Abstract
High‐normal albuminuria is related to the morbidity and mortality of cardiovascular disease. Arterial stiffness has been regarded as a predictor of cardiovascular disease. However, the relationship between high‐normal albuminuria and arterial stiffness is uncertain in Chinese population. A total of 1343 Chinese participants (aged 58.9 ± 12.1 years, 63.53% male) were included in this study. High‐normal albuminuria was defined as urinary albumin‐to‐creatinine ratio (UACR) above the median within normal albuminuria. Based on the level of UACR, all participants were divided into low‐normal albuminuria group (UACR < 6.36 mg/g, n = 580), high‐normal albuminuria group (6.36 mg/g ≤ UACR < 30 mg/g, n = 581), microalbuminuria (30 mg/g ≤ UACR < 300 mg/g, n = 162), and macroalbuminuria (UACR ≥ 300 mg/g, n = 20). Arterial stiffness was assessed by measuring carotid‐femoral pulse wave velocity (cfPWV). With the increment of UACR, the level of cfPWV was increased gradually (P < .001). Stepwise multiple regression analysis showed that systolic blood pressure, age, serum creatinine, heart rate, logarithmic (LG)‐transformed UACR, and fasting plasma glucose were independently associated with cfPWV in all subjects (P < .001). LG‐UACR was found to be related to cfPWV in high‐normal albuminuria and macroalbuminuria subjects. After further stratification in the high‐normal albuminuria subjects, their relation remained in male, elderly over 65 years old, or normotensives. In summary, UACR is associated with arterial stiffness in subjects with proteinuria excretion in high normal level. High‐normal albuminuria might be an early indicator of arterial stiffness, especially in male, elderly, or normotensives in Chinese population. Furthermore, age and blood pressure are still observed to be the most important risk factor of arterial stiffness.
Keywords: arterial stiffness, high‐normal albuminuria, hypertension, pulse wave velocity
1. INTRODUCTION
Microalbuminuria, a hazard factor of cardiovascular events in hypertensives, diabetics, and general population, 1 , 2 , 3 is defined as urinary albumin‐to‐creatinine ratio (UACR) between 30 and 300 mg/g. 4 It is generally believed that microalbuminuria is not only an indicator of glomerular endothelial damage representing microvascular injuries, but also reflects widespread vascular damage. 5 Actually, endothelial injury occurs at the same time in both large and small arteries. Arterial wall consists of endothelium, smooth muscle cells, adventitia fibroblasts, and extracellular matrix. 6 Recently, high‐normal albuminuria, a higher degree of albuminuria below the diagnostic threshold for microalbuminuria, was found to be related with adverse cardiovascular events, which can predict the morbidity and mortality of cardiovascular diseases. 7 , 8 Albuminuria is the earliest manifestation of vascular damage, and it is also presented throughout this pathological process. 5 While the earliest pathological change of arterial stiffness is vascular endothelial injury, 9 arterial stiffness goes simultaneously reflecting structural changes of large arteries. 10 Therefore, it is reasonable to assume that there is a connection between high‐normal albuminuria and arterial stiffness. However, the potential mechanisms by the effect of high‐normal albuminuria on cardiovascular diseases remain unclear.
Arterial stiffness is a significant index for rigidity of the arteries. Increased arterial stiffness is regarded as a predictor of cardiovascular diseases, including myocardial infarction, unstable angina pectoris, heart failure, and strokes. 11 Arterial stiffness may be assessed by measuring carotid‐femoral pulse wave velocity (cfPWV) and brachial‐ankle pulse wave velocity (baPWV). Compared with widely used baPWV, cfPWV has been recognized as a golden standard of arterial stiffness measurements. 12
In recent years, the association between high‐normal albuminuria and arterial stiffness has been investigated in several studies, and most studies have reported an independent positive association between them. 13 , 14 However, existing studies showed controversial results, 15 which suggested that arterial stiffness was independently related to urinary albumin excretion in African men, and this association was not found in Caucasian men. This inconsistency may be attributed to ethnic variation and the method for assessing arterial stiffness. Hence, it is necessary to investigate and have a deep look into this relationship in Chinese population. In China, the independent association between arterial stiffness assessed by baPWV and high‐normal albuminuria has been investigated in type 2 diabetics. 13 To our knowledge, no research has been reported to investigate this association using cfPWV in the general Chinese population. The objective of present study was to investigate whether there was a link between high‐normal albuminuria and cfPWV in the population from China.
