Abstract
Despite the availability of a numerous antihypertensive agents, hypertension treatment and control rates remain low in many countries. The role of the sympathetic nervous system has long been recognized, but recent sham control renal denervation studies demonstrated conflicting results. In this reviewe paper, the authors performed a systematic review and meta‐analysis to examine outcomes of sham‐controlled studies utilizing new technologies and procedures. Six published randomized, sham‐controlled studies were included in this meta‐analysis. Of those, three trials used the first‐generation radiofrequency renal denervation device and technique and the other three used second‐generation devices and techniques. In total, 981 patients with hypertension were randomized in all 6 trials to undergo renal denervation (n = 585) or sham procedure (n = 396). Overall, renal denervation resulted in a decrease of 24‐hours systolic ambulatory blood pressure (ABP) by 3.62 mm Hg (95% CI: −5.28‐−1.96; I2 = 0%), compared to sham procedure (GRADE: low). Renal denervation also reduced daytime systolic ABP by 5.51 mm Hg (95% CI: −7.79‐−3.23; I2 = 0%), compared to sham procedure but not nighttime systolic ABP. Office systolic blood pressure was reduced by 5.47 mm Hg (95% CI −8.10‐−2.84; I2 = 0%), compared to sham control. Further analysis demonstrated that second‐generation devices were effective in reducing blood pressure, whereas the first‐generation devices were not. These results indicate that effective renal denervation can result in significant and clinically meaningful blood pressure reduction. The second‐generation devices provide better renal nerve ablation.
Keywords: blood pressure, cardiovascular risk, device‐based antihypertensive treatment, hypertension, renal denervation
1. INTRODUCTION
Untreated or uncontrolled hypertension remains one of the most important cardiovascular risk factors, worldwide.1, 2, 3, 4 Despite the availability of a numerous antihypertensive agents, hypertension treatment and control rates are still low in many countries.5, 6 Poor patient compliance, medication intolerance, socioeconomic, and other factors contribute to inadequate hypertension control.
The role of the sympathetic nervous system in hypertension has long been recognized, and through the years, many attempts have been made to tackle its consequences.7, 8
Almost a 100 years ago, radical surgical sympathectomy was successfully employed to treat patients with malignant hypertension and surgical renal denervation was employed in a limited number of patients with mixed results.9, 10, 11, 12, 42
In recent years, catheter‐based techniques using radiofrequency/thermal energy placed the concept of renal denervation at the epicenter of cardiovascular medicine. Several single‐arm studies have shown significant blood pressure reduction in some patients but not in others.13, 14, 15 Although the early proof of concept studied published impressive blood pressure reduction, the largest sham‐controlled study (SYMPLICITY HTN‐3) failed to confirm similar findings.16
Results of SYMPLICITY HTN‐3 have been attributed to several factors that include poor drug adherence, inhomogeneity of patient population, and carryover diuretic effect.17, 18, 19, 20, 21 However, it has also been discussed that incomplete denervation due to catheter design, and/or operator inexperience and learning curve (ie, lack of appropriate circular four quadrant pattern and reduced number of ablation point) contributed to the failed outcomes.17, 18, 19, 20, 21, 22, 23 Since then, a great deal of basic and clinical research took place and findings have been incorporated in the design of new devices and new procedures utilized in the modern renal denervation era. In this reviewe paper therefore, we performed a systematic review and meta‐analysis to examine outcomes of sham‐controlled studies utilizing new technologies and procedures.
2. SYSTEMATIC REVIEW AND META‐ANALYSIS
2.1. Methods
This systematic review and meta‐analysis will be reported according to the Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) statement.24
2.1.1. Eligibility criteria
We searched for randomized, sham‐controlled trials prospectively enrolling adult patients with an established diagnosis of primary hypertension, which assessed the safety and efficacy of renal denervation on the management of hypertension.
We searched for parallel‐group randomized controlled trials, while we did not impose any restriction regarding study blinding (single‐blind, double‐blind, or open‐label), setting, and sample size.
2.1.2. Search strategy
We performed a systematic search in the two major electronic databases, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL), for eligible randomized controlled trials, from their inception to May 2019 (Table S1). MeSH term was used for hypertension, as well. We restricted our search to human studies. However, we did not impose any filter regarding language, text availability, and publication date.
Gray literature was searched, as well. We searched clinicaltrials.gov for eligible, completed randomized controlled trials with results, applying the corresponding filters. We also attempted to retrieve eligible completed randomized controlled trials searching the conference proceedings of leading scientific societies, namely the American Heart Association (AHA), the American College of Cardiology, the International Society of Hypertension (ISH), the European Society of Hypertension (ESH), and the European Society of Cardiology (ESC). Finally, we hand‐searched reference lists of all eligible studies. Search strategy was reviewed upon the PRESS 2015 Guideline Statement.25
2.1.3. Study selection
All retrieved reports were imported into reference software manager (Mendeley©) for deduplication. After that, remaining reports were reviewed at title and abstract level by two independent reviewers (KS and DP). Potentially eligible studies, based on the aforementioned eligibility criteria, were full‐text‐assessed. Any discrepancies among the two reviewers at any stage were resolved by discussion, consensus, or arbitration by a third senior reviewer (VP and MD). Eligible reports from gray literature were cross‐checked with the results retrieved from electronic databases. The study selection process is depicted in the flow diagram, as provided in Figure 1.
Figure 1.
