were analyzed using Student's (independent) t test and the Mann‐Whitney test when normal or abnormal distribution was assumed, respectively. All analyses were weighted to take into account the probability of inclusion and non‐participation bias.
Correlations between possessing a HBPM device and its determinants were performed by weighted logistic regression in participants aware of their hypertension. All determinants with a P‐value < .10 and all socioeconomic factors were considered for inclusion in the multivariate logistic regression model. The sub‐population of those aware of their hypertension was selected because they were more likely to possess a self‐monitoring device than persons unaware of their condition. The results of the logistic regression analyses were reported as odds ratios (ORs) with corresponding 95% confidence intervals (95% CI). All tests were 2‐sided. A P‐value < .05 was considered statistically significant. Statistical analyses were performed using SAS software (version 9.4; SAS Institute).
3. RESULTS
One in five (20.9%) participants in the study sample possessed a HBPM device. This value was 42.1% for hypertensive participants compared with 11.1% for the normotensive participants, P < .0001. The proportion of possession was also higher for those aware of their hypertension than those unaware of it (54.0% vs 27.0%, P < .0001). Among the former, the possession was higher in treated participants than those not treated (59.3% vs 44%, P = .004). No significant difference in possession was observed between treated controlled hypertensive participants and their treated uncontrolled counterparts (59.4% vs 59.2%, P = .975) and between adherent and non‐adherent (59.6% vs 59.1%, P = .942) (Figure 2).
Figure 2.
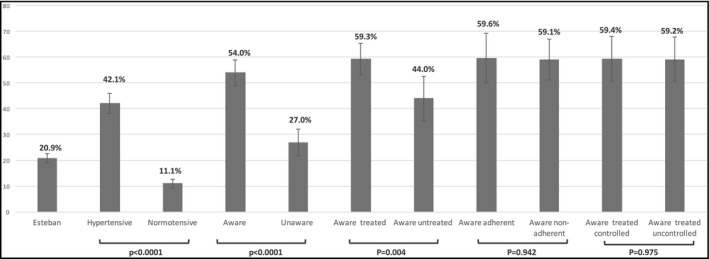
Possession rate of home blood pressure monitoring device among the different subgroups investigated
The characteristics of the 385 participants aware of their hypertension are displayed in Table 1.
Table 1.
Characteristics of adult participants aware of their hypertension according to HBPM device possession
| Aware of hypertension | ||||
|---|---|---|---|---|
|
Total N = 385 |
HBPM N = 221 |
No HBPM N = 164 |
P value | |
| Age | 60.0 (9.1) | 61.4 (8.0) | 58.4 (9.9) | <.0001 |
| SBP | 145 (19) | 144 (19) | 145 (18) | .621 |
| DBP | 85 (11) | 84 (10) | 87 (11) | .008 |
| Controlled hypertension a | 48.0% | 48.1% | 47.9% | .975 |
| Gender (female) | 47.4% | 55.5% | 37.9% | .0005 |
| BMI | 29.0 (6.1) | 28.5 (5.4) | 29.6 (6.8) | .094 |
| BMI class | ||||
| Normal | 25.3% | 24.8% | 25.9% | .489 |
| Overweight | 39.4% | 42.0% | 36.3% | |
| Obese | 35.3% | 33.2% | 37.8% | |
| Diabetes | 20.3% | 14.6% | 27.1% | .003 |
| Hypercholesterolemia | 36.8% | 40.2% | 32.6% | .137 |
| CKD | 4.5% | 5.8% | 3.0% | .192 |
| Previous CV events | 10.7% | 13.7% | 7.1% | .036 |
| Income | ||||
| Low | 31.2% | 31.6% | 30.8% | .181 |
| Moderate | 37.9% | 33.0% | 41.4% | |
| High | 31.8% | 35.4% | 27.8% | |
| Education | ||||
| Low | 21.4% | 26.5% | 15.6% | .064 |
| Moderate | 59.8% | 56.3% | 63.7% | |
| High | 18.8% | 17.1% | 20.7% | |
| DASH score | ||||
| Low | 25.1% | 22.6% | 28.0% | .034 |
| Moderate | 46.0% | 42.8% | 49.7% | |
| High | 28.9% | 34.6% | 22.3% | |
| Physical activity | ||||
| Low | 37.9% | 37.5% | 38.5% | .301 |
| Moderate | 56.0% | 58.2% | 53.5% | |
| High | 6.1% | 4.4% | 8.0% | |
| Living in a couple | 73.8% | 79.5% | 67.0% | .005 |
| Treatment for hypertension (yes) | 65.2% | 71.7% | 57.7% | .004 |
| Number of antihypertensive drugs | ||||
| 0 | 34.8% | 28.3% | 42.3% | .010 |
| 1 | 37.4% | 40.9% | 33.3% | |
| 2 or more | 27.8% | 30.8% | 24.4% | |
| Adherence a | 40.8% | 41.0% | 40.5% | .942 |
| Tobacco | ||||
| Current | 13.0% | 15.2% | 10.4% | .010 |
| No | 87.0% | 84.8% | 89.6% | |
| GP visit | 93.7% | 97.4% | 89.4% | .001 |
| Cardiologist visit | 14.1% | 12.7% | 15.7% | .399 |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CV, cardiovascular; DBP, diastolic blood pressure; GP, general practitioner; HBPM, home blood pressure monitoring; SBP, systolic blood pressure.
