Abstract
Lactate dehydrogenase (LDH) has been reported to be positively correlated with albuminuria assessed by urinary albumin‐to‐creatinine ratio (UACR) in patients with sickle cell disease; both LDH and albuminuria are positively associated with the severity of hypertension (HTN). Here, a cross‐sectional study was performed to investigate the association between LDH and albuminuria in Chinese hypertensives. A total of 1169 Chinese individuals (aged 58.0 ± 11.5 years, 60.4% male), who were admitted to our hospital, were included in this study. Based on the level of LDH, all hypertensives (n = 802) were divided into three groups: HTN1 (lowest tertile of LDH, n = 264), HTN2 (mediate tertile of LDH, n = 268), and HTN3 (highest tertile of LDH, n = 270). Hypertensives with hyperhomocysteinemia were defined as hypertensives with homocysteine ≥15μmol/L. Meanwhile, 367 normotensives served as controls. Compared with normotensives, the levels of LDH and UACR were significantly higher in hypertensives (p < .05). There was an increasing trend of albuminuria (UACR ≥30 mg/g) from control, HTN1, HTN2 to HTN3 group (4% vs. 12.1% vs. 14.9% vs. 19.6%, χ2 = 38.886, p < .001). Stepwise multiple regression analysis showed an independent association between LDH and UACR in patients with HTN (β = 0.085, p < .05), but not in normotensives. After further stratification in hypertensive patients, this correlation remained in the male (β = 0.161, p < .001), elderly (age ≥65 years, β = 0.174, p < .001) and especially hypertensives with hyperhomocysteinemia (β = 0.402, p < .001). LDH combined with white blood cell (WBC) counts was observed to have better discrimination for albuminuria than creatinine united with cystatin C in hypertensives according to receiver operation characteristic curves (area under curve: 0.637 vs. 0.535, z = 2.563, p = .0104). In conclusion, the level of LDH was associated with albuminuria in Chinese patients with HTN, particularly in hypertensives with hyperhomocysteinemia. LDH combined with WBC provided better prediction of albuminuria than routine renal function assessment in hypertensives. Further studies are needed to confirm LDH as an early marker for the risk of kidney involvement among hypertensives.
Keywords: albuminuria, hypertension, lactate dehydrogenase
The incidence of albuminuria was increased along with the increment of LDH level in patients with HTN. LDH correlated independently with UACR in the elderly, male and especially hypertensives with hyperhomocysteinemia. Moreover, LDH combined with WBC provides better prediction of albuminuria than creatinine united with cystatin C in hypertensives.
1. INTRODUCTION
According to the latest data of hypertension (HTN) survey, 1 over a quarter of Chinese adults are suffering from HTN, and the trend of prevalence is still in the rising stage. It is well recognized that the incidence of end‐stage renal disease (ESRD) increases significantly with elevated blood pressure, even in prehypertension. 2 In addition, HTN, including white‐coat and masked HTN, is significantly associated with an increased likelihood of albuminuria, defined as urinary albumin‐to‐creatinine ratio (UACR) levels ≥30 mg/g. 3 Furthermore, albuminuria is fully proved as an independent prognostic risk factor for adverse outcomes, including ESRD, myocardial infarction, and all‐cause mortality among individuals with preserved estimated glomerular filtration rates (eGFRs) as well as those with abnormal eGFRs. 4 , 5 , 6 Lactate dehydrogenase (LDH) presents as an ubiquitous cytoplasmic enzyme in all cells of the body that catalyzes the reversible conversion of pyruvate to lactate as a part of the lactic acid cycle. 7 Following cellular injury, LDH is released from impaired cells into serum. 8 It has been shown serum LDH is elevated in hypertensive rats induced by sodium fluoride indicating induction of oxidative stress, renal, and cardiac damage after exposure. 9 Furthermore, it has been demonstrated that LDH is positively correlated with the severity of HTN, especially in gestational hypertension. 10 In addition, albuminuria is considered as a result of glomerular endothelial dysfunction which leads to an abnormally increased glomerular filtration of albumin. 11 As far as we know, HTN is well known to be related to endothelial dysfunction. Hence, it is reasonable to assume that there is a relationship between LDH and albuminuria in hypertensives. Several attempts have been made to identify a significant correlation between serum LDH levels and albuminuria in patients with sickle cell disease. 12 , 13 , 14 Nevertheless, related data on HTN are not clearly shown. In this study, we aimed to investigate whether there was a link between LDH and albuminuria in hypertensives from a southern Chinese population.
2. METHODS
2.1. Statement of ethics
Our study was in accordance with the ethical standards formulated in the Helsinki Declaration, and the protocol was approved by Ethics Committee of the First Affiliated Hospital of Fujian Medical University. Informed consent was obtained from all patients involved.