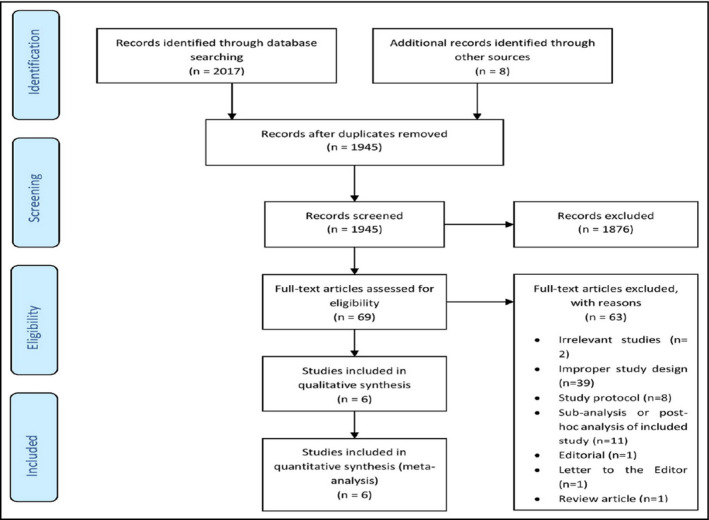
Flow diagram depicting the study selection process
2.1.4. Data extraction
Two independent reviewers (KS and DP) extracted the data from the eligible reports, by using a pilot tested, data extraction form developed in Microsoft Excel©.
Extracted information included source characteristics, study characteristics, participants' baseline characteristics, and interventions, comparators, and key efficacy and safety outcomes. Our primary efficacy outcome was change in 24‐hour ambulatory blood pressure monitoring (in mm Hg). Secondary efficacy outcome was defined as the change in office systolic and diastolic blood pressure (in mm Hg). Assessed safety outcomes were the changes in 24‐hour ambulatory and office heart rate (in beats per minute), in renal function, as estimated by glomerular filtration rate (in mL/min/1.73 m2), along with major adverse events, as recorded across the trials.
If results were reported in multiple articles or at different follow‐up time points, we preferred data extracted from peer review journal articles, and used the reports with longer duration of intervention. Data retrieved from intention‐to‐treat (ITT) analyses were used for each outcome of interest.
2.1.5. Risk of bias assessment
Two independent reviewers (DP and KI) assessed the following domains, by using the corresponding Cochrane Collaboration's tool: random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.26 Each domain was rated as low, unclear, or high risk of bias. The presence of adequate procedures in all domains rated a study as being of low risk of bias, while inadequate procedure in at least one domain rated a study as being of high risk of bias. In any other case, each study was determined as being of unclear risk of bias. When a study was rated as being of high risk of bias, it was subsequently excluded from the meta‐analysis. Discrepancies between reviewers were solved by discussion, consensus, or arbitration by a third senior reviewer (VP). Risk of bias assessment across the selected studies is provided in Table 1.
Table 1.
Risk of bias assessment
| Study | Random sequence generation | Allocation concealment | Blinding of participants and staff | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
|
Bhatt (2014) SYMPLICITY HTN‐3 16 |
Low | Low | High | Low | Low | Low | Selection bias (enrolled population) |
| Desch (2015)33 | Low | Low | Low | Low | Low | Low | |
|
Kandzari (2018) SPYRAL HTN‐ON MED 35 |
Low | Low | Low | Low | Low | Low | |
|
Townsend (2017) SPYRAL HTN‐OFF MED 34 |
Low | Low | Low | Low | Low | Low | |
|
Azizi (2018) RADIANCE‐HTN SOLO 36 |
Low | Low | Low | Low | Low | Low | |
|
Mathiassen (2016) ReSET 32 |
Low | Low | Low | Low | Low | Low |
Finally, we planned to assess the presence of publication bias for the primary efficacy outcome by inspecting a funnel plot for asymmetry (Figures S1, S2).27
2.1.6. Data synthesis and analysis
A meta‐analysis for each outcome of interest was conducted, when at least three randomized controlled trials provided adequate data.
For continuous outcome variables, we calculated weighted mean differences (WMD) with 95% confidence intervals (CI), using an inverse variance weighted random effects model. Regarding dichotomous variables, differences were calculated with the use of odds ratios (OR), with 95% CI, after implementation of the Mantel‐Haenszel (M‐H) random effects formula. For those studies not reporting standard deviation (SD), we calculated SD either from the sample size and the standard error (SE) or the 95% CI.
Statistical heterogeneity among studies was assessed by using I2 and chi‐square statistics, with I2 values >50% representing substantial heterogeneity.28, 29, 30
All analyses were performed at the 0.05 significance level, while they were undertaken with RevMan 5.3 software.
2.1.7. Grading of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach31 to assess the credibility of our summary estimates. Two reviewers (DP and KS) graded inconsistency, risk of bias, indirectness, imprecision, and publication bias for evidence related to the primary efficacy outcome. Discrepancies between reviewers were solved by discussion, consensus, or arbitration by a third senior reviewer (VP). We used GRADEpro (GRADE Working Group) to generate a summary of findings as shown in Table 3.
Table 3.