2. METHODS
2.1. Statement of ethics
Our study was in accordance with the ethical standards formulated in the Helsinki Declaration, and the protocol was approved by Ethics Committee of the First Affiliated Hospital of Fujian Medical University. Informed consent was obtained from all subjects involved.
2.2. Subjects
This paper was a cross‐sectional clinical study. The protocol for investigation was established before clinical data collection, and it was performed by well‐trained physicians. The outpatients or inpatients who visited the department of General Medicine and Geriatrics in the First Affiliated Hospital of Fujian Medical University were included from January 2016 to March 2018. Exclusion criteria included the following: (a) secondary hypertension; (b) coronary heart disease and stroke within 3 months; (c) acute infection or other inflammatory diseases; (d) serum creatinine >2.5 mg/dL; (e) the level of aminotransferase and bilirubin ≥ 3 times the upper limit of the normal; (f) congestive heart failure, severe arrhythmia, ischemic heart disease, hypertrophic cardiomyopathy, valvular heart diseases, restrictive cardiomyopathy; (g) chronic consumptive diseases and malignant tumors; and (h) connective tissue diseases.
In this study, 2106 subjects were screened, of which 252 subjects were excluded according to the exclusion criteria, and 511 subjects were further removed due to missing UACR data. Finally, a total of 1343 participants were included and analyzed (Figure 1).
FIGURE 1.
Participant screening for final analysis
2.3. Clinical data collection
General information, physical examination data, and laboratory assays were collected. Subjects were interviewed regarding their age, gender, smoking habits, hypertension, and diabetes history. Body weight and height were measured, and body mass index (BMI) was calculated as the ratio of the bodyweight (kg) to the square of height (m2). Current smoking was defined as consuming no less than one cigarette everyday for at least six months. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg, and/or taking antihypertensive medications. 16 Diabetes was diagnosed on the basis of the standard recommended by the American Diabetes Association in 2014. 17 Blood pressure and heart rate was measured using automated sphygmomanometer (HEM‐7052; Omron, Kyoto, Japan). Blood sample for laboratory assays was taken after an eight‐hour overnight fasting. Serum biochemistry criteria such as blood urea nitrogen (BUN) and creatinine (CREA) were examined by enzymatic method using automatic biochemistry analyzer (ADVIA 2400; Siemens, Germany), and uric acid (UA), fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high‐density lipoprotein‐cholesterol (HDL‐C), and low‐density lipoprotein‐cholesterol (LDL‐C) were measured by oxidase method using automatic biochemistry analyzer (ADVIA 2400; Siemens, Germany). Estimated glomerular filtration rate (eGFR) was calculated by experimental equation: estimated GFR (eGFR) mL/min·1.73 m2 = 186 × (serum creatinine [mmol/L] ×0.0113)–1.154 × (Age)–0.203 (×0.742 for female). Glycosylated hemoglobin (HbA1c) was determined by high‐performance liquid chromatography (HPLC) using automatic analyzer (VARIANTTM‐II; Bio‐Rad, USA). First voiding point urine samples in the early morning were collected, and urinary albumin and creatine were detected within two hours. Urinary creatine concentration was detected by colorimetry assay (Boehringer Mannheim/Hitachi 717 analyzer, Germany), and urinary albumin was determined by immunoturbidimetry method (Roche P800 automatic analyzer, USA). The value of UACR was obtained by calculating the ratio of urinary albumin (mg) to urinary creatine (g). The normal albuminuria was defined as UACR < 30 mg/g, microalbuminuria 30 mg/g ≤ UACR<300 mg/g, and macroalbuminuria UACR ≥ 300 mg/g. Normal albuminuria was further classified based on the median of UACR, and the level above the median was defined as high‐normal albuminuria. All subjects were divided into four groups: low‐normal albuminuria, high‐normal albuminuria, microalbuminuria, and macroalbuminuria.