Only estimated in treated patients.
Prevalence of previous CV events was higher in HBPM device owners than in non‐owners (13.7% vs 7.1%, P = .036). Furthermore, the former were older (61.4 years vs 58.4 years, P < .0001) and had a higher DASH score (34.6% vs 22.3% for the “high” classification, P = .034). However, they were less likely to have diabetes (14.6% vs 27.1%, P = .003). Owners visited their GP more than non‐owners (97.4% vs 89.4%, P = .001) but no significant difference was observed for consultations with cardiologists (12.7% vs 15.7%, P = .399) (Table 1).
Participants aware of their hypertension who possessed a HBPM device were more frequently treated than non‐owners aware of their hypertension (71.7% vs 57.7%, P = .004). However, no difference was observed regarding adherence to treatment (41.0% vs 40.5%, P = .942) and control of hypertension (48.1% vs 47.9%, P = .975) between the same two groups (Table 1).
In logistic regression, female gender (OR = 2.03, 95%CI [1.46; 2.60], P = .022), current smoking (OR = 2.33, 95%CI [1.51; 3.15], P = .007), antihypertensive drug treatment (OR = 1.75, 95%CI [1.06; 2.44], P = .048), and GP visits (OR = 3.28, 95%CI [1.84; 4.68], P = .006) were all positively associated with possessing a HBPM device. Diabetes (OR = 0.41, 95%CI [0.14; 0.68], P = .006) was negatively associated with possession. Neither education nor income was significantly associated with possessing a HBPM device (Table 2).
Table 2.
Logistic regression of possession of HBPM determinants among participants aware of their hypertension
| OR | 95%CI | P value | |
|---|---|---|---|
| Age | 1.01 | (0.99; 1.03) | .073 |
| Gender (female) | 2.03 | (1.46; 2.60) | .022 |
| Diabetes | 0.41 | (0.14; 0.68) | .006 |
| BMI | 0.86 | (0.50; 1.22) | .489 |
| Previous CV events | 2.17 | (0.93; 3.41) | .125 |
| DASH score | |||
| Low | Ref. | .491 | |
| Moderate | 1.06 | (0.82; 1.30) | .486 |
| High | 1.62 | (0.91; 2.33) | .248 |
| Education | |||
| Low | Ref. | .149 | |
| Moderate | 0.85 | (0.28; 1.42) | .345 |
| High | 0.76 | (0.23; 1.29) | .243 |
| Income | |||
| Low | Ref. | .467 | |
| Moderate | 0.77 | (0.34; 1.20) | .249 |
| High | 1.27 | (0.72, 1.82) | .355 |
| Living in a couple | 1.67 | (0.94; 2.40) | .108 |
| Treatment for hypertension | 1.75 | (1.06; 2.44) | .048 |
| Current smoker | 2.33 | (1.51; 3.15) | .007 |
| GP visit | 3.28 | (1.84; 4.68) | .006 |
Abbreviations: CV events, cardiovascular events; GP, general practitioner; HBPM, home blood pressure monitoring.
4. DISCUSSION
One in five (20.9%) participants in the overall study sample possessed a HBPM device. This value was 42.1% for those with hypertension, 54% for those aware they had the condition, and 59.3% for those who were both aware and were being treated. Female gender, current smoking, GP visits, and antihypertensive treatment were the main factors associated with possessing a device among participants aware of their hypertension.