Summary of findings
| Renal denervation compared to sham procedure for patients with uncontrolled hypertension |
| Patient population: Patients with uncontrolled hypertension |
| Intervention: Renal denervation |
| Comparison: Sham procedure |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with sham procedure | Risk with renal denervation | |||||
| 24‐h diastolic ambulatory blood pressure (24‐h diastolic ABP) follow‐up: range 2‐6 mo | MD 1.92 mm Hg lower (3.65 lower to 0.2 lower) | – | 425 (5 RCTs) |
⨁⨁◯◯ Low |
Inconsistency is downgraded by 1 level due to the relatively wide variance of point estimates across the included studies. Imprecision is downgraded by 1 level due to the differences in selected populations across the included studies. |
|
| 24‐h systolic ambulatory blood pressure (24‐h systolic ABP) follow‐up: range 2‐6 mo | MD 3.62 mm Hg lower (5.28 lower to 1.96 lower) | – | 916 (6 RCTs) |
⨁⨁◯◯ Low |
Inconsistency is downgraded by 1 level due to the relatively wide variance of point estimates across the included studies, both first generation and second generation. Imprecision is downgraded by 1 level due to the differences in selected populations across the included studies. | |
GRADE Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Abbreviations: CI, confidence interval; MD, mean difference
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
3. RESULTS
Overall, six randomized, sham‐controlled studies were included in this meta‐analysis.16, 31, 32, 33, 34, 35 All six sham‐controlled studies used radiofrequency or ultrasound devices to achieve renal denervation. Of those, three utilized the single‐tip catheter (Flex, Medtronic) and placed lesions in the main renal artery (first‐generation studies).16, 32, 33 The other three utilized either the multi‐electrode Spyral catheter (Medtronic) and placed lesions at the distal segment of the renal artery and into the branches, or focused ultrasound (ReCor) which can deliver thermal energy deeper into the adventitia of the main renal artery, thus achieving greater sympathetic fiber interruption (second‐generation studies).34, 35, 36
Thus, we first analyzed all sham‐controlled studies together and then the first‐ and second‐generation devices separately.
In total, 981 patients with hypertension were randomized in all six trials to undergo renal denervation (n = 585) or sham procedure (n = 396). Baseline demographics, blood pressure measurements, and other parameters of participants are depicted in Table 2.
Table 2.
Baseline demographic characteristics of the participants in the six included sham‐controlled studies included in our meta‐analysis
| Study | SYMPLICITY HTN‐3 14 | ReSET 15 | Desch et al 16 | SPYRAL HTN‐OFF MED 25 | SPYRAL HTN‐ON MED 26 | RADIANCE‐HTN SOLO 27 |
|---|---|---|---|---|---|---|
| N (RDN) | 535 (364) | 69 (36) | 71 (35) | 80 (38) | 80 (38) | 146 (74) |
| Condition | Resistant hypertension | Resistant hypertension | Resistant hypertension | Untreated hypertension | Uncontrolled hypertension on drugs | Untreated hypertension |
| Energy source | Radiofrequency | Radiofrequency | Radiofrequency | Radiofrequency | Radiofrequency | Ultrasound |
| Device | Symplicity Flex catheter | Symplicity Flex catheter | Symplicity Flex catheter | Symplicity Spyral and G3 | Symplicity Spyral and G3 | Paradise system |
| Follow‐up (months) | 6 | 6 | 6 | 3 | 6 | 2 |
| Age RDN/sham (years) | 57.9 ± 10.4/56.2 ± 11.2 | 54.3 ± 7.8/57.1 ± 9.6 | 64.5 ± 7.6/57.4 ± 8.6 | 55.8 ± 10.1/52.8 ± 11.5 | 53.9 ± 8.7/53 ± 10.7 | 54.4 ± 10.2/53.8 ± 10 |
| Female RDN/sham (%) | 40.9/35.7 | 25/23 | 23/31 | 31.6/26.2 | 13/19 | 38/46 |