2.4. cfPWV measurements
cfPWV was measured using an automatic atherosclerosis and cardiac function detection system (Complior Analysis, France), as described previously by our team 18 , 19 . Briefly, subjects were asked to lay down on an examination table in the supine position after 10 minutes rest. The mean of two measurements was adopted for each subject. If the deviation between the first two measurements was >0.5 m/s, another measurement was performed, and the mean value of three measurements was adopted. The value of cfPWV (m/s) was obtained by calculating the ratio of carotid‐femoral transmit distance (m) to transmit time (s). The linear distance between right carotid artery and right femoral artery was measured by an inelastic tape. According to the recent expert consensus, 12 carotid‐femoral distance was calculated by the equation: d = 0.8 × (surface tape length), while the time (t) referred to the pulse transit time which was automatically measured by the device. Each measurement was repeated no less than 10 cardiac cycles.
2.5. Statistical analysis
Gaussian distribution test and homogeneity test of variance were carried out for continuous variables. And continuous variables were presented as mean ± standard deviation for Gaussian distribution or median and interquartile distance for skewed distribution. Categorical variables were presented as percentages and absolute values. One‐way ANOVA or chi‐squared test was performed to compare the differences between the clinical characteristics of multiple groups. Associations between cfPWV levels and anthropometric data, blood pressure, heart rate, and laboratory parameters were assessed by Pearson correlation analysis. The stepwise multivariate linear regression analysis was used to determine the independent variables related to cfPWV. Statistical significance was defined as two‐side P < .05. Our data were analyzed by SPSS 20.0 statistical software package (NY, USA).
3. RESULTS
3.1. Clinical characteristics of the subjects
A total of 1343 subjects were analyzed in current research (aged 58.9 ± 12.1 years, male 61.36%, hypertension 65.82%, diabetes 29.41%), 1161 of whom showed normal albuminuria (86.45%), 162 microalbuminuria (12.06%), and only 20 macroalbuminuria (1.49%). The level of albuminuria below median and above median within the normal albuminuria ranged from 0 to 6.35 mg/g and from 6.36 to 30 mg/g. Clinical and biochemical data of all participants were presented in Table 1. Age, systolic blood pressure, cfPWV, fasting plasma glucose, glycosylated hemoglobin, and UACR of subjects with high‐normal albuminuria were higher, and uric acid and creatinine were lower than those with low‐normal albuminuria. cfPWV was significantly increased with the increment of UACR (8.99 ± 1.81, 9.38 ± 2.03, 10.09 ± 2.33, 12.58 ± 4.44 m/s, P < .001)(Figure 2). There was no significant difference in body mass index, diastolic blood pressure, heart rate, total cholesterol, triglycerides, high‐density lipoprotein‐cholesterol, and low‐density lipoprotein‐cholesterol among groups.
TABLE 1.