European and French guidelines suggest the use of HBPM in the diagnosis and therapeutic management of hypertension. 4 , 5 Several evidences have been shown for the emergent role of HBPM use in hypertension management. HBPM can be used to identify specific hypertension, as white coat hypertension and masked uncontrolled hypertension. 24 HBPM presents many advantages such as a large number of measurements, an usual environment of each individual, more reproducible measurements than office BP, closer association with preclinical organ damage and CV events than office BP, improvement of hypertension control rate, and compliance with drugs. 25 Hypertension awareness remained one of the main determinant of HBPM use. 26 Moreover, adults using HBPM were more likely to have diagnosed, treated and controlled hypertension and this, in specific population presenting comorbidities. 27
In the French Flahs survey in 2012, 21% of the study population and 41% of participants aware of their hypertension and being treated for it, had a HBPM. 28 Our study showed that this rate did not increase over time between 2012 and 2015 in the French general population. However, it did increase in hypertensive people aware of their condition (59.1% in our study vs 41% in the 2012 Flahs survey). In France, a growing number of health professionals are proposing HBPM to their hypertensive patients. 8 We found a positive association between visiting a GP and possessing a HBPM device among those aware of their hypertension. This highlights the importance of health professionals, and especially GP, in the management of this condition. Nevertheless, there is significant room for improvement.
Simply possessing a HBPM device is not enough; it must also be used and data must be shared with medical doctors. 8 , 28 , 29 Although participants in the Esteban study were asked about possessing (or not) a HBPM device, no data were collected on whether they used them. This could explain the absence of expected relationships between adherence to treatment and control of hypertension. Other specific surveys could be developed to study the percentage of HBPM data provided by patients to health professionals.
The rates of HBPM device possession in studies for other countries range from 24% to 70% among participants with hypertension. 11 , 30 , 31 , 32 Furthermore, between 50% and 73% of those with a device purchased it without a physician's advice and were not trained by a health professional about how to use it. 30
In our study, there was no difference in mean systolic BP or BP control rates between hypertensive patients aware of their condition who possessed a HBPM device and those without one. However, this result should be interpreted with caution considering the higher proportion of patients treated—in particular those taking several concomitant antihypertensive drugs—who owned a HBPM device. It is therefore likely that hypertensive patients with a device had higher initial BP levels than those with no device. However, no data on this were collected in Esteban.
In our study, there was no difference in adherence to antihypertensive therapy between hypertensive patients aware of their condition who possessed a HBPM device and those without one. Nevertheless, in other studies, HBPM use was associated with increased adherence to antihypertensive treatment 33 and may therefore have led to greater hypertension control. 34 , 35 , 36 However, a recent meta‐analysis of interventions to improve medication adherence showed that HBPM had no major effect on treatment adherence (HBPM vs. no HBPM, effect size: 0.381 vs. 0.216, P = .160). 37
In previous studies, HBPM had a marginal effect on BP when used in the absence of other interventions such as patient education, counseling by nurses or pharmacists, and telecounseling. 33 , 38 , 39 Finally, interventional studies showed that HBPM was not associated with improved BP control in patients with frequent medical visits. 40
Home blood pressure monitoring is already considered a better predictor of cardiovascular risk than clinical measurements. 41 , 42 Importantly, HBPM devices are less expensive than 24H ambulatory blood pressure monitors (ABPM) and are much more acceptable to patients for the management of their hypertension. 43 , 44 The use of HBPM devices could enhance the management of hypertension and reduce both health costs and the frequency of primary care consultations, by improving patients’ perception of health management. 36 , 45 , 46 , 47
Diabetes was associated with fewer HBPM device possession in our study. The association between diabetic status and HBPM possession rate remains unclear with studies showing no significant association 48 , 49 or positive association among US adults 26 and among male US veterans. 50 Although diabetic patients could be more natural candidates for HBPM possession due to their experience with self‐monitoring blood glucose, one possible explanation could be that, in our study, health practitioners place much more emphasis on glycaemic control than on BP control in the promotion of one monitoring device for hypertensive and diabetic patients. In France, HBPM is not reimbursed whereas it is the case for self‐monitoring blood glucose. The negative association between diabetes and HBPM in aware hypertensive adults in our study is worrisome. Firstly, there is more frequent masked hypertension in diabetic patients and the possession of HBPM in this population could allow better screening and monitoring of blood pressure. 51 Secondly, hypertensive and diabetic adults are at very high cardiovascular risk and should benefit from optimal management of their BP. It is essential to make efforts to increase the proportion of hypertensive diabetics who own and use these devices.
