Abstract
Findings from randomized trials addressing the effect of vitamin D supplementation and blood pressure are inconsistent and have been the subject of recent debate. This study aimed to assess the effect of vitamin D supplementation on primary hypertension. This double‐blind randomized clinical trial was conducted on patients aged 26‐84 years with essential hypertension from March 2017 to April 2019. Patients with vitamin D insufficiency (serum vitamin D levels 20‐30 ng/ml) or vitamin D deficiency (serum vitamin D levels <20 ng/ml) were enrolled in the study. Patients were randomly assigned to receive vitamin D supplementation or placebo. Systolic and diastolic blood pressure was measured before the intervention and one and two months thereafter. Of 208 patients enrolled, 171 patients remained for analysis. The effect of vitamin D supplementation on systolic blood pressure was statistically significant in the first and second months after the intervention (P=0.004 and P=0.024, respectively). The effect of vitamin D supplementation on diastolic blood pressure was statistically significant in the first month after the intervention (P=0.046), but not in the second month (P=0.885). No evidence of drug side effects was reported in the two groups. The results of this trial are suggestive of the potential benefits of vitamin D supplementation on blood pressure end points. Therefore, the use of vitamin D may be recommended as an adjuvant drug in the treatment of essential hypertension in patients with vitamin D deficiency because it is safe and well‐tolerated by the patients and can significantly reduce the systolic and diastolic blood pressure.
Trial registration: Iranian Registry of Clinical Trials registration number: IRCT201703129014N151.
Keywords: Blood Pressure, Hypertension, Vitamin D Deficiency, Clinical Trial
1. Introduction
Vitamin D, also known as calciferol, is a fat‐soluble vitamin that is naturally present in very few foods and available as a dietary supplement. The two major forms are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is largely human‐made and is usually added to certain foods. Vitamin D3 is produced endogenously in the skin of humans when ultraviolet rays from sunlight strike the skin and trigger vitamin D synthesis. It is also available in animal‐based foods. Both forms are produced commercially and are available as dietary supplements 1 , 2 .
At present, the available scientific data indicate that vitamin D has a key role in skeletal health including bone mineralization and maintenance of calcium homeostasis. Thus, vitamin D deficiency is well‐known for the disorders that involve the musculoskeletal system and bone metabolism and provide a sound basis for dietary reference intakes (DRIs) 1 . However, extra‐skeletal health outcomes related to vitamin D is not well understood yet.
Animal studies provided strong evidence that vitamin D level is inversely associated with the renin‐angiotensin‐aldosterone system (RAAS) activity 3 . Furthermore, vitamin D deficiency is associated with endothelial vasodilator dysfunction 4 . Thus, it is likely that the activity of vitamin D in animals contributes to a reduction in blood pressure 3 .
Based on recent epidemiological studies, there has been growing evidence that vitamin D deficiency plays a role in developing metabolic syndrome and cardiovascular diseases 5 , 6 . However, results from observational studies are conflicting and inconsistent. Several studies suggested that low levels of vitamin D are specifically associated with an increased risk of several diseases such as myocardial infarction 7 , abnormal vascular endothelial function 8 , vascular smooth muscle proliferation and vascular calcification 9 , elevated RAS activity 10 , and hypertension 11 , 12 , 13 , 14 , 15 . On the other hand, some observational studies did not support these findings 16 , 17 , 18 .
To date, the mechanism underlying the inverse relationship between vitamin D and blood pressure and the role of vitamin D deficiency as an independent risk factor for CVD is not completely understood 19 , 20 . Furthermore, findings from randomized trials addressing the association between vitamin D supplementation and hypertension are inconsistent. Thus, using supplementation with vitamin D to prevent or treat hypertension has been the subject of recent debate 17 , 19 , 20 , 21 . The present study was conducted to assess the effect of vitamin D supplemented with antihypertensive drugs on reducing blood pressure in hypertensive patients with vitamin D insufficiency or deficiency.
2. Methods
This double‐blind randomized clinical trial was conducted in Shahid Beheshti Hospital, affiliated with Hamadan University of Medical Sciences, in the west of Iran, from March 2017 to April 2019. Written informed consent was received from all patients. The Ethics Committee of the university approved the trial (IR.UMSHA.REC.1395.393). The protocol was registered with the Iranian Registry of Clinical Trials on March 12, 2017 (IRCT201703129014N151).
