Abstract
Hypertension is an important risk factor for non‐valvular atrial fibrillation (NVAF) and its prognosis. However, there is no study to clarify the impact of pre‐existing hypertension and BP control status before the onset of AF on the prognosis after the onset of AF. This retrospective real‐world cohort analysis used data from the Japan Medical Data Center Co., Ltd database. The presence of NVAF and hypertension, plus the occurrence of adverse events, was based on ICD‐10 codes in the database. The primary composite AF‐related cardiovascular event end point included ischemic stroke, hemorrhagic stroke, and acute myocardial infarction. Of the 21 523 patients identified as having new‐onset NVAF between January 2005 and June 2016, 7885 had blood pressure (BP) data before the onset of NVAF available and were included in the analysis (4001 with pre‐existing hypertension and 3884 without pre‐existing hypertension). The rate of primary composite end point events was 10.3 and 4.4 per 1000 patient‐years in patients with and without hypertension, respectively (P < .001). In addition, lower systolic BP (<120 mm Hg) before the onset of NVAF was associated with a lower incidence of cardiovascular events after the development of NVAF (P < .001). This highlights the importance of earlier and tighter 24‐hour BP control before the onset of NVAF in patients with hypertension, not only for reducing the occurrence of new‐onset of NVAF, but also for reducing both hemorrhagic and ischemic cardiovascular events after the onset of NVAF.
Keywords: atrial fibrillation, cardiovascular events, morbidity, mortality, pre‐existing hypertension, stroke
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is an important risk factor for ischemic cerebrovascular events.1 The prevalence of AF in the general population is 1‐3%,2, 3 but this is growing. The number of US adults with AF is predicted to at least double by 2050,1, 4 while by 2030 the estimated number of individuals with AF in Europe and Japan will be 14‐17 million and 1.08 million, respectively.3, 5, 6 These increases in AF prevalence are most likely a result of the aging demographic of the global population7 and the fact that cardiovascular disease mortality rates are generally falling.8 This is particularly relevant in Japan, which has the most rapidly aging population worldwide.9
Hypertension is fourth behind heart failure, aging, and valvular heart disease as a risk factor for AF.10 As the most prevalent of these conditions, hypertension contributes to a large number of cases of AF.11, 12 This is highlighted by the reported presence of hypertension in patients with AF enrolled in recent clinical trials, which was approximately 80%‐90%.13, 14, 15, 16, 17 Actually, both hypertension10, 18, 19, 20, 21 and pre‐hypertension20, 22 are risk factors for incident AF. Epidemiologic studies have shown that hypertension is associated with a 1.8‐fold increase in the risk of developing new‐onset AF and a 1.5‐fold increase in the risk of progression to permanent AF.10, 23 AF itself is associated with increased risk of stroke and systemic emboli, but patients with both AF and hypertension have a higher risk of stroke compared to those with either condition alone.24, 25 Thus, earlier detection and treatment of new‐onset AF are essential in patients with hypertension.26
Poor control of blood pressure (BP) in elderly patients with hypertension has been associated with a significantly higher incidence of new‐onset AF compared with normotensives or patients with hypertension who had good or moderate BP control (hazard ratio [HR] 7.0, 95% confidence interval [CI] 2.4‐20.2; P < .001),27 although BP levels may be underestimated in patients with AF.28 Furthermore, data from a large systematic review showed that antihypertensive therapy with angiotensin receptor blockers tended to reduce the rate of new‐onset AF in patients with hypertension (odds ratio [OR] 0.85, 95% CI 0.71‐1.01; P = .067).29 However, there are currently no data clarifying the impact of pre‐existing hypertension and BP control status before the onset of AF on prognosis after AF onset. Thus, we investigated the prognostic impact of pre‐existing hypertension and control status before the onset of AF in a real‐world database of patients with new‐onset non‐valvular atrial fibrillation (NVAF).
2. METHODS
2.1. Study design
This study was a retrospective, longitudinal, observational cohort study based on the Japan Medical Data Center Co., Ltd (JMDC) database. It was founded by the BMS/Pfizer Japan Thrombosis Investigator Initiated Research Program (JRISTA). All data in the database were anonymized, de‐identified, and compliant with the International Conference on Harmonization (ICH) guidelines regarding the protection of human patients in observational studies.30 The study received ethical approval from the Ethics Committees of Jichi Medical University.