Clinical characteristics of all subjects with different levels of albuminuria
| Variables | Normal albuminuria(UACR <30 mg/g) | Microalbuminuria(UACR ≥ 30 mg/g, but < 300 mg/g) | Macroalbuminuria UACR ≥ 300 mg/g) | P value | |
|---|---|---|---|---|---|
| Low‐normal albuminuria (0‐6.35 mg/g) | High‐normal albuminuria (6.36‐30 mg/g) | ||||
| N | 580 | 581 | 162 | 20 | |
| Male, n(%) | 416 (71.72%) | 298 (51.29%) | 99 (61.11%) | 11(55%) | <.001 |
| Age, years | 57.81 ± 11.09 | 59.81 ± 11.96 a | 59.93 ± 13.14 | 60.53 ± 19.18 | .017 |
| BMI, kg/m2 | 24.66 ± 2.95 | 24.69 ± 3.04 | 25.15 ± 3.26 a | 23.73 ± 2.9 | .202 |
| Smoking, n(%) | 198 (34.14%) | 144 (24.78%) | 53 (32.72%) | 4 (20%) | .003 |
| Hypertension, n(%) | 326 (56.21%) | 401 (69.02%) | 138 (85.19%) | 19 (95%) | <.001 |
| Diabetes, n(%) | 146 (25.17%) | 179 (30.81%) | 58 (35.8%) | 12 (60%) | <.001 |
| SBP, mmHg | 126.4 ± 14.83 | 133.6 ± 18.49 a | 143.16 ± 19.49 a | 157.53 ± 24.18 a | <.001 |
| DBP, mmHg | 73.12 ± 10.43 | 73.5 ± 14.94 | 74.41 ± 20.35 | 80.71 ± 10.88 | .277 |
| HR, bpm | 66.29 ± 10.71 | 66.65 ± 11.95 | 68.94 ± 13.65 a | 66.38 ± 10.77 | .198 |
| cfPWV, m/s | 8.99 ± 1.81 | 9.38 ± 2.03 a | 10.09 ± 2.33 a | 12.58 ± 4.44 a | <.001 |
| BUN, mmol/L | 5.29 ± 1.29 | 5.29 ± 1.37 | 5.62 ± 1.45 a | 7.46 ± 5.88 a | <.001 |
| UA, μmol/L | 377.89 ± 85.62 | 357.96 ± 94.81 a | 358.48 ± 110.68 | 408.55 ± 101.04 a | <.001 |
| CREA, μmol/L | 69.49 ± 13.52 | 64.99 ± 16.29 a | 68.91 ± 19.18 b | 91.44 ± 40.39 b | <.001 |
| FPG, mmol/L | 5.6 ± 1.46 | 6.04 ± 2.02 a | 6.58 ± 2.51 a | 6.04 ± 1.86 | <.001 |
| TC, mmol/L | 4.73 ± 1.14 | 4.78 ± 1.06 | 4.6 ± 1.05 | 4.97 ± 1.81 | .528 |
| TG, mmol/L | 1.29 (0.91‐1.77) | 1.3 (0.96‐1.8) | 1.36 (0.98‐1.78) | 1.23 (1.08‐1.82) | .551 |
| HDL‐C, mmol/L | 1.24 ± 0.38 | 1.26 ± 0.34 | 1.22 ± 0.34 | 1.31 ± 0.44 | .844 |
| LDL‐C, mmol/L | 2.97 ± 0.98 | 2.98 ± 0.99 | 2.89 ± 0.95 | 3.09 ± 1.39 | .846 |
| GFR, mL/min | 110.78 ± 25.59 | 109.43 ± 25.22 | 103.52 ± 24.73 a | 80.57 ± 26.08 a | <.001 |
| HbA1c, % | 5.97 ± 1.2 | 6.19 ± 1.35 a | 6.53 ± 1.56 a | 7.41 ± 2.31 a | <.001 |
| UACR, mg/g | 4.4 (3.38‐5.21) | 11.09 (8.06‐16.16) a | 52.64 (37.7‐89.31) a | 744.81 (457.06‐2189.25) a | <.001 |
Data are expressed as means ± SD or median (25th–75th).
Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; cfPWV, carotid‐femoral pulse wave velocity; CREA, creatinine; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; HR, heart rate; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; UACR, urinary albumin‐to‐creatinine ratio.
P < .05 vs low‐normal albuminuria group.
P < .05 vs high‐normal albuminuria group.
P < .05 vs microalbuminuria group.
FIGURE 2.