According to the results of a controlled trial conducted by Goel et al 22 , the mean (SD) systolic blood pressure after three months of Vitamin D supplementation was 141.6 (9.2) mmHg and 145.2 (8.3) mmHg in the intervention and control groups, respectively. Based on these results, we arrived at a sample size of 93 for each group and a total sample size of 186 at a 95% significance level and 80% statistical power. Anticipating a 10% loss to follow‐up, we increased the sample size to a maximum of 208, of which 104 were randomly allocated to the vitamin D supplementation group and 104 to the placebo group.
The study population included patients aged 18 to 75 years with systolic blood pressure equal to or greater than 140 mmHg or diastolic blood pressure equal to or greater than 90 mmHg who were vitamin D deficient or insufficient. Vitamin D insufficiency was defined as serum vitamin D levels between 20 and 30 ng/ml (50‐75 nmol/liter), and vitamin D deficiency was defined as serum vitamin D levels below 20 ng/ml (50 nmol/liter) 23 . Patients with any of the following characteristics were excluded from the study: (a) pregnancy; (b) secondary hypertension; (c) renal failure; (d) history of using vitamin D or calcium during the last three months; or (e) using thiazide‐type diuretics.
The eligible patients were randomly assigned to two groups using the balance block randomization method. For this purpose, we prepared four sheets of paper, writing on two sheets “A” for “vitamin D,” and on two “B” for “placebo.” The paper sheets were pooled, placed in the allocator’s white coat pocket, and randomly drawn one at a time for each patient without replacement until all four sheets were drawn. The four paper sheets were then placed back into her pocket and this action repeated until the sample size was reached. The allocations remained concealed during the study. For this purpose, the vitamin D and placebo pearls were put in envelops coded A and B, respectively. The shape of vitamin D and placebo pearls were perfectly the same. Therefore, neither the patients nor the physician who examined the patients was aware of the type of intervention. Thus, the trial was double‐blind.
Both groups received routine antihypertensive medications. The intervention group received vitamin D (Calciferol) pearls based on serum vitamin D levels. Patients, whose serum vitamin D level was less than 20 ng/mL, received one vitamin D pearl 50,000 U weekly for two months. Patients, whose serum vitamin D level was between 20 and 30 ng/mL, received one vitamin D pearl 1000 U weekly for two months 23 . The control group received a placebo pearl for two months.
The primary outcome of interest was systolic and diastolic blood pressure, which was evaluated before the intervention and at the end of the first and second months from the beginning of the intervention. Blood pressure was measured in the sitting position in the office after 10 minutes of rest using a mercury sphygmomanometer. The secondary outcome of interest was serum calciferol level before the intervention and two months after the intervention using the VIDAS kit manufactured by bioMérieux Co, France.
The independent t test was used for analysis. We also used analysis of variance to perform change score analysis adjusted for sex, age group, body mass index, fasting blood sugar (FBS), and serum cholesterol level. For this purpose, we compared the difference in blood pressure (systolic and diastolic) before the intervention and one and two months thereafter between the vitamin D and placebo groups. All statistical analyses were performed at a significance level of 0.05 using Stata software, version 14 (StataCorp).
3. Results
Of 368 patients identified, 80 were ineligible, 71 had normal serum vitamin D levels, and 9 declined to participate. The randomization was based on the remaining 208 patients, of whom 104 patients were allocated to the vitamin D Group and 104 to the control group. Thirty‐seven patients declined follow‐up including 7 patients in the vitamin D group and 30 patients in the control group. The analysis was based on data from the remaining 171 patients (66 men and 105 women) including 97 in the vitamin D group and 74 in the control group (Figure 1). The mean (SD) age of the patients was 55.88 (11.51) years, ranged from 26 to 84 years.
Figure 1.