2.2. Data source and patient selection
The JMDC claims database uses standardized disease classifications and anonymous record linkage.31 It provides information on the beneficiaries (including encrypted personal identifiers, age, sex, International Classification of Diseases 10th revision [ICD‐10] procedure and diagnostic codes) and the name, dose, and number of days supplied for prescribed and/or dispensed drugs.
The database for this study was constructed based on monthly claims from medical institutions and pharmacies submitted from January 2005 to June 2016, which included approximately 3.84 million insured persons (aged 0‐75 years), comprised mainly of company employees and their family members.
2.3. Characteristics and comorbidities
Hypertension was identified based on the ICD‐10 code I10‐15. AF was identified using the ICD‐10 code I48. Data on patient characteristics just before NVAF diagnosis were collected, including gender, age, and the presence of comorbidities (ICD‐10 code) such as coronary heart disease (I20‐25), cardiomyopathy (I42), hyperthyroidism (E05), hypothyroidism (E03), chronic obstructive pulmonary disease (J449), dyslipidemia (E78), chronic kidney disease (N18), congestive heart failure (I50), hypertension (I10‐I15), diabetes mellitus (E10‐14), and stroke/transient ischemic attack (I63/G45). Data on heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or TIA were used to calculate the CHADS2 score for each patient, which provides an estimation of stroke risk in patients with AF.24
2.4. End points
The primary composite AF‐related cardiovascular event end point included acute myocardial infarction (I21‐I22), hemorrhagic stroke including non‐traumatic subarachnoid hemorrhage (I60‐I62) and ischemic stroke (I63). Secondary AF‐related end points included gastrointestinal bleeding (K00‐K93 plus K920), all‐cause mortality (based on medical records and registry data), percutaneous coronary intervention (PCI, K546, K548, and K549), cardiac bypass surgery (Z951), and embolism or thrombosis (including retinal vascular occlusions [H34], aorta [I74], cerebral infarction [I65], occlusion and stenosis of precerebral arteries, not cerebral infarction [I65], occlusion and stenosis of cerebral arteries, not cerebral infarction [I66], portal vein thrombosis [I81], other venous thrombosis [I82], atrial thrombosis [I236], acute coronary thrombosis [I240], other secondary pulmonary hypertension [I272], intracardiac thrombosis [I513], nonpyogenic thrombosis of the intracranial venous system [I676], sequelae of cerebral infarction [I693], acute vascular disorders of the intestine [K550], thrombotic microangiopathy [M311], and ischemia and infarction of kidney [N280]). Event rates were calculated for NVAF patients with vs without hypertension, and in patient subgroups based on age (<60 or ≥60 years); the timing of events after NVAF diagnosis was also determined.
2.5. Statistical analyses
Categorical variables are presented as number and percentage. Continuous variables are expressed as mean ± SD. The event rate was calculated per 1000 person‐years. The cumulative incidence of events was estimated using the Kaplan‐Meier method and compared between new‐onset NVAF patients with and without hypertension. All statistical analyses were performed using SAS software (Ver9.4; SAS Institute Inc, Cary, NCI). In all analyses, a two‐side P‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Subjects
A total of 21 523 patients had a diagnosis of new‐onset of NVAF between January 2005 and June 2016. A total of 7885 patients who had BP data before the onset of NVAF available were included in the analysis (4001 with pre‐existing hypertension and 3884 without pre‐existing hypertension). Patients with hypertension were significantly older, significantly more likely to be male, had significantly higher rates of most comorbidities, and significantly more likely to have higher CHADS2 scores compared to those without hypertension (Table 1).
Table 1.