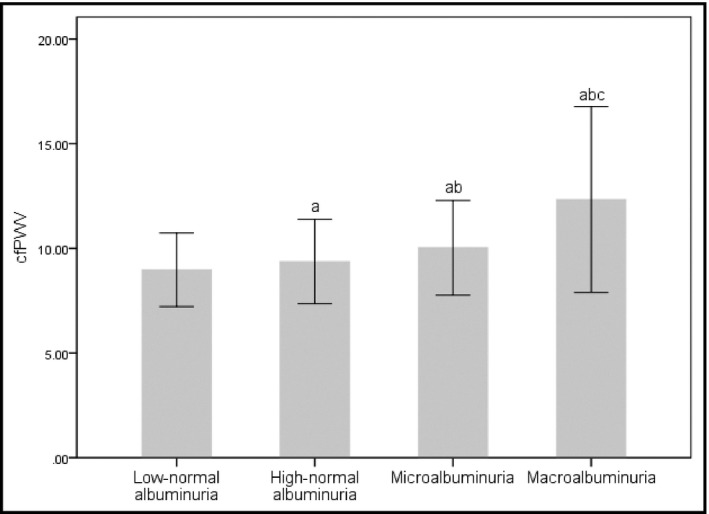
The comparison of carotid‐femoral pulse wave velocity (cfPWV) among subjects with different urinary albumin‐to‐creatinine ratio (UACR) groups. cfPWV increased progressively with the increase of UACR. a p < .05 vs low‐normal albuminuria, b p < .05 vs high‐normal albuminuria, c p < .05 vs microalbuminuria
3.2. Correlations between cfPWV and clinical profile
Pearson's correlation analysis showed that gender, age, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, blood urea nitrogen, creatinine, uric acid, fasting plasma glucose, total cholesterol, high‐density lipoprotein‐cholesterol, glomerular filtration rate, glycosylated hemoglobin, and LG‐UACR were significantly correlated with cfPWV in all subjects (Table 2). As shown in Figure 3, LG‐UACR level and creatinine (r = .214, p < .001) had a significantly mild positive correlation with cfPWV (r = .261, P < .001), while age (r = .486, P < .001), systolic blood pressure (r = .497, P < .001) moderately correlated with cfPWV, diastolic blood pressure (r = .067, P = .017), body mass index (r = .061, P = .031), heart rate (r = .092, P = .001), blood urea nitrogen (r = .186, P < .001), uric acid (r = .091, P < .001), fasting plasma glucose (r = .147, P < .001), and glycosylated hemoglobin (r = .198, P < .001) were only slightly positively correlated with cfPWV. However, total cholesterol (r = −.087, P = .002), high‐density lipoprotein‐cholesterol (r = −.102, P < .001), and glomerular filtration rate (r = −.254, P < .001) were mildly negatively correlated with cfPWV, and female gender (r = −.087, P = .002) seemed a protective factor for increased arterial stiffness.
TABLE 2.
Pearson correlation and stepwise multivariate linear regression analysis of clinical profile and cfPWV in all subjects
| Variables | Pearson Correlation | Stepwise multivariate linear regression | ||
|---|---|---|---|---|
| r | P Value | β (SE) | P value | |
| Gender (male:0; female:1) | −.087 | .002 | ‐ | ‐ |
| Age | .486 | <.001 | 0.392 (0.004) | <.001 |
| BMI | .061 | .031 | ‐ | ‐ |
| SBP | .497 | <.001 | 0.342 (0.003) | <.001 |
| DBP | .067 | .017 | ‐ | ‐ |
| HR | .092 | .001 | 0.117 (0.004) | <.001 |
| BUN | .186 | <.001 | ‐ | ‐ |
| CREA | .214 | <.001 | 0.154 (0.003) | <.001 |
| UA | .091 | .001 | ‐ | ‐ |
| FPG | .147 | <.001 | 0.084 (0.026) | <.001 |
| TC | −.087 | .002 | ‐ | ‐ |
| HDL‐C | −.102 | <.001 | ‐ | ‐ |
| GFR | −.254 | <.001 | ‐ | ‐ |
| HbA1c | .198 | <.001 | ‐ | ‐ |
| LG‐UACR | .261 | <.001 | 0.098 (0.106) | <.001 |
Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; CREA, creatinine; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; HR, heart rate; LG‐UACR, logarithmic transformed urinary albumin‐to‐creatinine ratio; SBP, systolic blood pressure; TC, total cholesterol; UA, uric acid.
FIGURE 3.