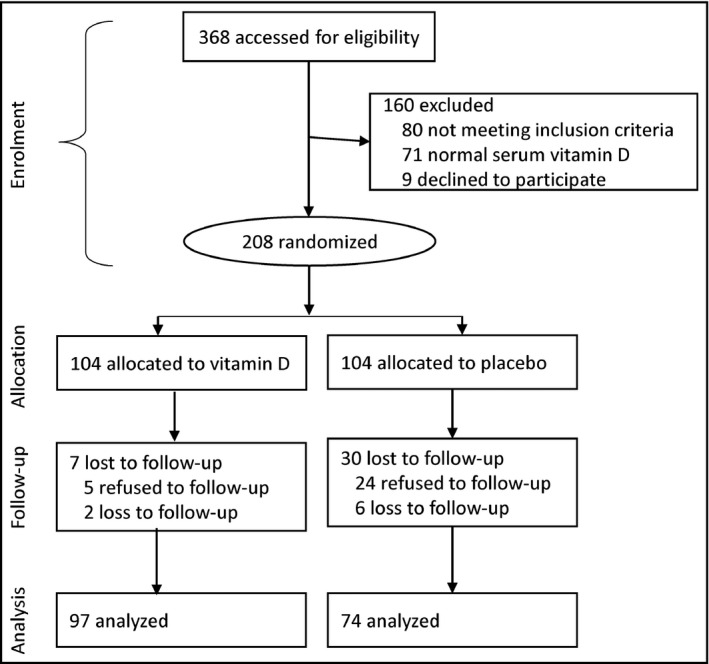
Flowchart of progress through the trial
The baseline characteristics of the intervention (vitamin D) and control (placebo) groups are given in Table 1. There were no statistically significant differences between the age, body mass index, duration of hypertension, serum vitamin levels, fasting blood sugar, total cholesterol, creatinine, sodium (Na), potassium (K), calcium (Ca), and phosphor (P) between the two groups.
Table 1.
Baseline characteristics of the intervention (vitamin D) and control (placebo) groups
| Placebo group | Vitamin D group | ||||
|---|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD | P‐value |
| Age (yr) | 54.41 | 10.70 | 57.01 | 12.02 | 0.145 |
| Body mass index (kg/m 2 ) | 27.13 | 4.05 | 26.69 | 5.01 | 0.544 |
| Duration of hypertension (yr) | 8.12 | 8.92 | 8.83 | 7.45 | 0.576 |
| Vitamin D (ng/ml) at baseline | 14.58 | 6.22 | 13.21 | 6.70 | 0.173 |
| Fasting blood sugar (mg/dl) | 93.40 | 10.34 | 93.70 | 8.72 | 0.839 |
| Total cholesterol (mg/dl) | 205.56 | 44.13 | 201.63 | 46.19 | 0.575 |
| Creatinine (mg/dl) | 0.96 | 0.14 | 0.97 | 0.17 | 0.566 |
| Na (meq/l) | 137.60 | 3.27 | 136.30 | 4.06 | 0.026 |
| K (meq/l) | 3.99 | 0.36 | 4.04 | 0.32 | 0.295 |
| Ca (mg/dl) | 8.91 | 0.47 | 8.85 | 0.57 | 0.418 |
| P (mg/dl) | 3.32 | 0.51 | 3.35 | 0.48 | 0.719 |
The effects of vitamin D supplementation vs placebo on systolic and diastolic blood pressure are given in Table 2. At baseline, there were no statistically significant differences in the systolic and diastolic blood pressure between the vitamin D and placebo groups (P=0.233 and P=0.843, respectively). The effect of vitamin D supplementation on systolic blood pressure was statistically significant in the first and second months after the intervention (P=0.003 and P=0.037, respectively). However, the effect of vitamin D supplementation on diastolic blood pressure was not statistically significant in the first and second months after the intervention (P=0.053 and P=0.740, respectively).
Table 2.