Patient characteristics at baseline, overall and in patients with or without hypertension before the onset of non‐valvular atrial fibrillation
| Overall (n = 7885) | Pre‐existing hypertension | |||
|---|---|---|---|---|
| No (n = 3884) | Yes (n = 4001) | P‐value | ||
| Age, years | 53.8 ± 10.0 | 50.9 ± 10.4 | 56.6 ± 8.7 | <.001 |
| Age ≥75 y, n (%) | 20 (0.3) | 2 (0.1) | 18 (0.5) | <.001 |
| Female, n (%) | 1491 (18.9) | 874 (22.5) | 617 (15.4) | <.001 |
| SBP, mm Hg | 126.4 ± 17.2 | 120.8 ± 14.8 | 131.7 ± 17.7 | <.001 |
| DBP, mm Hg | 78.5 ± 12.1 | 75.1 ± 10.8 | 81.9 ± 12.3 | <.001 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 4364 (55.4) | 1646 (42.4) | 2718 (67.9) | <.001 |
| Dyslipidemia | 3587 (45.5) | 1223 (31.5) | 2364 (59.1) | <.001 |
| Chronic kidney disease | 182 (2.3) | 24 (0.6) | 158 (4.0) | <.001 |
| COPD | 125 (1.6) | 48 (1.2) | 77 (1.9) | .014 |
| Hyperthyroidism | 2041 (25.9) | 1012 (26.1) | 1029 (25.7) | .733 |
| Hypothyroidism | 809 (10.3) | 354 (9.1) | 455 (11.4) | <.001 |
| Coronary heart disease | 3484 (44.2) | 1405 (36.2) | 2079 (52.0) | <.001 |
| Cardiomyopathy | 335 (4.3) | 98 (2.5) | 237 (5.9) | <.001 |
| Congestive heart failure | 3759 (47.7) | 1484 (38.2) | 2275 (56.9) | <.001 |
| Stroke/TIA | 1367 (17.3) | 479 (12.3) | 888 (22.2) | <.001 |
| CHADS2 score at NVAF diagnosis, n (%) | ||||
| 0 | 1481 (18.8) | 1481 (38.1) | 0 | <.001 |
| 1 | 1811 (23.0) | 1144 (29.5) | 667 (16.7) | |
| 2 | 2027 (25.7) | 949 (24.4) | 1078 (26.9) | |
| 3 | 1682 (21.3) | 192 (4.9) | 1490 (37.2) | |
| 4 | 455 (5.8) | 118 (3.0) | 337 (8.4) | |
| 5 | 427 (5.4) | 0 | 427 (10.7) | |
| 6 | 2 (0.1) | 0 | 2 (0.1) | |
Values are mean ± standard deviation, or number of patients (%).
Abbreviations: COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; TIA, transient ischemic attack.
3.2. Event rates
Overall, there were 109 primary composite end point events, an event rate of 7.1 per 1000 patient‐years. Corresponding event rates in new‐onset NVAF patients with and without pre‐existing hypertension were 10.3 and 4.4 per 1000 patient‐years, respectively (P < .001) (Table 2). The higher rate of events in patients with hypertension was attributable to a significantly higher rate of both ischemic stroke and hemorrhagic stroke (Table 2). Rates for all secondary end point events, especially percutaneous coronary intervention, cardiac bypass and thrombotic or embolic events, were significantly higher in patients with vs without hypertension (Table 2).
Table 2.
Rate of primary and secondary end point events, overall and in patients with or without hypertension before the onset of non‐valvular atrial fibrillation
| Incidence per 1000 patient/years | Overall (n = 7885) | Pre‐existing hypertension | ||
|---|---|---|---|---|
| No (n = 3884) | Yes (n = 4001) | P‐value | ||
| Primary composite end point | 7.1 | 4.4 | 10.3 | <.001 |
| Ischemic stroke | 4.0 | 2.3 | 6.0 | <.001 |
| Hemorrhagic stroke | 1.3 | 0.6 | 2.1 | .010 |
| AMI | 2.2 | 1.9 | 2.5 | .432 |
| Secondary end points | ||||
| GI hemorrhage | 1.7 | 1.1 | 2.5 | .034 |
| All‐cause mortality | 6.7 | 5.3 | 8.2 | .025 |
| PCI | 9.4 | 2.8 | 17.3 | <.001 |
| Cardiac bypass | 3.2 | 0.5 | 6.3 | <.001 |
| Thrombosis or embolism | 22.9 | 16.7 | 30.1 | <.001 |
Abbreviations: AMI, acute myocardial infarction; GI, gastrointestinal; PCI, percutaneous coronary intervention.