| Caucasians RDN/sham (%) | 73/69.6 | 97/97 | 100/100 | 26.3/23.8* | 34/35* | 81/72 |
| Smokers RDN/sham (%) | 9.9/12.3 | 19/15 | 17/11 | 10.5/23.8 | 21/26 | NR |
| BMI RDN/sham(kg/m2) | 34.2 ± 6.5/33.9 ± 6.4 | 28.2 ± 5/28.8 ± 3.9 | 31.9 ± 4.4/31.2 ± 4.6 | 29.8 ± 5.1/30.2 ± 5.1 | 31.4 ± 6.4/32.5 ± 4.6 | 29.9 ± 5.9/29 ± 5 |
| Heart Rate RDN/sham (beats/min) | NR | 71 ± 10/70 ± 11 | 67 ± 11/68 ± 12 | 72.3 ± 10.9/75.5 ± 11.5 | 75.5 ± 11.4/76.2 ± 10.2 | 72 ± 12.1/72.6 ± 12.3** |
| RDN 24‐h ABPM (mm Hg) | 159.1 ± 13.2/88 ± 14 | 152 ± 12/91 ± 9 | 140.2 ± 4.6/78.2 ± 7.4 | 153.4 ± 9/99.1 ± 7.7 | 151.9 ± 7.1/96.9 ± 6.9 | 142.6 ± 8.1/87.3 ± 5 |
| sham 24‐h ABPM (mm Hg) | 159.5 ± 15.3/90.9 ± 14.4 | 153 ± 13/89 ± 11 | 140.4 ± 5.6/80.6 ± 7.1 | 151.6 ± 7.4/98.7 ± 8.2 | 151.1 ± 6.8/97.6 ± 8.3 | 143.8 ± 10.4 |
| RDN OBP (mm Hg) | 179.7 ± 16.1/96.5 ± 16.6 | 160 ± 20/95 ± 15 | NR | 162 ± 7.6/99.9 ± 6.8 | 164.6 ± 7.1/99.6 ± 6.9 | 154.5 ± 12.4/99.7± |
| Sham OBP (mm Hg) | 180.2 ± 16.8/98.9 ± 15.8 | 166 ± 19/90 ± 17 | NR | 161.4 ± 6.4/101.5 ± 7.5 | 163.1 ± 7.2/102.3 ± 8 | 153.6 ± 15.7/99.1 ± 9.4 |
| RDN HBP (mm Hg) | 169 ± 15.9/89.6 ± 15.9 | NR | NR | NR | NR | 147.5 ± 8.8/94.8 ± 6.9 |
| sham HBP (mm Hg) | 169.1 ± 16.3/92.9 ± 16.4 | NR | NR | NR | NR | 147.7 ± 12.3/94.6 ± 7 |
| RDN daytime ABPM (mm Hg) | NR | 159 ± 12/96 ± 9 | 144.4 ± 4.8/80.6 ± 7.8 | NR | 156.4 ± 8.1/101 ± 7.1 | 150.3 ± 7.8/93.1 ± 4.8 |
| Sham daytime ABPM (mm Hg) | NR | 159 ± 14/93 ± 12 | 143 ± 4.7/82.9 ± 7.3 | NR | 157.4 ± 8.4/102.7 ± 9.3 | 150 ± 9.8/93.5 ± 5.5 |
| RDN nighttime ABPM (mm Hg) | NR | 136 ± 17/79 ± 11 | 130.5 ± 9.7/69.7 ± 8 | NR | 144.9 ± 11/90.5 ± 10.6 | 130.3 ± 11.9/78.2 ± 8 |
| sham nighttime ABPM (mm Hg) | NR | 141 ± 18/80 ± 10 | 132.3 ± 11.7/73.2 ± 8.4 | NR | 141 ± 8.5/89.5 ± 8.9 | 132.5 ± 13.7/80 ± 8.1 |
| eGFR RDN/sham (ml/min/1.73 m2) | 72.8 ± 15.7/74 ± 18.7 | NR | 79 ± 20/84 ± 20 | 80.9 ± 16.7/88.3 ± 20.5 | 81.9 ± 15.3/82 ± 19.7 | 84.7 ± 16.2/83.2 ± 16.1 |
| ISH RDN/sham (%) | NR | NR | 66/56 | 0/0 | 0/0 | 0/0 |
| Diabetes RDN/sham (%) | 47/40.9 | 25/31 | 54/36 | 2.6/7.1 | 13/19 | 3/7 |
| Antihypertensive agents RDN/sham (n) | 5.1 ± 1.4/5.2 ± 1.4 | 4.1 ± 1.2/4.1 ± 1.1 | 4.4 ± 1.3/4.3 ± 1.3 | 0/0 | 2.2 ± 0.9/2.3 ± 0.8 | 0/0 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; eGFR, estimated glomerular filtration rate; HBP, home blood pressure; ISH, isolated systolic hypertension; NR, not reported; OBP, office blood pressure; RDN, renal denervation.
Not reported in more than 40% of study participants.
Values prior to the washout‐not baseline.
3.1. Ambulatory blood pressure monitoring
Overall, renal denervation resulted in a decrease of 24‐hours systolic ambulatory blood pressure (ABP) by −3.62 mm Hg (95% CI: −5.28‐−1.96; I2 = 0%), compared to sham procedure (Figure 2A). Certainty of evidence using the GRADE system is considered low (Table 3).
Figure 2.
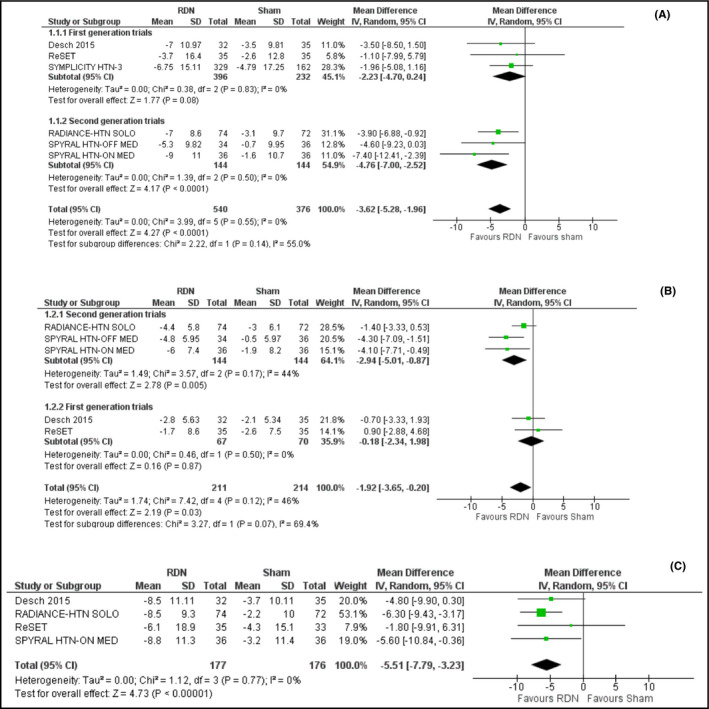
A, Effect of renal denervation on 24‐h systolic ambulatory blood pressure monitoring compared to sham procedure. B, Effect of renal denervation on 24‐h diastolic ambulatory blood pressure monitoring compared to sham procedure. C, Effect of renal denervation on daytime systolic ambulatory blood pressure monitoring compared to sham procedure. D, Effect of renal denervation on nighttime systolic ambulatory blood pressure monitoring compared to sham procedure
In total, renal denervation also resulted in a decrease of 24‐h diastolic ABP by −1.92 mm Hg (95% CI: −3.65‐−0.20; I2 = 46%) compared to the sham procedure (Figure 2B). Grade of evidence is considered as low.