Effect of vitamin D supplementation versus placebo on systolic and diastolic blood pressure using change score analysis adjusted for sex, age group, body mass index, fasting blood sugar (FBS), and serum cholesterol level
| Placebo group | Vitamin D group | ||||
|---|---|---|---|---|---|
| Blood pressure (mmHg) | Mean | SD | Mean | SD | P‐value |
| Systolic blood pressure at baseline | 149.45 | 14.34 | 152.93 | 16.42 | 0.233 |
| Diastolic blood pressure at baseline | 87.50 | 8.72 | 87.37 | 9.38 | 0.843 |
| Systolic blood pressure at 1st month | 138.37 | 12.05 | 134.93 | 13.16 | 0.003 |
| Diastolic blood pressure at 1st month | 83.78 | 6.86 | 82.06 | 5.76 | 0.053 |
| Systolic blood pressure at 2nd month | 130.97 | 12.82 | 127.65 | 13.38 | 0.037 |
| Diastolic blood pressure at 2nd month | 80.40 | 6.50 | 80.51 | 5.22 | 0.740 |
| Vitamin D (ng/ml) at 2nd month | 15.86 | 10.31 | 35.21 | 12.21 | 0.001 |
The effects of various doses of vitamin D supplementation vs placebo on systolic and diastolic blood pressure are given in Table 3. At baseline, there was a statistically significant difference in the systolic but not diastolic blood pressure among various doses of vitamin D and placebo groups (P=0.001 and P=0.424, respectively). The effect of various doses of vitamin D supplementation on systolic blood pressure was statistically significant in the first month but not in the second month after the intervention (P=0.012 and P=0.107, respectively). The effect of various doses of vitamin D supplementation on diastolic blood pressure was not statistically significant both in the first and second months after the intervention (P=0.157 and P=0.146, respectively).
Table 3.
Effect of various doses of vitamin D supplementation versus placebo on systolic and diastolic blood pressure using change score analysis adjusted for sex, age group, and body mass index
| Placebo |
Vitamin D 1000U |
Vitamin D 50,000U |
|||||
|---|---|---|---|---|---|---|---|
| Blood pressure (mmHg) | Mean | SD | Mean | SD | Mean | SD | P‐value |
| Systolic blood pressure at baseline | 149.45 | 14.34 | 145.55 | 15.80 | 157.29 | 15.29 | 0.001 |
| Diastolic blood pressure at baseline | 87.50 | 8.72 | 85.55 | 9.69 | 88.44 | 9.10 | 0.424 |
| Systolic blood pressure at 1st month | 138.37 | 12.05 | 131.91 | 4.12 | 136.72 | 2.34 | 0.012 |
| Diastolic blood pressure at 1st month | 83.78 | 6.86 | 81.38 | 6.16 | 82.45 | 5.52 | 0.157 |
| Systolic blood pressure at 2nd month | 130.97 | 12.82 | 125.63 | 12.06 | 128.85 | 14.06 | 0.107 |
| Diastolic blood pressure at 2nd month | 80.40 | 6.50 | 79.58 | 6.36 | 81.06 | 4.38 | 0.146 |
| Vitamin D (ng/ml) at 2nd month | 15.86 | 10.31 | 34.10 | 11.74 | 35.87 | 12.52 | 0.001 |
As shown in Figure 2, systolic blood pressure was slightly higher at baseline in the vitamin D group than in the control group. However, it decreases after much more in the vitamin D group than the control group after vitamin D supplementation. The effect of vitamin D supplementation on the diastolic blood pressure was not noticeable.
Figure 2.
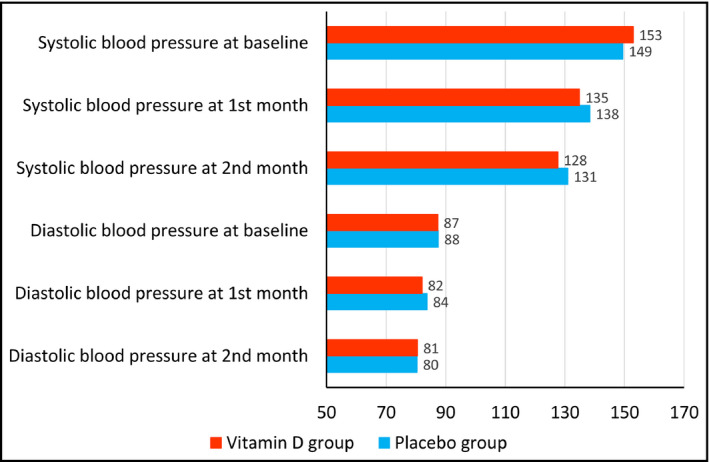
Effect of vitamin D supplementation versus placebo on systolic and diastolic blood pressure
Neither of the patients in the vitamin D or control groups reported any signs or symptoms of drug complications such as nausea, vomiting, abdominal pain, diarrhea, and constipation.
4. Discussion
The use of vitamin D supplementation is taken into consideration in the treatment of essential hypertension because it is safe and well‐tolerated by the patients and can significantly reduce systolic and diastolic blood pressure.