Differences in the overall rate of events in patients with vs without pre‐existing hypertension were evident within 1 month after diagnosis of NVAF (Figure 1A), and the event rate was higher in older patients (age ≥60 vs <60 years) (Figure 1B). The event rate in younger new‐onset NVAF patients with pre‐existing hypertension was similar to that in older patients without hypertension. In addition, lower systolic BP (<120 mm Hg) before the onset of NVAF was significantly associated with a lower incidence of cardiovascular events after NVAF onset (P < .001; Figure 1C).
Figure 1.
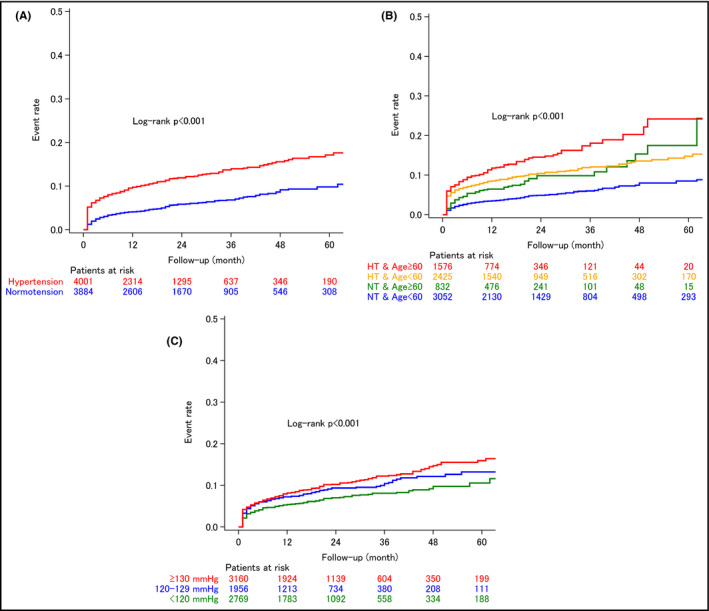
Kaplan‐Meier curves showing the rate of cardiovascular events (ischemic stroke, hemorrhagic stroke, and acute myocardial infarction) in non‐valvular atrial fibrillation (NVAF) patients with vs without pre‐existing hypertension before the onset of NVAF, overall (A), in patients aged <60 and ≥60 y with and without pre‐existing hypertension (B), and in subgroups based on systolic blood pressure before NVAF onset (C). HT, hypertension; NT, normotension
The proportion of patients with hypertension who were receiving antihypertensive therapy at 2 months after the diagnosis of AF was 86.0%. The anticoagulant prescription rate at 2 months after diagnosis was significantly higher in patients with vs without pre‐existing hypertension (48.0% vs 26.9%, respectively; P < .001); corresponding rates for warfarin prescription were 16.6% and 12.6%, respectively; P < .001.
4. DISCUSSION
This retrospective real‐world adult cohort study was the first to find a significantly higher primary end point event rate in new‐onset NVAF patients with pre‐existing hypertension compared to those who had been were normotensive up until the time of AF diagnosis. Furthermore, lower SBP before NVAF diagnosis was associated with a lower incidence of cardiovascular events after the onset of NVAF. Hypertension is a well‐known risk factor for the onset of NVAF and the occurrence of cardiovascular events. Calculation of both the CHADS2 and HAS‐BLED scores includes the presence of hypertension, and recent guidelines recommend strict BP control to <130/80 mm Hg in NVAF patients on antithrombotic agents.32 However, there are currently no data showing the influence of hypertension history and BP control status prior to developing NVAF on prognosis after NVAF onset.
In our study, the higher event rate in NVAF patients with vs without pre‐existing hypertension was largely due to a higher rate of both ischemic stroke and hemorrhagic stroke in those with pre‐existing hypertension. Event rates for all secondary end points were also higher in the group with pre‐existing hypertension vs normotension. The increase in events was evident within the first month after diagnosis of NVAF, and those with an end point event had more cardiovascular risk factors than those who were event free. In addition, event rates were higher in older (age ≥60 years) vs younger (age <60 years) patients with new‐onset NVAF.