3.1.1. Daytime and nighttime ambulatory blood pressure monitoring
Overall renal denervation reduced daytime systolic ABP by −5.51 mm Hg (95% CI: −7.79‐−3.23; I2 = 0%), compared to sham procedure (Figure 2C) and nighttime systolic ABP by a non‐significant −3.06 mm Hg (95% CI: −8.69‐2.56; I2 = 73%), compared to sham procedure (Figure 2D).
Further analysis showed that renal denervation significantly decreased daytime diastolic (3a) ABP level by −1.90 mm Hg (95% CI: −3.48‐−0.32; I2 = 4%) but failed to induce significant reduction in nighttime diastolic ABP (−0.80 mm Hg; 95% CI: −3.61‐2.02; I2 = 57%), compared to sham procedure (Figure 3B).
Figure 3.
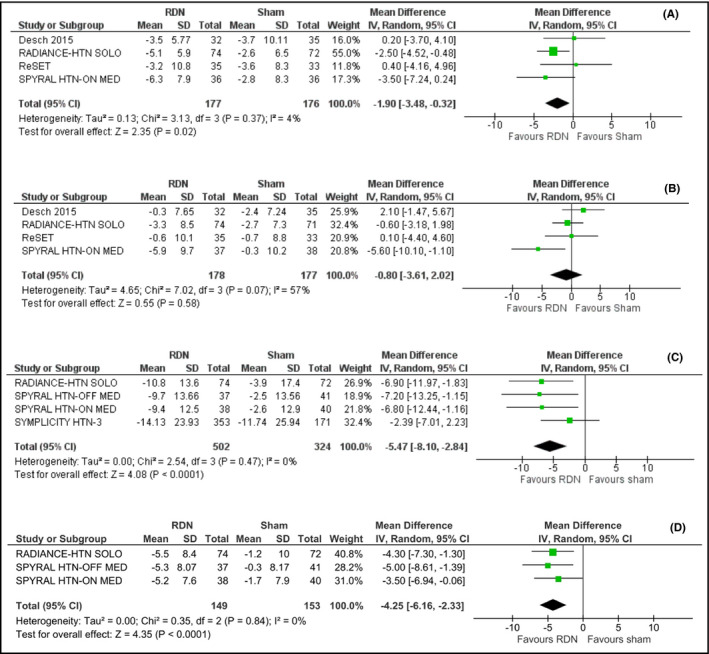
A, Effect of renal denervation on daytime diastolic ambulatory blood pressure monitoring compared to sham procedure. B, Effect of renal denervation on nighttime diastolic ambulatory blood pressure monitoring compared to sham procedure. C, Effect of renal denervation on systolic office blood pressure compared to sham procedure. D, Effect of renal denervation on diastolic office blood pressure compared to sham procedure
Thus, we might conclude that, while renal denervation was associated with a significant reduction in both systolic and diastolic daytime ABP, it did not consistently induce a substantial reduction in nighttime ABP levels.
3.2. Office blood pressure
Results indicate that renal denervation effected a significant decrease in office systolic blood pressure level by −5.47 mm Hg (95% CI −8.10‐−2.84; I2 = 0%), compared to sham (Figure 3C).
Moreover, RDN resulted in reduction in diastolic office blood pressure level by −4.25 mm Hg (95% CI −6.16‐−2.33; I2 = 0%), compared to sham (Figure 3D).
3.3. First and second generation of renal denervation devices and techniques
The separate analysis of the ABP outcomes between the first‐ and second‐generation devices of renal denervation is shown in Figure 4A, B, C and D.
Figure 4.