Correlation of LG‐UACR with cfPWV in a scatter plot (n = 1343)
3.3. Stepwise multivariate linear regression analysis of cfPWV
In order to correct the influences of confounding variables on cfPWV, stepwise multivariate linear regression analysis was performed to determine the independent variables related to cfPWV. As shown in Table 2, systolic blood pressure (β = 0.342, P < .001), age (β = 0.392, P < .001), creatinine (β = 0.154, P < .001), heart rate (β = 0.117, P < .001), LG‐UACR (β = 0.098, P < .001), and fasting plasma glucose (β = 0.084, P < .001) were observed to be associated independently with cfPWV in all subjects. In the low‐normal albuminuria group, age (β = 0.386, P < .001), systolic blood pressure (β = 0.268, P < .001), heart rate (β = 0.179, P < .001), uric acid (β = 0.100, P = .006), and fasting plasma glucose (β = 0.081, P = .027) had the independent relationship with cfPWV. In the high‐normal albuminuria group, systolic blood pressure (β = 0.382, P < .001), age (β = 0.421, P < .001), female gender (β=−0.097, P = .012), LG‐UACR (β = 0.093, P = .008), uric acid (β = 0.107, P = .006), and fasting plasma glucose (β = 0.095, P = .007) were independently associated with cfPWV. In the microalbuminuria group, age (β = 0.482, P < .001) and systolic blood pressure (β = 0.420, P < .001) were independent related variables affecting cfPWV. In the macroalbuminuria group, age (β = 0.652, P < .001) and LG‐UACR (β = 0.485, P = .002) correlated independently with cfPWV (Table 3). After further stratification in the high‐normal albuminuria group according to gender, age, the presence of hypertension or diabetes, a significant correlation between LG‐UACR and cfPWV was revealed in male (β = 0.141, P = .004), subjects over 65 years (β = 0.151, P = .009) or normotensives (β = 0.149, P = .019), and this association was not observed in either diabetics or non‐diabetics (Table 4).
TABLE 3.
Stepwise multivariate linear regression analysis of cfPWV in different levels of UACR
| Subgroups (N) | Variables | β (SE) | P value |
|---|---|---|---|
| Low‐normal albuminuria | Age | 0.386 (0.006) | <.001 |
| (580) | SBP | 0.268 (0.005) | <.001 |
| HR | 0.179 (0.006) | <.001 | |
| UA | 0.100 (0.001) | .006 | |
| FPG | 0.081 (0.045) | .027 | |
| High‐normal albuminuria | SBP | 0.382 (0.004) | <.001 |
| (581) | Age | 0.421 (0.006) | <.001 |
| Gender | ‐0.097 (0.157) | .012 | |
| LG‐UACR | 0.093 (0.391) | .008 | |
| UA | 0.107 (0.001) | .006 | |
| FPG | 0.095 (0.036) | .007 | |
| Microalbuminuria | Age | 0.482 (0.011) | <.001 |
| (162) | SBP | 0.420 (0.008) | <.001 |
| Macroalbuminuria | Age | 0.652 (0.029) | <.001 |
| (20) | LG‐UACR | 0.485 (1.501) | .002 |
Abbreviations: FPG, fasting plasma glucose; HR, heart rate; LG‐UACR, logarithmic transformed urinary albumin‐to‐creatinine ratio; SBP, systolic blood pressure; UA, uric acid.
TABLE 4.
Stepwise multivariate linear regression analysis with cfPWV as a dependent variable and LG‐UACR as an independent variable in high‐normal albuminuria group stratified by gender, age and presence of hypertension or diabetes
| Subgroups (N) | β (SE) | P value |
|---|---|---|
| Gender | ||
| Male (298) | 0.141 (0.546) | .004 |
| Female (283) | ‐ | ‐ |
| Age | ||
| <65 (391) | ‐ | ‐ |
| ≥65 (190) | 0.151 (0.732) | .009 |
| Hypertension | ||
| No (180) | 0.149 (0.606) | .019 |
| Yes (401) | ‐ | ‐ |
| Diabetes | ||
| No (402) | ‐ | ‐ |
| Yes (179) | ‐ | ‐ |
4. DISCUSSION
In the present study, high‐normal albuminuria was observed to be associated with cfPWV in the population from China. With the increasing of UACR, the level of cfPWV was increased gradually. In addition, systolic blood pressure, age, creatinine, heart rate, LG‐UACR, and fasting plasma glucose correlated independently with cfPWV in all subjects. After further stratification, it was found that LG‐UACR also correlated independently with cfPWV in high‐normal albuminuria group, especially in male, the elderly, or normotensives. Our data suggested that even if the UACR level was not high enough to meet the diagnostic criteria for microalbuminuria, it was still of clinical significance to predict the presence of cardiovascular diseases.