Based on the data from this study, it is not possible to clearly identify the mechanisms by which pre‐existing hypertension and BP control status before the onset of NVAF increases the risk of adverse cardio‐ and cerebrovascular events in these patients. As noted above, baseline differences between NVAF patients with and without hypertension are indicative of higher baseline cardiovascular risk, particularly with respect to comorbidities. This is also reflected in the higher CHADS2 scores in patients with hypertension. One possible contributor to adverse events in hypertensive patients with AF is vascular dysfunction. It has previously been reported that the presence of AF in patients with hypertension was associated with arterial stiffness, determined by measuring brachial‐ankle pulse wave velocity.33 It has also been suggested that hypertension may be associated remodeling of the left atrium, which could in turn promote AF via several electrophysiological mechanisms.34 In addition, high BP was linked to advancing atrial interstitial fibrosis, increased left atrial pressure, decreased atrial refractory period, and progression of coronary atherosclerosis.34
In our study, the higher rate of thrombosis/embolism in NVAF patients with hypertension was seen within 1 month after NVAF diagnosis. This may be due to inadequate anticoagulant treatment in the early period after diagnosis of AF. Although prescription of anticoagulant therapy at 2 months after diagnosis was significantly more common in patients with vs without hypertension in our study, more than half of all patients with hypertension and nearly three‐quarters of those without hypertension had not been prescribed anticoagulant therapy 2 months after being diagnosed with AF. In contrast, 87.3% of patients with AF participating in the J‐RHYTHM registry were taking warfarin,35 compared with 12.6% of patients overall in our study. Therefore, inadequate anticoagulation could have contributed to the early excess of stroke events in patients with hypertension and AF in this group.
This study included a population of patients with a new diagnosis of NVAF and presents important information about cardiovascular risk and treatment patterns. However, our findings need to be interpreted in the context of a number of limitations. The study had a retrospective design and included a population that was selected from beneficiaries covered by the employees' health insurance system in Japan. This population may not be fully representative of the overall Japanese population, and there was a relative lack of data for patients aged ≥75 years. Because of the nature of the dataset, we were not able to verify the accuracy of physician diagnoses of AF and hypertension, and the risk of misclassification cannot be ruled out. It is also possible that the ICD‐10 code entered into the claims database does not accurately reflect clinical manifestations.
5. CONCLUSIONS
These data contribute to addressing the knowledge gap around the role of hypertension in the lifetime trajectory of cardiovascular prognosis in patients with AF. Event rates, particularly early events, were higher in NVAF patients with vs without hypertension before the onset of NVAF. In addition, lower systolic BP before NVAF diagnosis was associated with a lower risk of cardiovascular events in patients with new‐onset NVAF. This highlights the importance of earlier and more tight control of 24‐hour BP before the onset of NVAF, not only for reducing the rate of new‐onset of NVAF, but also for decreasing the rate of both hemorrhagic and ischemic cardiovascular events after a diagnosis of NVAF.
CONFLICTS OF INTERESTS
This study was funded by the BMS/Pfizer Japan Thrombosis Investigator Initiated Research Program (JRISTA). All the authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
KK supervised the conduct of the study and data analysis, and had the primary responsibility of writing this paper. TA wrote the Introduction, Methods, Results, and Discussion sections. HK conducted the data analysis, and wrote the Methods and Results sections.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Nicola Ryan, independent medical writer.
Kario K, Abe T, Kanegae H. Impact of pre‐existing hypertension and control status before atrial fibrillation onset on cardiovascular prognosis in patients with non‐valvular atrial fibrillation: A real‐world database analysis in Japan. J Clin Hypertens. 2020;22:431–437. 10.1111/jch.13755
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370‐2375. [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461‐468. [DOI] [PubMed] [Google Scholar]
- 3. Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119‐125. [DOI] [PubMed] [Google Scholar]
- 5. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746‐2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohsawa M, Okayama A, Sakata K, et al. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular diseases in 1980, 1990 and 2000. J Epidemiol. 2005;15:194‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . World population ageing report (2015). http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf. Accessed May 30, 2019.