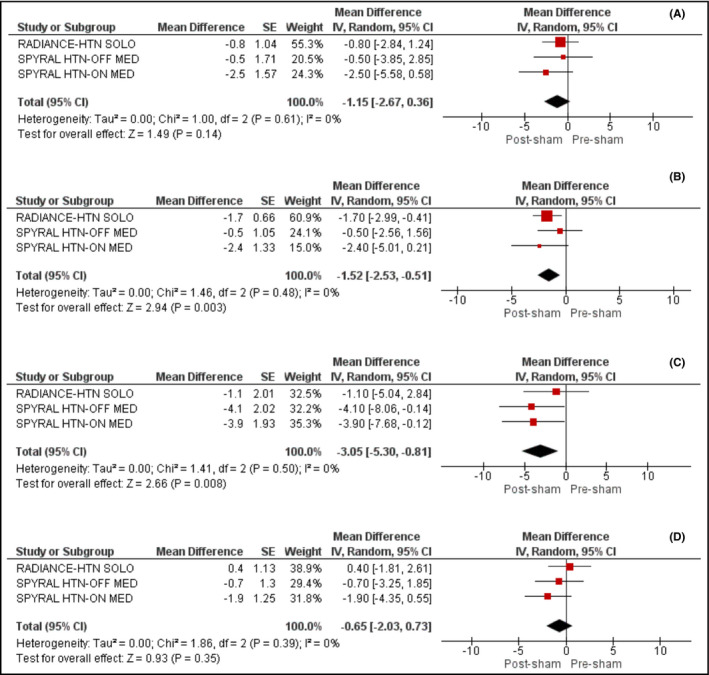
A, Effect of sham on 24‐h systolic ambulatory blood pressure across the second‐generation trials. B, Effect of sham on 24‐h diastolic ambulatory blood pressure across the second‐generation trials. C, Effect of sham on office systolic blood pressure across the second‐generation trials. D, Effect of sham on office diastolic blood pressure across the second‐generation trials
The first‐generation studies 16, 31, 32 failed to show any significant reduction in either systolic (−2.23 mm Hg; 95% CI: −4.70‐0.24, I2 = 0%) or diastolic (−0.18; 95% CI: −2.34‐1.98, I2 = 0%) ABP levels. In contrast, the second‐generation devices 33, 34, 35 resulted in a significant reduction of both systolic and diastolic ABP by −4.76 mm Hg (95% CI: −7.00‐−2.52, I2 = 0%) and −2.94 mm Hg (95% CI: −5.01‐−0.87, I2 = 44%), respectively.
3.4. Blood pressure lowering effect of sham procedure
We also evaluated the effect of sham procedure on 24‐hours systolic and diastolic ABP levels across the second‐generation trials. We documented that sham procedure did not affect significantly 24‐hours systolic ABP levels (MD: −1.15, 95% CI: −2.67‐0.36, I2 = 0%), as shown in Figure 4A; however, it induced a significant decrease in 24‐hours diastolic ABP levels (4b) by 1.52 mm Hg (95% CI: −2.53‐−0.51, I2 = 0%). In addition, we demonstrated that sham resulted in a significant decrease in systolic OBP by 3.05 mm Hg (95% CI: −5.30‐−0.81, I2 = 0%) and a non‐significant decrease in diastolic OBP by 0.65 mm Hg (95% CI: −2.03 to 0.73, I2 = 0%), as shown in Figure 4C and D.
3.5. Ambulatory and office heart rate
Insufficient reporting across included sham‐controlled trials did not permit us to perform a meta‐analysis, concerning the impact of renal denervation compared to sham procedure on ambulatory or office heart rate.
3.6. Renal function
Renal denervation was associated with a non‐significant decrease in estimated glomerular filtration rate by 0.24 mL/min/1.73 m2 (95% CI: −1.95‐1.47; I2 = 0%), compared to sham procedure (Figure 5A).
Figure 5.
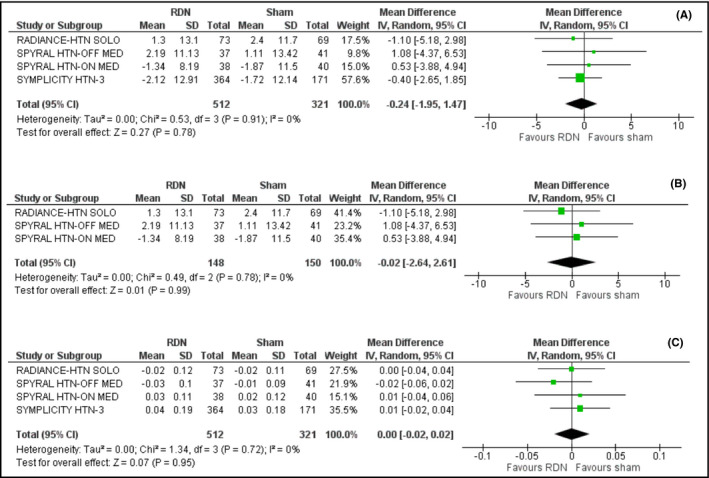
A, Effect of renal denervation on estimated glomerular filtration rate compared to sham procedure. B, Effect of renal denervation on estimated glomerular filtration rate compared to sham procedure across the second‐generation trials. C, Effect of renal denervation on serum creatinine levels compared to sham procedure
We also observed that inclusion of trials using novel renal denervation devices and techniques, namely the SPYRAL HTN‐ON MED, the SPYRAL HTN‐OFF MED, and the RADIANCE‐HTN SOLO trials, did not alter significantly renal function (mean difference =−0.02; 95% CI: −2.64‐2.62; I2 = 0%), as shown in Figure 5B.
All studies reported the effect of RDN or sham procedure on renal function according to the Modification of Diet in Renal Disease (MDRD) formula.
Finally, no difference was detected in serum creatinine levels, when renal denervation was compared to sham (mean difference = 0.00; 95% CI: −0.02‐0.02; I2 = 0%), as shown in Figure 5C.
3.7. Major adverse events
No major adverse events (including all‐cause death, major cardiovascular events, peri‐procedural complications, significant renal impairment, or hypotensive/hypertensive crisis) were reported in the active arm of any of the novel sham control trials (SPYRAL HTN‐ON MED, the SPYRAL HTN‐OFF MED, and the RADIANCE‐HTN SOLO).34, 35, 36
One case of stroke and one case of unstable angina requiring percutaneous coronary intervention were recorded in the sham group in the ReSET trial 32; however, both events occurred several weeks after the performance of the sham procedure, and they were not considered as procedure‐related events.
SYMPLICITY HTN‐3 trial—the largest sham‐controlled study,16 was also considered safe. There was no significant difference in adverse events between the two treatment groups.