Microalbuminuria is strongly associated with the morbidity and mortality of cardiovascular diseases in hypertensives, diabetics, and general population. 1 , 2 , 3 Epidemiological data suggested that UACR levels are low in the general population, usually below the diagnostic criteria for microalbuminuria, 20 which was consistent with our data, only a small percentage of subjects were diagnosed with microalbuminuria (12.06%) or macroalbuminuria (1.49%). High‐normal albuminuria, which was below the diagnostic criteria for microalbuminuria, was reported to be capable of predicting the morbidity and mortality of cardiovascular diseases 7 , and UACR level was proved to be related with pulse wave velocity as well. 13 , 14 Pulse wave velocity is recognized as an indicator of generalized arterial stiffness and an independent risk factor for adverse cardiovascular events. 21 In the study of Huang et al, 13 it was indicated that the level of UACR in high normal range was associated with atherosclerosis in young adult with type 2 diabetes. In the study of Liu et al, 14 high‐normal albuminuria was found to be associated with atherosclerosis and increased risk in adverse cardiac events in diabetics. Conversely, arterial stiffness was independently related to UACR in African men with normal renal function, but not existed in Caucasian men. 15 To the best of our knowledge, no study has investigated this association using carotid‐femoral pulse wave velocity, a golden standard of arterial stiffness, in Chinese population. If this association persists in the population from China, high‐normal albuminuria may serve as a biomarker to identify early mild vascular damage. Our data showed that cfPWV was increased with the increment of UACR. In Pearson’s correlation analysis, LG‐UACR was positively correlated with cfPWV (r = .261, P < .001). However, after further adjusting for blood pressure and other traditional cardiovascular risk factors in stepwise multivariate linear regression analysis, the strength of the relationship between LG‐UACR and cfPWV was attenuated (β = 0.098, P < .001). A possible explanation is that cfPWV is partially affected by hemodynamic mechanisms. PWV has been reported to be pressure‐dependent and varied with blood pressure fluctuation, but in reality, transient blood pressure fluctuation did not change the intrinsic artery elasticity. 22 So, we speculate that adjustments in blood pressure at the time of measurement could result in underestimation of the association between LG‐UACR and cfPWV. Although this correlation was modest, it was independent of other cardiovascular risk factors. The positive correlation between UACR and cfPWV was observed in high‐normal albuminuria group and macroalbuminuria group, even after adjusting for age, gender, body mass index, blood pressure, fasting plasma glucose, total cholesterol, and other traditional cardiovascular risk factors by stepwise multivariate linear regression analysis. In addition, the correlation between UACR and cfPWV became weak in low‐normal albuminuria group and microalbuminuria group, suggesting that other factors such as age or blood pressure had greater influence on cfPWV than UACR. The small sample size might be another reason explaining the correlation between UACR and cfPWV was not observed in the microalbuminuria group. In the subgroups stratified by gender, age, and the presence of hypertension or diabetes, high‐normal albuminuria was an independent factor of cfPWV in male, subjects over 65 years old or normotensives. The correlation between albuminuria and atherosclerosis in different gender remains controversial. A cross‐sectional study of Japanese adult males indicated that high‐normal albuminuria was associated with asymptomatic atherosclerosis. 23 In contrast, Huang et al. 24 indicated that the significant association between UACR and intima‐media thickness of carotid artery only observed in females. Difference between gender regarding this association may be related to different study population. Our previous study suggested that the association between uric acid and flow‐mediated dilatation, a marker of endothelial function, only exists in male hypertensives with metabolic syndrome. 