A great reduction in systolic and diastolic blood pressure was observed in both intervention and control groups. The reduction in blood pressure in the control group was reasonable. Although they did not receive vitamin D, they did receive routine antihypertension medications. However, the reduction in systolic blood pressure was greater among the intervention group who received antihypertensive medication plus vitamin D supplementation. Antihypertensive benefits of vitamin D can be attributable to suppression of RAAS, an anti‐protein uric effect, a direct effect on endothelial cells due to vitamin D receptor expression in endothelial cells, vascular smooth muscle cells, and cardiac myocytes 3 , 4 , 9 .
Several studies have shown the inverse association of 25‐hydroxy vitamin D [25(OH)D] levels and blood pressure in both normal and hypertensive individuals. Forman et al. showed that 25(OH)D had an inverse relationship with the risk of hypertension 24 . Demir et al. indicated the association of vitamin D deficiency and blood pressure and its relationship with hypotension during sleep 25 .
Goel et al conducted a clinical trial to assess the role of vitamin D supplementation in patients with hypertension. One hundred hypertensive patients (group I) were given conventional antihypertensive drugs while another 100 patients (group II), also, were supplemented with vitamin D3 (33,000 IU, after every 2 weeks, for 3 months). Besides diastolic and systolic blood pressure, serum calcium, phosphorus, alkaline phosphatase, albumin, albumin‐corrected calcium, and 24‐hour urinary creatinine levels were estimated in both the groups before the start of treatment and after 3 months. Vitamin D supplementation showed a more significant decrease in systolic blood pressure. The results of the study confirm that vitamin D supplementation has a role in reducing blood pressure in hypertensive patients and that it should be supplemented with antihypertensive drugs 22 .
Forman et al. performed a study on 208 black people and showed a significant decrease in systolic blood pressure after the consumption of vitamin D3 26 . Another randomized controlled trial conducted by Toxqui et al. indicated that 200 units of vitamin D3 supplementation per day for 16 weeks resulted in a decrease in systolic and diastolic blood pressure compared to placebo 27 .
In a study on 23 heart failure patients with 25(OH)D deficiency (<30 ng/mL), Dalbeni et al. showed that systolic blood pressure decreased significantly in the group receiving 4,000 units of vitamin D per day for 6 months (129.6‐122.7) compared with the placebo group 28 . Wang et al. administered 1 µg oral paricalcitol for 52 weeks to 62 patients with chronic kidney disease and found a decrease of 4.49 mmHg in systolic blood pressure in the vitamin D supplementation group and of 3.03 mmHg in the placebo receiving group 29 .
A meta‐analysis performed by He and Hao showed that oral intake of vitamin D had no significant effect on blood pressure in vitamin D–deficient normotensive individuals, but it decreased systolic blood pressure in patients over 50 years and obese patients. Supplementation of vitamin D lowers diastolic and systolic blood pressure in people with hypertension and vitamin D deficiency 30 .
Several investigations have been carried out to explain the possible mechanism of vitamin D deficiency‐induced hypertension. There are currently three theories. First, animal studies have shown increased production of renin and angiotensin II in mice lacking vitamin D receptors 31 , 32 . Second, vitamin D deficiency can lead to hyperparathyroidism; serum levels of 25(OH)D and parathormone are inversely correlated so that elevated levels of parathormone lead to hypertension 33 . Third, vitamin D deficiency leads to vascular endothelial dysfunction. Studies have shown that 1,25 dihydroxy vitamin D [1,25(OH)2 D] reduces the deleterious effects of advanced glycation end product on the endothelium 34 , 35 . Furthermore, vitamin D improves the activity of the nitric acid system and reduces inflammatory and atherosclerotic factors, and its deficiency is a risk factor for hypertension 36 .
Jablonski et al. investigated the association of vitamin D deficiency with endothelium‐dependent vasodilatation by measuring flow‐dependent vasodilatation. They concluded that vitamin D deficiency can impair endothelial cell function 37 .