2.2. Patients
This was a cross‐sectional clinical study. The protocol for investigation was established before clinical data collection, and it was performed by well‐trained physicians. According to the criteria of 2018 Chinese guidelines for the management of arterial hypertension, 15 hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or taking antihypertensive medications. Hypertensives with hyperhomocysteinemia were defined as hypertensives with homocysteine ≥15 μmol/L. Diabetes was defined as taking antihyperglycemic medications or establishing a new diagnosis of diabetes. The diagnosis of diabetes was based on the criteria recommended by the Chinese Diabetes Society in 2017. 16 Exclusion criteria included (1) secondary hypertension; (2) diabetes; (3) coronary heart disease and stroke within 3 months; (4) serum creatinine >2.5 mg/dl; (5) the level of aminotransferase and bilirubin ≥3 times the upper limit of the normal; (6) congestive heart failure, severe arrhythmia, ischemic heart disease, hypertrophic cardiomyopathy, valvular heart diseases, restrictive cardiomyopathy; (7) chronic consumptive diseases and malignant tumors; (8) acute or chronic infection or other inflammatory diseases; (9) connective tissue diseases; (10) mild or severe anemia; (11) those who are recently taking folic acid, vitamin B6 and vitamin B12; and (12) women during menstruation or pregnancy. In this study, 2903 patients, who were from the outpatients or inpatients of the department of General Medicine and Geriatrics in the First Affiliated Hospital of Fujian Medical University from January 2016 to March 2019, were screened. And 892 patients were excluded according to the exclusion criteria, 842 patients were further removed due to incomplete data of LDH, UACR, or homocysteine. Finally, a total of 1169 participants were included and analyzed. The patients included 802 hypertensives and 367 normotensives. Based on the level of LDH, the hypertensives were divided into three group: HTN1 (lowest tertile of LDH, n = 264), HTN2 (mediate tertile of LDH, n = 268), and HTN3 (highest tertile of LDH, n = 270).
2.3. Clinical data collection
General information, physical examination data, and laboratory assays were collected. Patients were interviewed regarding age, sex, smoking habits, hypertension, and diabetes history. Body weight and height were measured, and body mass index (BMI) was calculated as the ratio of the body weight (kg) to the square of height (m2). Waist circumference (WC) was measured around midway between the lower rib margin and iliac crest with tape. Current smoking was defined as consuming no less than one cigarette every day for at least 6 months. All participants were prohibited from consuming strong tea and coffee or take vigorous activities half an hour before measurement. After resting for at least 5 min in a sitting position, the heart rate was taken and the blood pressure was measured using an automated sphygmomanometer (HEM‐7052, Omron). The average reading of three measurements was used for the subsequent analysis. Blood sample for laboratory assays was taken after 8‐h overnight fasting. The levels of LDH, fasting plasma glucose, creatinine, uric acid, total bilirubin (TBil), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total cholesterol, triglycerides, high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C) were determined using an autoanalyzer (ADVIA 2400, Siemens). eGFR was calculated by experimental equation: eGFR (ml/min·1.73 m2) = 186 × (serum creatinine [mmol/L] × 0.0113)–1.154 × (Age)–0.203 (×0.742 for female). Latex enhanced immunoprojection turbidimetric assay was used to detect cystatin C. Glycosylated hemoglobin (HbA1c) was determined by high performance liquid chromatography using automatic analyzer (VARIANTTM‐II, Bio‐Rad). Plasma homocysteine levels were estimated by chemiluminescence microparticle immunoassay according to the manufacturer's protocol (Abbott GmbH & Co.KG). White blood cell (WBC) counts were performed with analyze (ADVIA2120). First voiding point urine samples in the early morning were collected, and urinary albumin and creatinine were detected within two hours. Urinary creatinine concentration was detected by colorimetry assay (Boehringer Mannheim/Hitachi 717 analyzer), and urinary albumin was determined by immunoturbidimetry method (Roche P800 automatic analyzer). The value of UACR was obtained by calculating the ratio of urinary albumin (mg) to urinary creatinine (g). Albuminuria was defined as UACR levels ≥30 mg/g.
2.4. Statistical analysis
Gaussian distribution test and homogeneity test of variance were carried out for continuous variables. Continuous variables were presented as mean ± standard deviation for Gaussian distribution or median and interquartile distance for skewed distribution. Categorical variables were presented as percentages and absolute values. Comparisons among groups were performed by analysis of variance (ANOVA) if the variables were in a Gaussian distribution and equal variance. If the variables were in a skewed distribution or not equal variance, comparisons among groups were made by Wilcoxon signed‐rank test. Associations between UACR levels and anthropometric data, blood pressure, heart rate, and laboratory parameters were assessed by Spearman correlative analysis. The stepwise multivariate linear regression analysis was used to determine the independent variables related to UACR. Receiver operation characteristic (ROC) curves were used to assess the predictive efficiency of LDH combined with WBC or CREA united with cystatin C for albuminuria. Delong test was used to compare the difference between two ROC curves. Statistical significance was defined as two‐side p < .05. Our data were analyzed by SPSS 20.0 statistical software package.
3. RESULTS
3.1. Clinical characteristics of the patients
A total of 1169 Chinese participants (aged 58.0 ± 11.5 years, 60.4% male) were included in this study. Clinical and biochemical data of all participants are summarized in Table 1. Compared with normotensives, the levels of LDH [176 (157–199) U/L vs. 183 (163–206) U/L, p < .05] and UACR[5.35 (3.47–8.65) mg/g vs. 7.76 (4.55–17.47) mg/g, p < .05] were significantly higher in hypertensives. Age, BMI, WC, duration of hypertension, systolic blood pressure, diastolic blood pressure, WBC, ALT, AST, GGT, ALP, uric acid, creatinine, cystatin C, eGFR, triglyceride, fasting plasma glucose, homocysteine, the use of angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB), Ca2+ channel blocker (CCB), and statin in hypertensives were markedly higher than normotensives as well. Whereas, total cholesterol, HDL‐C, and LDL‐C were lower in patients with HTN than those without HTN. No apparent difference in smoking rate or heart rate was found among the four groups. There was an increasing trend of albuminuria (UACR ≥30 mg/g) from control, HTN1, HTN2 to HTN3 group (4.1% vs. 12.1% vs. 14.9% vs. 19.6%, χ2 = 38.886, p < .001; Figure 1) and among subgroups in hypertensives (χ2 = 5.724, p = .017). LDH, age, AST, and ALP in hypertensives were distinctly increased with the rising tertile of LDH in hypertensives. The level of UACR in HTN2 and HTN3 groups was obviously higher than that of the HTN1 group, whereas UACR in HTN3 group did not significantly differ from HTN2. There was no significant difference in BMI, WC, smoking rate, systolic blood pressure, diastolic blood pressure, heart rate, GGT, creatinine, triglyceride, HbA1c, homocysteine, and use of medication among three subgroups.