- 8. Berge T, Lyngbakken MN, Ihle‐Hansen H, et al. Prevalence of atrial fibrillation and cardiovascular risk factors in a 63–65 years old general population cohort: the Akershus cardiac examination (ACE) 1950 study. BMJ Open. 2018;8:e021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Japan Ministry of Health LaW .Statistics 2015. http://www.mhlw.go.jp/toukei_hakusho/index.html. Accessed May 30, 2019.
- 10. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham heart study. JAMA. 1994;271:840‐844. [PubMed] [Google Scholar]
- 11. Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123:1501‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau YF, Yiu KH, Siu CW, Tse HF. Hypertension and atrial fibrillation: epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens. 2012;26:563‐569. [DOI] [PubMed] [Google Scholar]
- 13. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139‐1151. [DOI] [PubMed] [Google Scholar]
- 14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981‐992. [DOI] [PubMed] [Google Scholar]
- 15. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883‐891. [DOI] [PubMed] [Google Scholar]
- 16. Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93‐100. [DOI] [PubMed] [Google Scholar]
- 17. Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711‐716. [DOI] [PubMed] [Google Scholar]
- 18. Kokubo Y, Watanabe M, Higashiyama A, et al. Interaction of blood pressure and body mass index with risk of incident atrial fibrillation in a Japanese urban cohort: the Suita study. Am J Hypertens. 2015;28:1355‐1361. [DOI] [PubMed] [Google Scholar]
- 19. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455‐2461. [DOI] [PubMed] [Google Scholar]
- 20. Grundvold I, Skretteberg PT, Liestol K, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle‐aged men: a 35‐year follow‐up study. Hypertension. 2012;59:198‐204. [DOI] [PubMed] [Google Scholar]
- 21. Kodani E, Kaneko T, Fujii H, et al. Prevalence and incidence of atrial fibrillation in the general population based on national health insurance special health checkups‐ TAMA MED project‐AF. Circ J. 2019;83:524‐531. [DOI] [PubMed] [Google Scholar]
- 22. Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725‐731. [DOI] [PubMed] [Google Scholar]
- 24. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864‐2870. [DOI] [PubMed] [Google Scholar]
- 25. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 26. Kabutoya T, Imai Y, Hoshide S, Kario K. Diagnostic accuracy of a new algorithm to detect atrial fibrillation in a home blood pressure monitor. J Clin Hypertens (Greenwich). 2017;19:1143‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe T, Kawasaki M, Tanaka R, et al. Association among blood pressure control in elderly patients with hypertension, left atrial structure and function and new‐onset atrial fibrillation: a prospective 2‐year study in 234 patients. Hypertens Res. 2013;36:799‐806. [DOI] [PubMed] [Google Scholar]
- 28. Olbers J, Jacobson E, Viberg F, et al. Systolic blood pressure increases in patients with atrial fibrillation regaining sinus rhythm after electrical cardioversion. J Clin Hypertens (Greenwich). 2019;21:363‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webb AJ, Rothwell PM. Blood pressure variability and risk of new‐onset atrial fibrillation: a systematic review of randomized trials of antihypertensive drugs. Stroke. 2010;41:2091‐2093. [DOI] [PubMed] [Google Scholar]
- 30. Dixon JR Jr. The international conference on harmonization good clinical practice guideline. Qual Assur. 1998;6:65‐74. [DOI] [PubMed] [Google Scholar]
- 31. Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 33. Shi D, Meng Q, Zhou X, et al. Factors influencing the relationship between atrial fibrillation and artery stiffness in elderly Chinese patients with hypertension. Aging Clin Exp Res. 2016;28:653‐658. [DOI] [PubMed] [Google Scholar]
- 34. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. 2018;122:352‐368. [DOI] [PubMed] [Google Scholar]
- 35. Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa H. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J‐RHYTHM Registry. Circ J. 2011;75:1328‐1333. [DOI] [PubMed] [Google Scholar]