3.8. Risk of bias assessment
In total, all included trials were considered as low risk of bias across each assessed domain, as described in Cochrane Collaboration's tool. Risk of bias assessment is presented in Table 1.
4. DISCUSSION
Although other meta‐analyses have been previously published,37 we believe that this analysis offers new insides and new information. Beyond the usual analysis for office BP changes and ambulatory BP changes, we preformed analyses separating the studies into first and second generation and also looked at changes in the sham control arm.
Findings of this meta‐analysis confirm that renal denervation is associated with a statistically significant and clinically meaningful reduction in ambulatory and office blood pressure levels. Collectively, data were extracted from six sham‐controlled randomized studies that enrolled 981 patients with uncontrolled hypertension. The overall benefit noted can be attributed mostly to the second‐generation studies, that demonstrated a reduction in ABP of −4.8 (95% CI: −7‐−2.5)/‐2.9 (95% CI: −5‐−0.9) mm Hg, whereas the change in ambulatory or office blood pressure in the first‐generation studies did not achieve statistical significance. It is reasonable to assume that the first‐generation studies achieved less renal denervation compared to the second‐generation studies potentially due to the following reasons: (a) The first‐generation studies utilized a single‐tip radiofrequency catheter which is difficult to achieve circumferential lesioning, (b) fewer lesions were placed in the main renal artery, and c. lesions were placed randomly in the main renal artery in areas where not all sympathetic fibers were reachable. The second‐generation studies utilized multi‐electrode catheters and placed lesions in the distal segments of the renal arteries, and to the branches where fibers are closer to the lumen, and therefore, easily reachable. Focus ultrasound can potentially reach or achieve better denervation, since it can penetrate in greater depth than radiofrequency energy. It is reasonable therefore to assume that one of the important differences between the first‐ and the second‐generation studies is the degree of renal denervation achieved.38 Nevertheless, changes are relatively small and comparable to adding another dug to the regimen. This should be taken into consideration when renal denervation is recommended as a therapeutic option.
We have also shown that in the second‐generation trials, sham procedure resulted in mild but significant reduction of systolic OBP (−3.05 mm Hg) and diastolic ABP (−1.52 mm Hg). This outcome has specifically been investigated to clarify the suggestion of the European Society of Hypertension that the potential sham effect can be used as an incentive for patients to participate in sham‐controlled studies.39
Results indicate that procedures were safe for both the first‐ and second‐generation studies with no evidence of any deterioration in renal function.
Another significant finding of this meta‐analysis is the relatively small difference between the change in office and ambulatory systolic blood pressure, achieved with renal denervation (5.5 vs 4.6 mm Hg). Previous studies suggested that this difference between the two measurement methods can be substantial (up to 20 mm Hg) and may depend on the pre‐treatment blood pressure level.40
Furthermore, results of this meta‐analysis indicate a non‐significant decrease in nighttime systolic blood pressure, a finding that can be explained by associated high heterogeneity. In contrast, other recent studies demonstrated significant reduction in nighttime systolic blood pressure: The recently published secondary analysis of RADIANCE‐HTN SOLO, in which participants could be initiated on antihypertensive therapy after integration into the main study, found that renal denervation resulted in a significant reduction in nighttime systolic ABP by –4.7 (95% CI: −8.2‐−1.2) mm Hg compared to sham‐controlled group.41 In addition, the SPYRAL HTN‐ON MED trial demonstrated that renal denervation resulted in a significant reduction in nighttime systolic blood pressure compared to sham procedure by –11.90 (95% CI: −18.2‐−5.6) mm Hg.34
Our meta‐analysis has some significant strengths. Our meta‐analysis assessed the impact of renal denervation on office, 24 hours, daytime, and nighttime blood pressure and compare first‐ and second‐generation studies. Moreover, our primary and most secondary outcomes are characterized with zero or quite acceptable heterogeneity. In contrast, the small number of the included randomized controlled trials (n = 6), the relatively small sample size (n = 981), the short follow‐up period (up to 6 months), and small number of studies may be considered as the main limitations of the present meta‐analysis. In addition, we did not perform meta‐regression analyses for the investigation of the observed heterogeneity, due to the small number of included trials.
Nevertheless, results are useful, positive, and a good building block for further exploration of the field of renal denervation. There is no doubt that further, larger, adequately powered randomized controlled trials are needed to provide more precise insights into the role of renal denervation in the treatment of primary hypertension.
5. CONCLUSIONS
Results of this meta‐analysis suggest that renal denervation works in the short term and may contribute to better management and control of uncontrolled hypertension. Nonetheless, the effect is relatively small and most likely diluted by non‐responders. Further, well‐designed studies are needed to better define the role of renal denervation in the treatment of hypertension in the general population.
CONFLICT OF INTEREST
None.
AUTHORS' CONTRIBUTIONS
KS, MD, and VP conceived and designed the study. DP, KI, and KS performed the scientific literature search. AK, KD, and CT did literature screening. DP and KS extracted data. AK and KI did quality assessment of the included studies. DP did the analyses. KS wrote the first draft of the report. All authors contributed to interpretation and edited the draft report.