TABLE 1.
Clinical characteristics of all patients with and without hypertension
| Variables | Normotensives | HTN1 | HTN2 | HTN3 | p value |
|---|---|---|---|---|---|
| N | 367 | 264 | 268 | 270 | |
| Male, n (%) | 209 (56.9%) | 178 (64.5%) a | 182 (68.7%) a | 137 (52.5%) b , c | <.001 |
| Age (years) | 54.8 ± 9.2 a | 57.3 ± 12.0 a | 59.4 ± 12.1 a , b | 61.8 ± 11.9 a , b , c | <.001 |
| BMI (kg/m2) | 23.78 ± 2.72 | 24.95 ± 3.54 a | 25.27 ± 3.10 a | 25.24 ± 3.38 a | <.001 |
| WC (cm) | 84.4 ± 8.5 | 88.2 ± 9.7 a | 89.3 ± 8.0 a | 89.1 ± 9.3 a | <.001 |
| Current smoking [n (%)] | 74 (20.2%) | 56 (20.3%) | 51 (19.2%) | 37 (14.2%) | .291 |
| Duration of hypertension (years) | 0 (0–0) | 5.0 (1.3–10.0) a | 6.0 (1.0–10.0) a | 7.0 (2.0–10.0) a , b , c | <.001 |
| Systolic blood pressure (mmHg) | 117.2 ± 10.3 | 134.9 ± 16.1 a | 136.9 ± 15.5 a | 137.3 ± 16.3 a | <.001 |
| Diastolic blood pressure (mmHg) | 71.9 ± 7.9 | 81.1 ± 10.9 a | 80.3 ± 11.1 a | 80.5 ± 10.8 a | <.001 |
| Heart rate (bp) | 66.7 ± 10.1 | 68.1 ± 10.9 | 69.1 ± 10.9 | 67.6 ± 10.3 | .056 |
| WBC (109/L) | 5.76 ± 1.32 | 5.92 ± 1.43 | 6.09 ± 1.45 a | 6.18 ± 1.43 a , b | <.001 |
| TBil (μmol/L) | 13.48 ± 5.84 | 12.52 ± 4.80 a | 13.53 ± 6.10 | 14.38 ± 6.9 b | .031 |
| ALT (U/L) | 22.0 (16.0–30.0) | 21.0 (16.0–28.0) | 24.0 (18.0–34.0) a , b | 25.0 (19.0–33.0) a , b | <.001 |
| AST (U/L) | 22.0 (18.0–25.0) | 19.0 (16.0–23.0) a | 23.0 (18.0–27.8) a , b | 24.0 (21.0–29.3) a , b , c | <.001 |
| LDH (U/L) | 176.0 (157.0–199.0) | 154.0 (142.3–162.0) a | 182.0 (177.0–190.0) a , b | 215.0 (205.0–235.0) a , b , c | <.001 |
| GGT (U/L) | 22.0 (16.0–32.0) | 25.0 (17.0–41.0) a | 28.0 (19.3–43.8) a | 28.0 (20.0–42.5) a | <.001 |
| ALP (U/L) | 62.0 (53.0–74.0) | 63.0 (52.8–77.0) | 71.0 (60.0–83.0) a , b | 74.0 (62.0–89.0) a , b , c | <.001 |
| Uric acid (μmol/L) | 359.8 ± 92.5 | 369.9 ± 94.8 | 387.4 ± 102.5 a , b | 384.6 ± 96.9 a | .004 |
| Creatinine (μmol/L) | 65.5 ± 13.3 | 69.6 ± 16.6 a | 71.0 ± 17.7 a | 69.6 ± 18.5 a | <.001 |
| Cystatin C (mg/L) | 0.82 (0.72–0.92) | 0.84 (0.76–0.95) a | 0.88 (0.78–1.01) a , b | 0.88 (0.78–1.01) a , b | <.001 |
| eGFR (ml/min) | 114.6 ± 24.3 | 109.7 ± 27.9 a | 105.6 ± 29.2 a | 104.90 ± 26.8 a , b | <.001 |
| UACR (mg/g) | 5.35 (3.47–8.65) | 6.99 (4.30–14.22) a | 8.43 (4.71–18.65) a , b | 8.10 (4.65–19.80) a , b | <.001 |
| Total cholesterol (mmol/L) | 5.05 ± 1.04 | 4.58 ± 1.00 a | 4.84 ± 1.10 a , b | 4.82 ± 1.01 b , c | <.001 |
| Triglyceride (mmol/L) | 1.17 (0.86–1.69) | 1.25 (0.91–1.75) | 1.30 (0.98–1.79) a | 1.34 (0.95–1.89) a | .023 |
| HDL‐C (mmol/L) | 1.34 ± 0.36 | 1.21 ± 0.32 | 1.25 ± 0.32 | 1.35 ± 0.41 | <.001 |
| LDL‐C (mmol/L) | 3.24 ± 0.96 | 2.89 ± 0.91 a | 3.14 ± 1.05 b | 2.98 ± 0.96 a | <.001 |
| Fasting plasma glucose (mmol/L) | 5.06 ± 0.56 | 5.26 ± 0.64 a | 5.22 ± 0.63 a | 5.13 ± 0.63 b | <.001 |
| HbA1c (%) | 5.53 ± 0.39 | 5.59 ± 0.44 | 5.60 ± 0.43 | 5.65 ± 0.44 a | .014 |
| Homocysteine (μmol/L) | 9.36 (7.79–11.27) | 10.2 (8.3–12.1) a | 10.4 (8.6–13.2) a | 10.5 (8.7–13.3) a | <.001 |
| Use of ACEI/ARB [n (%)] | 0 (0%) | 92 (33.3%) a | 106 (40.0%) a | 98 (37.5%) a | <.001 |
| Use of Ca2+ channel blocker [n (%)] | 0 (0%) | 107 (38.8%) a | 104 (39.2%) a | 116 (44.4%) a | <.001 |
| Use of statin [n (%)] | 16 (4.4%) | 40 (14.5%) a | 55 (20.8%) a | 50 (19.2%) a | <.001 |
Data are expressed as means ± SD or median (25th–75th).