The result regarding the positive association between smokers and HBPM possession was unexpected. Few studies have reported results on the determinants of device ownership and the results reported in the literature were inconsistent regarding smoking. One hypothesis to explain this result could be that smokers had more difficult to control hypertension and thus had more HBPM than non‐smokers. However, because of low statistical power, we cannot exclude that this result was due to hazard.
In our study, we did not find any association between HBPM device possession and education or income level. However, given the modest statistical power, we cannot exclude that socioeconomic factors might have played a role, especially since the cost of these devices is not reimbursed by the French healthcare system.
4.1. Strengths and limitations
The main strength of our study is that participants in Esteban are representative of the general French population. Moreover, data were collected according to standardized protocols, and this adds validity to our results. Information about the names, ATC codes, and frequency of pharmacological treatments, as well as the dates of practitioner (GP and cardiologist) consultations, was collected using the SNDS, France's exhaustive national database for treatment delivery. Using this database ensured, we could accurately estimate treatment adherence of each treated adult without declarative bias. However, our study also had limitations. First, although Esteban is representative for the French population, we cannot assume that the hypertensive Esteban population is representative for the French hypertensive population. Since the sample is quite small, some rare hypertensive subgroups may not be represented; for example, rare cause of hypertension. Second, its cross‐sectional design means that reverse causation cannot be excluded. Third, the sample size was rather small, limiting the statistical power of the analyses. Finally, the study design did not allow the collection of significant information such as the type of home BP monitor (validated or not), the use of appropriate cuff size, the use of a recommended home BP monitoring schedule, the frequency, or potential proper use. These details are crucial for the optimal use of home BP monitoring in terms of hypertension management and might account, at least in part, for the absent effect of a home BP monitor possession on adherence or BP control in this study.
5. CONCLUSION
One in five French adults owned a HBPM device in our study. This proportion rose to one in two for participants aware of their hypertension. Among the latter, female gender, GP visits, and antihypertensive drug use were positively and independently associated with possessing a device. In contrast, income was not. The potential benefit of owning and using a HBPM device could be to better diagnose people in the general population who are unaware they are hypertensive. Moreover, increasing HBPM device possession among hypertensive people could enhance the management of this condition, as long as people use their device and share their BP values with their GP.
CONFLICT OF INTEREST
JB has received research support or has served on advisory boards or as a speaker for Abbott, Amgen, Astellas, Astra‐Zeneca, Bayer, Boehringer Ingelheim, Bouchara‐Recordati, Daiichi Sankyo, Ferring, Gilead, Icomed, Medexact, Medtronic, Novartis, Novo Nordisk, Quantum Genomics, Saint Jude, Sanofi Aventis, and Servier outside of this work.
AUTHOR CONTRIBUTIONS
AV, JB, and VO designed the study. AV performed the statistical analyses and wrote the manuscript. AV, AG, CG, HL, JB, and VO validated the article.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
IRB APPROVAL
The study was registered in the French National Agency for Medicines and Health Products Safety (No. 2012‐A00456‐34) and was approved by the Advisory Committee for Protection of Persons in Biomedical Research.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
ACKNOWLEDGMENTS
The authors thank the Centers for Health Examinations, the Cetaf and the laboratories involved in the collection, as well as the entire Esteban team and study participants. The Esteban study, from the National Biosafety launches, is financed by the Ministry of Solidarities and Health and the Ministry of Ecological and Solidarity Transition.