There were a few limitations and potential biases in this trial as follows. Patients were enrolled in the study from a single‐center study. This issue may raise the possibility of selection bias. In this study, loss to follow‐up was nearly 18%. The most common reasons for loss to follow‐up were being unreachable due to change of address or immigration, refusal of follow‐up, treatment discontinuation, and non‐compliance to adhere to the study protocol. If dropouts had not occurred, the difference between the trial groups could be indicated more clearly with less possibility of random error. The relative short‐term period is another obstacle to our study. Also, our study sample included patients who were vitamin D deficient or insufficient; therefore, our results may not be generalizable to hypertensive patients with normal serum vitamin D levels. Despite its limitations, this trial was able to efficiently assess and compare the efficacy of vitamin D supplementation for the treatment of primary hypertension.
5. Conclusions
The results of this trial are suggestive of the potential benefits of vitamin D supplementation on blood pressure end points. Therefore, the use of vitamin D supplementation may be taken into consideration in the treatment of essential hypertension in patients with vitamin D deficiency because it is safe and well‐tolerated by the patients and can significantly reduce the systolic and diastolic blood pressure and help patients return to normal serum vitamin D levels.
Conflict of interest
Zahravi Pharmaceutical Company provided vitamin D and placebo pearls for this study free of charge. However, the authors have no conflict of interest to declare.
Author Contributions
Vida Sheikh contributed to the study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision. Azadeh Mozaianimonfared contributed to the study design, the acquisition of data, and critical revision. Mohsen Gharakhani contributed to the study design and critical revision. Jalal Poorolajal contributed to the study conception and design, analysis and interpretation of data, drafting of the manuscript, and critical revision.
Acknowledgments
We would like to appreciate the Center for Clinical Research Development Unit of Shahid Beheshti Hospital the Vice‐Chancellor for Research and Technology of the Hamadan University of Medical Sciences for approval of this work. Also, we thank Zahravi Pharmaceutical Company for providing the study medication free of charge.
Sheikh V, Mozaianimonfared A, Gharakhani M, Poorolajal J, Ph.D. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: a double‐blind randomized clinical trial. J Clin Hypertens. 2020;22:1867–1873. 10.1111/jch.13926
Sources of funding
The Vice‐Chancellor of Research and Technology, Hamadan University of Medical Sciences, funded this study (9510075751).
References
- 1. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes calcium vitamin D. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.National Institutes of Health. D.etary supplement fact sheet: vitaminD.2011 [up D. te D. June 24, 2011; cite D. April 4, 2012]; Available from: http://oD. .oD.nih.gov/factsheets/VitaminD. HealthProfessional/.
- 3. Vaidya A, Williams JS. The relationship between vitamin D and the renin‐angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2011; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011;589:4777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sood A, Arora R. Vitamin D deficiency and its correlations with increased cardiovascular incidences. Am J Ther. 2010;17:e105–e9. [DOI] [PubMed] [Google Scholar]
- 6. Vanga SR, Good M, Howard PA, Vacek JL. Role of vitamin D in cardiovascular health. Am J Cardiol. 2010;106:798–805. [DOI] [PubMed] [Google Scholar]
- 7. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25‐hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chitalia N, Recio‐Mayoral A, Kaski JC, Banerjee D. Vitamin D deficiency and endothelial dysfunction in non‐dialysis chronic kidney disease patients. Atherosclerosis. 2012;220:265–8. [DOI] [PubMed] [Google Scholar]
- 9. Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kota SK, Kota SK, Jammula S, Meher LK, Panda S, Tripathy PR, et al. Renin‐angiotensin system activity in vitamin D deficient, obese individuals with hypertension: an urban Indian study. Indian. J Endocrinol Metab. 2011;S395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ringrose JS, PausJenssen AM, Wilson M, Blanco L, Ward H, Wilson TW. Vitamin D and hypertension in pregnancy. Clin Invest Med. 2011;34:147–54. [DOI] [PubMed] [Google Scholar]
- 12. Griffin FC, Gadegbeku CA, Sowers MR. Vitamin D and subsequent systolic hypertension among women. Am J Hypertens. 2011;24:316–21. [DOI] [PubMed] [Google Scholar]