Abbreviations: ACEI, Angiotensin‐converting enzyme inhibitor; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDH, lactate dehydrogenase; LDL C, low‐density lipoprotein‐cholesterol; TBil, total bilirubin; UACR, urinary albumin‐to‐creatinine ratio; WBC, white blood cell counts; WC, waist circumference.
p < .05 versus normotensives.
p < .05 versus HTN1 group.
p < .05 versus HTN2 group.
FIGURE 1.
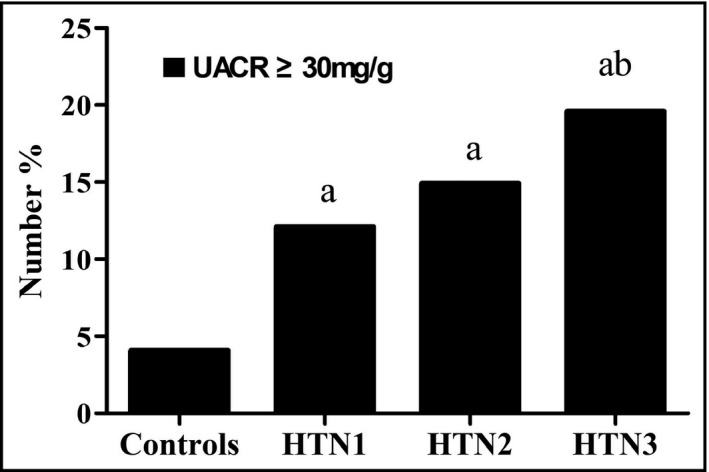
Percentage of albuminuria in normotensives and hypertensives with elevated tertile of LDH level (n = 1169). a p < 0.05 versus normotensives, b p < 0.05 versus hypertensives with lowest tertile of LDH level
3.2. Correlations between UACR and clinical profile
Spearman correlation analysis showed that LDH slightly correlated with UACR both in all patients (r = .112, p < .001) and in hypertensives (r = .099, p = .005; Table 2). Meanwhile, BMI, duration of hypertension, systolic blood pressure, diastolic blood pressure, heart rate, WBC, ALP, total cholesterol, triglyceride, and fasting plasma glucose were positively related to UACR in all patients. In addition, duration of hypertension, systolic blood pressure, diastolic blood pressure, heart rate, WBC, ALP, total cholesterol, triglyceride, LDL‐C, and fasting plasma glucose were positively associated with UACR in hypertensives as well.
TABLE 2.
Correlation of clinical profile and UACR in all patients and hypertensives
| Variables | All patients (n = 1169) | Hypertensives (n = 802) | ||
|---|---|---|---|---|
| r | p value | r | p value | |
| Age | .022 | .458 | −.038 | .289 |
| BMI | .060 | .040 | .043 | .219 |
| WC | .001 | .963 | −.020 | .580 |
| Duration of hypertension | .182 | <.001 | .031 | .381 |
| Systolic blood pressure | .293 | <.001 | .258 | <.001 |
| Diastolic blood pressure | .214 | <.001 | .212 | <.001 |
| Heart rate | .130 | <.001 | .127 | <.001 |
| WBC | .169 | <.001 | .199 | <.001 |
| TBil | −.003 | .913 | .003 | .941 |
| ALT | .024 | .403 | .043 | .223 |
| AST | .020 | .493 | .020 | .566 |
| LDH | .112 | <.001 | .099 | .005 |
| GGT | .016 | .581 | .019 | .588 |
| ALP | .171 | <.001 | .132 | <.001 |
| Uric acid | −.010 | .741 | .024 | .493 |
| Creatinine | .038 | .200 | .036 | .307 |
| Cystatin C | .050 | .093 | .038 | .290 |
| eGFR (ml/min) | −.036 | .218 | −.033 | .352 |
| Total cholesterol | .062 | .035 | .111 | .002 |
| Triglyceride | .095 | .001 | .087 | .014 |
| HDL‐C | .014 | .622 | .042 | .236 |
| LDL‐C | .046 | .119 | .095 | .007 |
| Fasting plasma glucose | .108 | <.001 | .101 | .004 |
| HbA1c | .042 | .157 | .025 | .494 |
| Homocysteine | −.006 | .849 | −.043 | .221 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; LDH, lactate dehydrogenase; LDL‐C, low‐density lipoprotein‐cholesterol; TBil, total bilirubin; UACR, urinary albumin‐to‐creatinine ratio; WBC, white blood cell counts; WC, waist circumference.