Vallée A, Gabet A, Grave C, Lelong H, Blacher J, Olié V. Home blood pressure monitoring in France: Device possession rate and associated determinants, the Esteban study. J. Clin. Hypertens. 2020;22:2204–2213. 10.1111/jch.14055
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959‐968. [DOI] [PubMed] [Google Scholar]
- 3. Blood Pressure Lowering Treatment Trialists’ Collaboration . Blood pressure‐lowering treatment based on cardiovascular risk: a meta‐analysis of individual patient data. Lancet Lond Engl. 2014;384:591‐598. [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284‐2309. [DOI] [PubMed] [Google Scholar]
- 5. Blacher J, Halimi J‐M, Hanon O, et al. Management of hypertension in adults: the 2013 French Society of Hypertension guidelines. Fundam Clin Pharmacol. 2014;28:1‐9. [DOI] [PubMed] [Google Scholar]
- 6. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163:691‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725‐731. [DOI] [PubMed] [Google Scholar]
- 8. Dugelay G, Kivits J, Desse L, Boivin J‐M. Implementation of home blood pressure monitoring among French GPs: A long and winding road. PLoS One. 2019;14:e0220460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao H, Zeng F, Wang X, Wang L. Prevalence, risk factors, and prognostic significance of masked hypertension in diabetic patients. Medicine (Baltimore). 2017;96:e8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta‐analysis of randomised trials. BMJ. 2004;329:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baral‐Grant S, Haque MS, Nouwen A, Greenfield SM, McManus RJ. Self‐monitoring of blood pressure in hypertension: a UK primary care survey. Int J Hypertens. 2012;2012:582068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logan AG, Dunai A, McIsaac WJ, Irvine MJ, Tisler A. Attitudes of primary care physicians and their patients about home blood pressure monitoring in Ontario. J Hypertens. 2008;26:446‐452. [DOI] [PubMed] [Google Scholar]
- 13. Balicco A, Oleko A, Boschat L, et al. Esteban design: A cross‐sectional health survey about environment, biomonitoring, physical activity and nutrition (2014–2016). Toxicologie Analytique & Clinique. 2017;29:517‐537. [Google Scholar]
- 14. Vallée A, Gabet A, Grave C, Sorbets E, Blacher J, Olié V. Patterns of hypertension management in France in 2015: The ESTEBAN survey. J Clin Hypertens. 2020;22(4):663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kish L. A Procedure for Objective Respondent Selection within the Household. J Am Stat Assoc. 1949;44:380‐387. [Google Scholar]
- 16. Vallée A, Gabet A, Deschamps V, Blacher J, Olié V. Relationship between Nutrition and Alcohol Consumption with Blood Pressure: The ESTEBAN Survey. Nutrients. 2019;11(6):1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vallée A, Perrine A‐L, Deschamps V, Blacher J, Olié V. Relationship between dynamic changes in body weight and blood pressure: the ESTEBAN survey. Am J Hypertens. 2019;32(10):1003‐1012. [DOI] [PubMed] [Google Scholar]
- 18. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149‐S167. [DOI] [PubMed] [Google Scholar]
- 19. Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457‐464. [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider S.The International Standard Classification of Education 2011. Emerald Group Publishing Limited. Birkelund GE, editor, 2013.
- 21. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH‐style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713‐720. [DOI] [PubMed] [Google Scholar]
- 22. Besson H, Brage S, Jakes RW, Ekelund U, Wareham NJ. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self‐report in adults. Am J Clin Nutr. 2010;91:106‐114. [DOI] [PubMed] [Google Scholar]
- 23. Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease‐Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann Intern Med. 2018;168:422‐430. [DOI] [PubMed] [Google Scholar]
- 24. Kario K, Shimbo D, Hoshide S, Wang J‐G, Asayama K, Ohkubo T, et al. Emergence of Home Blood Pressure‐Guided Management of Hypertension Based on Global Evidence. Hypertens Dallas Tex. 1979 2019: HYPERTENSIONAHA11912630. [DOI] [PMC free article] [PubMed]
- 25. Stergiou GS, Kario K, Kollias A, et al. Home blood pressure monitoring in the 21st century. J Clin Hypertens Greenwich Conn. 2018;20:1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostchega Y, Zhang G, Kit BK, Nwankwo T. Factors Associated With Home Blood Pressure Monitoring Among US Adults: National Health and Nutrition Examination Survey, 2011–2014. Am J Hypertens. 2017;30:1126‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang O, Foti K, Miller ER, Appel LJ, Juraschek SP. Factors associated with physician recommendation of home blood pressure monitoring and blood pressure in the US population. Am J Hypertens. 2020;33(9):852‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaïsse B, Mourad J‐J, Girerd X, et al. Flash Survey 2012: the use of self‐measurement in France and its evolution since 2010. Ann Cardiol Angeiol (Paris). 2013;62:200‐203. [DOI] [PubMed] [Google Scholar]