- 13. Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25‐hydroxyvitamin D concentration and hypertension: a meta‐analysis. J Hypertens. 2011;29:636–45. [DOI] [PubMed] [Google Scholar]
- 14. Kim MK, Il Kang M, Won OhK, Kwon HS, Lee JH, Lee WC, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle‐aged Korean subjects. Clin Endocrinol. 2010;73:330–8. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle‐aged and older women. Hypertension. 2008;51:1073–9. [DOI] [PubMed] [Google Scholar]
- 16. Sumriddetchkajorn K, Chailurkit L, Thakkinstian A, Sritara P. Hypertension is statistically associated with higher body mass index but not with vitamin D level in a Thai population. Eur J Clin Nutr. 2012;66:405–7. [DOI] [PubMed] [Google Scholar]
- 17. Geleijnse JM. Vitamin D and the prevention of hypertension and cardiovascular diseases: a review of the current evidence. Am J Hypertens. 2011;24:253–62. [DOI] [PubMed] [Google Scholar]
- 18. Forman JP, Bischoff‐Ferrari HA, Willett WC, Stampfer MJ, Curhan GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. 2005;46:676–82. [DOI] [PubMed] [Google Scholar]
- 19. Cosenso‐Martin LN, Vilela‐Martin JF. Is there an association between vitamin D and hypertension? Recent Pat Cardiovasc Drug Discov. 2011;6:140–7. [DOI] [PubMed] [Google Scholar]
- 20. Liss Y, Frishman WH. Vitamin D: a cardioprotective agent? Cardiol Rev. 2012;20:38–44. [DOI] [PubMed] [Google Scholar]
- 21. Vaidya A, Forman JP. Vitamin D and vascular disease: the current and future status of vitamin D therapy in hypertension and kidney disease. Curr Hypertens Rep. 2012;14:111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goel RK, Lal H. Role of vitamin D supplementation in hypertension. Indian J Clin Biochem. 2011;26:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drezner MK. Patient education: Vitamin D deficiency (Beyond the Basics) . UpToDate; 2016 [cited 30 November 2016]; Available from: http://www.uptodate.com/contents/vitamin‐d‐deficiency‐beyond‐the‐basics.
- 24. Forman JP, Curhan GC, Taylor EN. Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demir M, Gunay T, Ozmen G, Melek M. Relationship between vitamin D deficiency and nondipper hypertension. Clinical and experimental hypertension, vol. 2013. (New York, NY: 1993:45–9. [DOI] [PubMed] [Google Scholar]
- 26. Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toxqui L, Blanco‐Rojo R, Wright I, Perez‐Granados AM, Vaquero MP. Changes in blood pressure and lipid levels in young women consuming a vitamin D‐fortified skimmed milk: a randomised controlled trial. Nutrients. 2013;5:4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalbeni A, Scaturro G, Degan M, Minuz P, Delva P. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double‐blind controlled trial. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24:861–8. [DOI] [PubMed] [Google Scholar]
- 29. Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. Journal of the American Society of Nephrology : JASN. 2014;25:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He S, Hao X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: A system review and meta‐analysis. Medicine. 2019;98:e15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Independent association between 1,25‐dihydroxyvitamin D, 25‐hydroxyvitamin D and the renin‐angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clinica chimica acta; international journal of clinical chemistry. 2010;411:1354–60. [DOI] [PubMed] [Google Scholar]
- 32. Forman JP, Williams JS, Fisher ND. Plasma 25‐hydroxyvitamin D and regulation of the renin‐angiotensin system in humans. Hypertension. 2010;55:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodman WG. Recent developments in the management of secondary hyperparathyroidism. Kidney International. 2001;59:1187–201. [DOI] [PubMed] [Google Scholar]
- 34. Sebekova K, Sturmer M, Fazeli G, Bahner U, Stab F, Heidland A. Is vitamin D deficiency related to accumulation of advanced glycation end products, markers of inflammation, and oxidative stress in diabetic subjects? BioMed research international. 2015;2015:958097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Omidian M, Djalali M, Javanbakht MH, Eshraghian MR, Abshirini M, Omidian P, et al. Effects of vitamin D supplementation on advanced glycation end products signaling pathway in T2DM patients: a randomized, placebo‐controlled, double blind clinical trial. Diabetology & metabolic syndrome. 2019;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wakasugi M, Noguchi T, Inoue M, Kazama Y, Tawata M, Kanemaru Y, et al. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42:127–36. [DOI] [PubMed] [Google Scholar]
- 37. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25‐Hydroxyvitamin D deficiency is associated with inflammation‐linked vascular endothelial dysfunction in middle‐aged and older adults. Hypertension. 2011;57:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