3.3. Stepwise multivariate linear regression analysis of UACR
In order to correct confounding variables on UACR, stepwise multivariate linear regression analysis was performed to determine the independent variables related to UACR. As shown in Table 3, LDH was slightly but independently associated with UACR in all patients (β = 0.071, p < .05) and patients with HTN (β = 0.085, p < .05), but not in normotensives. After further stratification for hypertensive patients, the association remained in the male (β = 0.161, p < .05), elderly (age ≥65 years, β = 0.174, p < .05) and especially hypertensives with hyperhomocysteinemia (β = 0.402, p < .05). The relationship between LDH and lgUACR in hypertensives with hyperhomocysteinemia was shown in Figure 2. Meanwhile, WBC, LDL‐C, WC, and AST were independently associated with UACR as well in hypertensives with hyperhomocysteinemia.
TABLE 3.
Stepwise multivariate linear regression analysis of UACR before and after stratification of hypertension, age, sex, and HCY
| N | Variables | B | SE | β | t | p value | |
|---|---|---|---|---|---|---|---|
| All patients | 1169 | LDH | 1.222 | 0.526 | 0.071 | 2.323 | .020 |
| Normotensives | 367 | GGT | 0.099 | 0.035 | 0.157 | 2.858 | .005 |
| sex | 5.824 | 1.957 | 0.162 | 2.976 | .003 | ||
| SBP | 0.240 | 0.094 | 0.137 | 2.539 | .012 | ||
| Hypertensives | 802 | LDH | 1.848 | 0.799 | 0.085 | 2.314 | .021 |
| Age <65 years | 547 | – | – | – | – | – | – |
| Age ≥65 years | 255 | LDH | 3.756 | 1.414 | 0.174 | 2.656 | .008 |
| Male | 497 | LDH | 2.783 | 0.793 | 0.161 | 3.512 | <.001 |
| Female | 305 | – | – | – | – | – | – |
| HCY <15μmol/L | 702 | – | – | – | – | – | – |
| HCY ≥15μmol/L | 100 | LDH | 7.991 | 2.203 | 0.402 | 3.628 | <.001 |
| WBC | 106.790 | 57.384 | 0.180 | 1.861 | .066 | ||
| LDL‐C | 219.851 | 81.002 | 0.259 | 2.714 | .008 | ||
| WC | 17.402 | 7.861 | 0.202 | 2.214 | .030 | ||
| AST | −26.329 | 11.493 | −0.250 | −2.291 | .024 |
Sex: male = 1, female = 2.
Abbreviations: AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; HCY, Homocysteine; LDH, lactate dehydrogenase; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; UACR, urinary albumin‐to‐creatinine ratio; WBC, white blood cell counts; WC, waist circumference.
FIGURE 2.
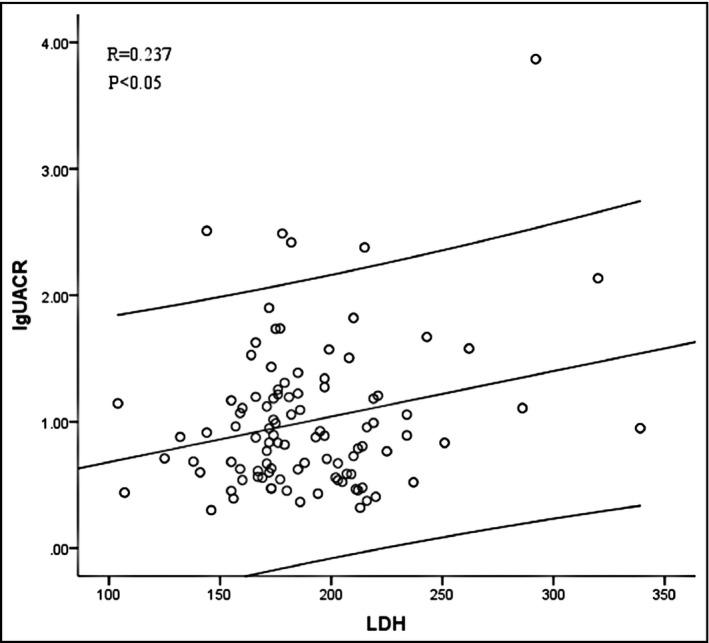
Relationship between lgUACR and LDH levels in hypertensives with hyperhomocysteinemia in a scatter plot (n = 100)
3.4. ROC curves of creatinine combined with cystatin C and LDH combined with WBC for discrimination of albuminuria in hypertensives
ROC curves and their characteristics are shown in Figure 3 and Table 4. Obviously, creatinine united with cystatin C did not predict albuminuria in hypertensives (area under curve: 0.538, p = .217). Although LDH significantly provided a discrimination for albuminuria, but only to a very low extent (area under curve: 0.577, p = .006), the predictive efficiency of LDH for albuminuria did not significantly differ from creatinine united with cystatin C (area under curve: 0.577 vs. 0.538, z = 0.984, p = .325), whereas LDH combined with WBC provides better discrimination of albuminuria than creatinine combined with cystatin C in hypertensives (area under curve: 0.637 vs. 0.535, z = 2.563, p = .0104).
FIGURE 3.