Supporting information
Stavropoulos K, Patoulias D, Imprialos K, et al. Efficacy and safety of renal denervation for the management of arterial hypertension: A systematic review and meta‐analysis of randomized, sham‐controlled, catheter‐based trials. J Clin Hypertens. 2020;22:572–584. 10.1111/jch.13827
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics‐2019 update: a report from the american heart association. Circulation. 2019;139:e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165‐182. [DOI] [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 5. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959‐968. [DOI] [PubMed] [Google Scholar]
- 6. Banegas JR, López‐García E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32:2143‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grassi G, Pisano A, Bolignano D, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta‐analyses. Hypertension. 2018;72:483‐491. [DOI] [PubMed] [Google Scholar]
- 8. Bradford JR. The innervation of the renal blood vessels. J Physiol. 1889;10(5):358‐432.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neurosurgical treatment, indications and results (chapter7, methods of operation). J Intern Med. 1947;127:72‐76. [Google Scholar]
- 10. Adson AW, McCraig W, Brown GE. Surgery in its relation to hypertension. Surg Gynecol Obstet. 1936;62:314‐331. [Google Scholar]
- 11. Sen SK. Some observations on decapsulation and denervation of the kidney. Brit J Urol. 1936;8:319‐328. [Google Scholar]
- 12. Page IH, Heuer GJ. The effect of renal denervation on patients suffering from nephritis. J Clin Invest. 1935;14:443‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krum H, Schlaich M, Whitbourn R, et al. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009;373:1275‐1281. [DOI] [PubMed] [Google Scholar]
- 14. HTN‐2 Investigators , Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010;376:1903‐1909. [DOI] [PubMed] [Google Scholar]
- 15. Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi‐electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393‐1401. [DOI] [PubMed] [Google Scholar]
- 17. Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri‐arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635‐643. [DOI] [PubMed] [Google Scholar]
- 18. Henegar JR, Zhang Y, Hata C, et al. Catheter‐based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens. 2015;28:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN‐3 trial. Eur Heart J. 2015;36:219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tzafriri AR, Mahfoud F, Keating JH, et al. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahfoud F, Edelman ER, Böhm M. Catheter‐based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol. 2014;64:644‐666. [DOI] [PubMed] [Google Scholar]
- 22. Papademetriou V, Tsioufis C, Doumas M. Renal denervation and Symplicity HTN‐3: "Dubium sapientiae initium" (doubt is the beginning of wisdom). Circ Res. 2014;115:211‐214. [DOI] [PubMed] [Google Scholar]
- 23. Papademetriou V, Rashidi AA, Tsioufis C, et al. Renal nerve ablation for resistant hypertension: how did we get here, present status, and future directions. Circulation. 2014;129:1440‐1451. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sedgwick P, Marston L. How to read a funnel plot in a meta‐analysis. BMJ. 2015;351:h4718. [DOI] [PubMed] [Google Scholar]
- 28. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analyzing data and undertaking meta‐analyses. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011]. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 29. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schünemann HBJ, Guyatt G, Oxman A.GRADE handbook for grading quality of evidence and strength of recommendations (Updated October 2013). The GRADE Working Group; 2013.
- 32. Mathiassen ON, Vase H, Bech JN, et al. Renal denervation in treatment‐resistant essential hypertension. A randomized, SHAM‐controlled, double‐blinded 24‐h blood pressure‐based trial. J Hypertens. 2016;34:1639‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desch S, Okon T, Heinemann D, et al. Randomized sham‐controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202‐1208. [DOI] [PubMed] [Google Scholar]
- 34. Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter‐based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN‐OFF MED): a randomised, sham‐controlled, proof‐of‐concept trial. Lancet. 2017;390:2160‐2170. [DOI] [PubMed] [Google Scholar]
- 35. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6‐month efficacy and safety results from the SPYRAL HTN‐ON MED proof‐of‐concept randomised trial. Lancet. 2018;391:2346‐2355. [DOI] [PubMed] [Google Scholar]
- 36. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE‐HTN SOLO): a multicentre, international, single‐blind, randomised, sham‐controlled trial. Lancet. 2018;391:2335‐2345. [DOI] [PubMed] [Google Scholar]
- 37. Sardar P, Bhatt DL, Kirtane AJ, et al. Sham‐controlled randomized trials of catheter‐based renal denervation in patients with hypertension. J Am Coll Cardiol. 2019;73(13):1633‐1642. [DOI] [PubMed] [Google Scholar]
- 38. Papademetriou V, Stavropoulos K, Doumas M, et al. Now that renal denervation works, how do we proceed? Circ Res. 2019;124:693‐695. [DOI] [PubMed] [Google Scholar]
- 39. Schmieder RE, Mahfoud F, Azizi M, et al. European Society of hypertension position paper on renal denervation 2018. J Hypertens. 2018;36:2042‐2048. [DOI] [PubMed] [Google Scholar]
- 40. Schmieder RE, Schmidt ST, Riemer T, et al. Disproportional decrease in office blood pressure compared with 24‐h ambulatory blood pressure with antihypertensive treatment: dependency on pretreatment blood pressure levels. Hypertension. 2014;64:1067‐1072. [DOI] [PubMed] [Google Scholar]
- 41. Azizi M, Schmieder RE, Mahfoud F, et al. Six‐month results of treatment‐blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE‐HTN SOLO Trial. Circulation. 2019;391(10137):2335-2345. [DOI] [PubMed] [Google Scholar]
- 42. Page IH, Heuer GJ. The effect of renal denervation on the level of arterial blood pressure and renal function in essential hypertension. J Clin Invest. 1935;14:27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