- 29. Boivin J‐M, Tsou‐Gaillet T‐J, Fay R, Dobre D, Rossignol P, Zannad F. Influence of the recommendations on the implementation of home blood pressure measurement by French general practitioners: a 2004–2009 longitudinal survey. J Hypertens. 2011;29:2105‐2115. [DOI] [PubMed] [Google Scholar]
- 30. Tan NC, Khin LW, Pagi R. Home blood‐pressure monitoring among hypertensive patients in an Asian population. J Hum Hypertens. 2005;19:559‐564. [DOI] [PubMed] [Google Scholar]
- 31. Zahid H, Amin A, Amin E, et al. Prevalence and Predictors of Use of Home Sphygmomanometers Among Hypertensive Patients. Cureus. 2017;9(4): e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bancej CM, Campbell N, McKay DW, Nichol M, Walker RL, Kaczorowski J. Home blood pressure monitoring among Canadian adults with hypertension: results from the 2009 Survey on Living with Chronic Diseases in Canada. Can J Cardiol. 2010;26:e152‐e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens Greenwich Conn. 2006;8:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fletcher BR, Hartmann‐Boyce J, Hinton L, McManus RJ. The Effect of Self‐Monitoring of Blood Pressure on Medication Adherence and Lifestyle Factors: A Systematic Review and Meta‐Analysis. Am J Hypertens. 2015;28:1209‐1221. [DOI] [PubMed] [Google Scholar]
- 35. McManus RJ, Mant J, Franssen M, et al. Efficacy of self‐monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet Lond Engl. 2018;391:949‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bray EP, Holder R, Mant J, McManus RJ. Does self‐monitoring reduce blood pressure? Meta‐analysis with meta‐regression of randomized controlled trials. Ann Med. 2010;42:371‐386. [DOI] [PubMed] [Google Scholar]
- 37. Conn VS, Ruppar TM, Chase J‐AD, Enriquez M, Cooper PS. Interventions to Improve Medication Adherence in Hypertensive Patients: Systematic Review and Meta‐analysis. Curr Hypertens Rep. 2015;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tucker KL, Sheppard JP, Stevens R, et al. Self‐monitoring of blood pressure in hypertension: A systematic review and individual patient data meta‐analysis. PLoS Medicine. 2017;14:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheppard JP, Tucker KL, Davison WJ, et al. Self‐monitoring of blood pressure in patients with hypertension related multi‐morbidity: Systematic review and individual patient data meta‐analysis. Am J Hypertens. 2020;33(3):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hosseininasab M, Jahangard‐Rafsanjani Z, Mohagheghi A, et al. Self‐monitoring of blood pressure for improving adherence to antihypertensive medicines and blood pressure control: a randomized controlled trial. Am J Hypertens. 2014;27:1339‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama, Japan. J Hypertens. 1998;16:971‐975. [DOI] [PubMed] [Google Scholar]
- 42. Niiranen TJ, Hänninen M‐R, Johansson J, Reunanen A, Jula AM. Home‐measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn‐Home study. Hypertens Dallas Tex. 1979;2010(55):1346‐1351. [DOI] [PubMed] [Google Scholar]
- 43. Jones MI, Greenfield SM, Bray EP, et al. Patients’ experiences of self‐monitoring blood pressure and self‐titration of medication: the TASMINH2 trial qualitative study. Br J Gen Pract J R Coll Gen Pract. 2012;62:e135‐e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nasothimiou EG, Karpettas N, Dafni MG, Stergiou GS. Patients’ preference for ambulatory versus home blood pressure monitoring. J Hum Hypertens. 2014;28:224‐229. [DOI] [PubMed] [Google Scholar]
- 45. Fletcher BR, Hinton L, Bray EP, et al. Self‐monitoring blood pressure in patients with hypertension: an internet‐based survey of UK GPs. Br J Gen Pract J R Coll Gen Pract. 2016;66:e831‐e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self‐measured blood pressure monitoring in the management of hypertension: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:185‐194. [DOI] [PubMed] [Google Scholar]
- 47. McManus RJ, Mant J, Roalfe A, et al. Targets and self monitoring in hypertension: randomised controlled trial and cost effectiveness analysis. BMJ. 2005;331:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ayala C, Tong X, Neeley E, Lane R, Robb K, Loustalot F. Home blood pressure monitoring among adults‐American Heart Association Cardiovascular Health Consumer Survey, 2012. J Clin Hypertens Greenwich Conn. 2017;19:584‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viera AJ, Cohen LW, Mitchell CM, Sloane PD. Use of home blood pressure monitoring by hypertensive patients in primary care: survey of a practice‐based research network cohort. J Clin Hypertens Greenwich Conn. 2008;10:280‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thorpe CT, Oddone EZ, Bosworth HB. Patient and social environment factors associated with self blood pressure monitoring by male veterans with hypertension. J Clin Hypertens Greenwich Conn. 2008;10:692‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertens Dallas Tex. 1979;2013(61):964‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]