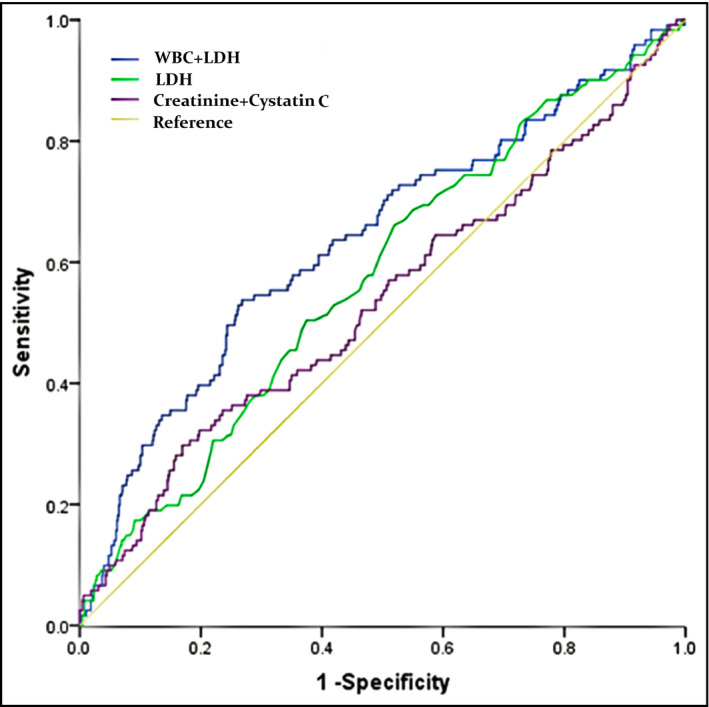
ROC curves of creatinine combined with cystatin C and LDH combined with WBC for discrimination of albuminuria in hypertensives (n = 802)
TABLE 4.
Characteristics of receiver operation characteristic curve of CREA creatinine combined with cystatin C and LDH combined with WBC for discrimination of albuminuria in hypertensives
| Model | Associated criterion | AUC (95% CI) | p value of curve | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| Creatinine + cystatin C | 17.5% | 0.538 (0.502–0.573) | .217 | 0.295 | 0.832 | 0.127 |
| LDH | 14.7% | 0.577 (0.542–0.612) | .006 | 0.664 | 0.477 | 0.141 |
| LDH + WBC | 17.4% | 0.634 (0.599–0.668) | <.0001 | 0.536 | 0.731 | 0.267 |
Abbreviations: LDH, lactate dehydrogenase; WBC, white blood cell.
4. DISCUSSION
To the best of our knowledge, this is the first study exploring the association between LDH and albuminuria in Chinese hypertensives. The incidence of albuminuria was increased along with the increment of LDH level in patients with HTN. After further stratification, it was found that LDH correlated independently with UACR in the elderly, male, and especially hypertensives with hyperhomocysteinemia. Although this correlation was modest, it was independent of other cardiovascular risk factors. Moreover, LDH combined with WBC provides better prediction of albuminuria in hypertensives than routine renal tests.
Most of the albumin filtered through glomerulus is reabsorbed in renal tubules, thus, generally urinary excretion of albumin was virtually undetectable. Nevertheless, imbalance between filtration and reabsorption of albumin in the kidney could result in an increase in urinary excretion of albumin and glomerular endothelial dysfunction is recognized as a primary cause for an abnormally increased glomerular filtration of albumin. 11 Increased albumin excretion is related to endothelial alterations in the glomerular capillaries in patients with essential HTN. 17 So far, it is believed that microalbuminuria is not only an indicator of glomerular endothelial damage representing microvascular injuries, but also reflects widespread vascular damage. 18 LDH is an enzyme widespreadly detectable in the cytoplasm of almost every kind of cells in the human body and is a sensitive indicator of cell injury and/or increased cell membrane permeability. The process of atherosclerosis begins with endothelial cell impairment and increased cell membrane permeability, resulting in the release of LDH into circulation. In atherosclerosis rabbit models, it was found that serum LDH gradually increased with the progress of atherosclerosis, suggesting that LDH is related to pathogenesis of atherosclerosis. 19 Patients with HTN are well known to be more vulnerable for endothelial dysfunction, which may simultaneously lead to albuminuria and atherosclerotic disease. 20 The multi‐ethnic study of atherosclerosis, including 6774 asymptomatic individuals, demonstrated an increased risk of incident coronary artery calcification (CAC) as well as greater CAC progression among those with microalbuminuria. 21 Hence, vascular endothelial injury and atherosclerosis may be the explanation for the relevance of LDH and UACR. However, as LDH exists in all the cells of human body, it may be affected not only by renal lesion, but also damaged condition of other organs, which may explain the relatively weak association of LDH with UACR.
In the present study, the association between LDH and UACR preserved only in the male, the elderly hypertensives. It is in accordance with our previous study, indicating UACR was associated with arterial stiffness, assessed by measuring carotid‐femoral pulse wave velocity, in the male and the elderly. 22 In addition, our previous research has also shown that the LDH correlates positively with intima‐media thickness of carotid artery, which is considered as a predictor for atherosclerotic disease, in the male hypertensives and those aged between 60 and 70 years old. 23 In the study of Kong et al, 24 slight elevation of albuminuria was associated with carotid artery intima‐media thickness in elderly people with normal renal function, which was similar to our current result. For the elderly, cell injury and arteriosclerosis are usually more obvious than the young‐ and middle‐aged, which could be the reason for inconsistent results for different age subgroups. Additionally, the differences between the sexes may be explained by the protective effects of estrogen on cardiovascular system for female.
At present, it has been confirmed that abnormal increase of homocysteine level was related with injury of vascular endothelial cells and lead to endothelial dysfunction. 25 Meanwhile, our previous work has consistently shown that the plasma homocysteine levels were independently associated with arteriosclerosis in hypertension. 26 Furthermore, it has been demonstrated that homocysteine was inversely associated eGFR. 27 Also, LDH leakage was observed to be evidently increased in cultured vascular smooth muscle cells treated with homocysteine. 28 Those mentioned above may elucidate the more significant correlation between LDH level and UACR in hypertensives with hyperhomocysteinemia. Since the existing research data are limited, relevant mechanism needs to be further explored. Some previous studies have investigated the association of markers of low‐grade inflammation, such as C‐reactive protein, IL‐6, and TNF‐α to the occurrence and progression of microalbuminuria and an increased risk for atherosclerotic disease. 29 And WBC was reported to be related to UACR in steady state children with sickle cell anemia. 13 Moreover, in Fangfang Fan's study, 30 WBC predicts the odds of kidney function decline in a Chinese community‐based population. Consistently, WBC was positively associated with UACR in hypertensives with hyperhomocysteinemia in the present study. Besides, LDH combined with WBC provided prediction of albuminuria in hypertensives to a certain extent. Additionally, LDL‐C and waist circumference were both positively correlated with UACR in hypertensives with hyperhomocysteinemia, which was consistent with former researches. 31 , 32 Increased LDL‐C and abdominal obesity are both well‐known risk factors for arteriosclerosis, which may explain the association. It is noteworthy that AST was negatively associated with UACR in hypertensives with hyperhomocysteinemia. In Anthony J G Hanley’ study, higher level of AST‐to‐ALT ratio predicts reduced risk of metabolic syndrome, 33 and metabolic syndrome was observed to be associated with albuminuria. 34 In contrary, serum ALT and AST levels were reported to be independently positively associated with the risk and severity of premature coronary artery disease in younger patients. 35 At present, related studies showed controversial results, and the mechanisms underlying the relationship between AST and UACR are still unclear.
Creatinine and eGFR are commonly used to assess renal function; however, in this study, LDH outperformed those two parameters in early detection of kidney lesions, according to multiple linear regression for UACR analysis and ROC curves for discrimination of albuminuria. Cystatin C also comes from nucleated cells and widely exists in human body. It is considered to be relatively stable, as the secretion would not be influenced by age and sex, and its metabolism will not be disturbed by diet. In this study, the level of plasma cystatin C was significantly increased in hypertensives, compared with normotensive, and it rose with the increment of LDH. However, LDH rather than CysC was determined as an independent relative factor for UACR in all subgroups.
Several limitations should be acknowledged in this study. Firstly, our study was cross‐sectional, and correlation coefficient between LDH and UACR was relatively small; no causal relationship can be inferred. Both of an increase in LDH and albuminuria might be the results of physiological malfunction. In addition, there are five isozyme forms of LDH, of which LDH1 and LDH2 are most common in kidney. In order to investigate the relationship between LDH and UACR, it is better to test LDH1 and LDH2 as well; unfortunately, we do not run these tests as a routine in our hospital, which needs further investigation. Secondly, the sample size in this study was relatively small, and this may explain the lack of difference in UACR of HTN2 and HTN3 group. Therefore, a prospective study with larger sample size is required to verify these results. In addition, urinary albumin excretion was measured on a single voided urine collection, which may not have accurately reflected the true level of albuminuria. However, it was reported suggested that a single‐void urine UACR correlated highly with the 24‐h urinary albumin excretion with a high specificity and sensitivity; therefore, it can be used to estimate quantitative microalbuminuria. 36 Thirdly, the patients were not randomly selected from the general population and do not represent the entire Chinese population. Fourthly, we did not exclude those who received the pharmacological therapies. The more frequent use of statin in hypertensives may explain the lower levels of total cholesterol and LDL‐C in this subgroup. However, we did put the use of ACEI/ARB, CCB, and statin into consideration in the liner regression to exclude the influence of medication. Fifthly, although the conclusion “the combination of LDH and WBC is a better predictor of UACR than the combination of creatinine and cystatin C” may not provide much value in clinic, as creatinine and cystatin C reflect GFR and are not directly related to UACR, besides, measuring UACR is simple. As far as we know, UACR is not a routine test or even not tested at all in some primary hospitals. LDH combined with WBC may be a little useful for the prediction of UACR in those institutes.
In conclusion, the level of LDH was associated with albuminuria in Chinese patients with HTN, particularly in hypertensives with hyperhomocysteinemia. This correlation was independent of age, sex, blood pressure and creatinine, CysC, and eGFR. LDH combined with WBC provided better prediction of albuminuria than routine renal function assessment in hypertensives. This research sheds new light on the relationship between LDH and albuminuria.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Xiaoqi Cai contributed to the conception and design of the work; analysis and interpretation of data for the work; and drafting the work. Tingjun Wang contributed to the design of the work and critical revision for important intellectual content. Chaoyi Ye contributed to the acquisition and analysis of data and critical revision for important intellectual content. Guoyan Xu contributed to the acquisition of data and critical revision for important intellectual content. Liangdi Xie contributed to critical revision of the manuscript and final version approval.
Cai X, Wang T, Ye C, Xu G, Xie L. Relationship between lactate dehydrogenase and albuminuria in Chinese hypertensive patients. J Clin Hypertens.2021;23:128–136. 10.1111/jch.14118
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy.
REFERENCES
- 1. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in china: results from the China hypertension survey, 2012–2015. Circulation. 2018;137(22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 2. Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 3. Hata J, Fukuhara M, Sakata S, et al. White‐coat and masked hypertension are associated with albuminuria in a general population: the Hisayama study. Hypertens Res. 2017;40(11):937‐943. [DOI] [PubMed] [Google Scholar]
- 4. Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423‐429. [DOI] [PubMed] [Google Scholar]
- 5. Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta‐analysis. PLoS Medicine. 2008;5(10):e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung KC, Kim BJ, Ryu S. An association of a variety of cardiovascular risk factors with low grade albuminuria in Korean men. Atherosclerosis. 2008;196(1):320‐326. [DOI] [PubMed] [Google Scholar]
- 7. Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb Protoc. 2018;(6):465–468. [DOI] [PubMed] [Google Scholar]
- 8. Liaw C, Wang C, Huang J, et al. Serum lactate dehydrogenase level in patients with nasopharyngeal carcinoma. Acta Oncol. 1997;36(2):159‐164. [DOI] [PubMed] [Google Scholar]
- 9. Oyagbemi AA, Omobowale TO, Asenuga ER, et al. Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol. 2017;32(4):1089‐1101. [DOI] [PubMed] [Google Scholar]
- 10. Burwick RM, Rincon M, Beeraka SS, et al. Evaluation of hemolysis as a severe feature of preeclampsia. Hypertens. 2018;72(2):460‐465. [DOI] [PubMed] [Google Scholar]
- 11. Ballermann BJ, Stan RV. Resolved: capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol. 2007;18(9):2432‐2438. [DOI] [PubMed] [Google Scholar]
- 12. Alzahri MS, Mousa SA, Almomen AM, et al. Lactate dehydrogenase as a biomarker for early renal damage in patients with sickle cell disease. Saudi J Kidney Dis Transpl. 2015;26(6):1161‐1168. [DOI] [PubMed] [Google Scholar]
- 13. Itokua KE, Makulo JR, Lepira FB, et al. Albuminuria, serum antioxidant enzyme levels and markers of hemolysis and inflammation in steady state children with sickle cell anemia. BMC Nephrol. 2016;17(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurkan S, Scarponi KJ, Hotchkiss H, et al. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010;25(10):2123‐2127. [DOI] [PubMed] [Google Scholar]
- 15. Joint Committee for Guideline Revision . 2018 Chinese guidelines for prevention and treatment of hypertension‐a report of the revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16(3):182‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang W, Shu L, Zhao H, et al. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin J Pract Intern Med. 2018;38(4):292‐344. [Google Scholar]
- 17. Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low‐grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157‐1165. [DOI] [PubMed] [Google Scholar]
- 18. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219‐226. [DOI] [PubMed] [Google Scholar]
- 19. Jing Z, Zhu Q, Zhou Q, et al. The dynamic analysis of lactate dehydrogenase and creatine kinase in the serum of atherosclerotic model rabbit. Acta Univ Med Anhui. 2004;39(1):12‐15. [Google Scholar]
- 20. Kramer H, Jacobs DR Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. The multi‐ethnic study of atherosclerosis. Hypertens. 2005;46(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 21. DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3(6):595‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ye C, Gong J, Wang T, et al. Relationship between high‐normal albuminuria and arterial stiffness in Chinese population. J Clin Hypertens. 2020;00:1‐8. 10.1111/jch.13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai X, Zhang L, Huang X, et al. The relationship between vascular overload index and CA‐IMT in Hypertensive Patients. Chin J Hypertens. 2014;22(9):830‐835. [Google Scholar]
- 24. Kong X, Jia X, Wei Y, et al. Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima‐media in elderly patients with normal renal function. BMC Nephrol. 2012;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Xu Y, Zhang H, et al. Coronary flow velocity reserve is impaired in hypertensive patients with hyperhomocysteinemia. J Hum Hypertens. 2014;28(12):743‐747. [DOI] [PubMed] [Google Scholar]
- 26. Wang T, Xu G, Cai X, et al. Association of homocysteine with carotid‐femoral pulse wave velocity in a southern Chinese population. Aging. 2019;11(21):9709‐9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, Tsai Y, Chen S, et al. The association of leptin and homocysteine with renal function impairment in a population of Taiwanese adults. Clin Nutr. 2015;34(5):943‐950. [DOI] [PubMed] [Google Scholar]
- 28. Chang L, Xu J, Zhao J, et al. Taurine antagonized oxidative stress injury induced by homocysteine in rat vascular smooth muscle cells. Acta Pharmacol Sin. 2004;25(3):341‐346. [PubMed] [Google Scholar]
- 29. Bakker SJ, Gansevoort RT, Stuveling EM, et al. Microalbuminuria and C‐reactive protein: similar messengers of cardiovascular risk? Curr Hypertens Rep. 2005;7(5):379‐384. [DOI] [PubMed] [Google Scholar]
- 30. Fan F, Jia J, Li J, et al. White blood cell count predicts the odds of kidney function decline in a Chinese community‐based population. BMC Nephrol. 2017;18(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Y, Sun G, Liu R, et al. Plasma triglyceride levels and central obesity predict the development of kidney injury in Chinese community older adults. Ren Fail. 2019;41(1):946‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam GE, Han K, Kim DH, et al. Relationship between dyslipidemia and albuminuria in prediabetic adults: the Korea National Health and Nutrition Examination Survey 2011–2012. Endocrine. 2015;48(2):557‐565. [DOI] [PubMed] [Google Scholar]
- 33. Hanley AJ, Williams K, Festa A, et al. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54(11):3140‐3147. [DOI] [PubMed] [Google Scholar]
- 34. Saito T, Mochizuki T, Uchida K, et al. Metabolic syndrome and risk of progression of chronic kidney disease: a single‐center cohort study in Japan. Heart Vessels. 2013;28(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 35. Masoudkabir F, Karbalai S, Vasheghani‐Farahani A, et al. The association of liver transaminase activity with presence and severity of premature coronary artery disease. Angiology. 2011;62(8):614‐619. [DOI] [PubMed] [Google Scholar]
- 36. Nathan DM, Rosenbaum C, Protasowicki VD. Single‐void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10(4):414‐418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy.