25 This difference between the gender may be explained by the protective effects of estrogen on cardiovascular system. In the study of Kong et al, 26 slight elevation of albuminuria was associated with carotid artery intima‐media thickness in elderly people with normal renal function, which was similar to our current result. Our data showed that high‐normal albuminuria was associated with cfPWV in subjects with age ≥ 65 years, whereas this relationship became weak in subjects with age <65 years. In addition, we also observed a correlation between high‐normal albuminuria and cfPWV in normotensives; however, there was no significant correlation in hypertensives. Ma et al. 27 reported that high‐normal albuminuria was independently correlated with carotid atherosclerosis in normotensive Chinese individuals, which was, to some extend, similar to our findings. The mechanisms linking high‐normal albuminuria with arterial stiffness remain unclear, but it is generally believed that excretion of albuminuria represents generalized vascular damage rather than that albuminuria induces arterial stiffness directly. 5 Increased arterial stiffness can reduce the aortic‐peripheral pressure gradient and transmit high‐pressure pulsatile energy to kidney capillary blood vessels, which could lead to hyperfunction of glomerular filtration, glomerulosclerosis, and decreased renal function gradually. 28
In this study, systolic blood pressure, age, serum creatinine, resting heart rate, and fasting plasma glucose were observed to be associated independently with cfPWV in all participants as well, which was in line with some previous clinical studies. Our previous study showed that blood pressure and age were important in the progression of arterial stiffness, and mechanical stress and “vascular aging” may be the mediator of arterial stiffness, respectively. 18 Serum creatinine, a common indicator of renal function, comes from the decomposition of creatine in human muscle. It is excreted to urine after glomerular filtration. Gu et al. 29 found that the level of baPWV was increased with the elevation of creatinine, and creatinine was an independent risk factor of arterial stiffness in hypertensive. Resting heart rate has recently been regarded as cardiovascular risk factor in the general population as well as in individual persons suffered from cardiovascular diseases. 30 , 31 Many previous researches suggested that heart rate was positively correlated with arterial stiffness. 32 , 33 Fasting plasma glucose was also reported to be associated with vascular damage in some previous studies. 34 , 35 Elevated levels of plasma glucose has been reported to promote the formation of advanced glycation end products (AGEs), and higher AGEs may enhance the cross‐linking of collagen; thus, the elasticity of the vascular wall was reduced and arterial stiffness was increased. 36 , 37
These findings of our study may have significant clinical implications for those patients with high‐normal albuminuria. Increased arterial stiffness is frequently associated with the occurrence of myocardial infarction, unstable angina, heart failure, and stroke. 11 If high‐normal albuminuria is an early indicator of arterial stiffness in Chinese population, particularly in male, elderly, or normotensives, people with high‐normal albuminuria should accept screening for arterial stiffness or other major cardiovascular diseases and initiate intensive multifactorial interventions including non‐pharmacological interventions and pharmaceutical interventions in early stage. 38 These measures will be beneficial to primary prevention of cardiovascular diseases in the population from China.
There were still some limitations in this study. Firstly, our study was a cross‐sectional study and the sample size was moderate, relatively small. Secondly, the subjects in our study were outpatients or inpatients from Fujian Province of China; hence, selection bias may not be avoided and the results were underrepresented. Additionally, a moderate sample size may, to some extend, reduce the sampling bias. Thirdly, our study did not exclude the possible influence of medications on the measurement of arterial stiffness. Therefore, a cohort study with sufficient samples is necessary to confirm our conclusions in the future.
In conclusion, the level of UACR was associated with the increase of arterial stiffness in subjects with albuminuria, even when the level of UACR did not reach the diagnostic threshold for microalbuminuria. It was suggested that the high‐normal albuminuria may serve as a biomarker to identify the subjects with vascular damage, especially in male, elderly, or normotensives in the population from China. Furthermore, age and blood pressure are still found to be the most important contributor to arterial stiffness and cannot be ignored.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
C.Y. and L.X. participated in the design of this research. C.Y., J.G., L.L., G.L., H.W., and W.C. collected the clinical data. C.Y., J.G., and T.W. analyzed the results. C.Y. wrote the manuscript. C.Y., T.W., J.G., and L.X. revised and provided critical comments. All authors read and approved the manuscript.
Ye C, Gong J, Wang T, et al. Relationship between high‐normal albuminuria and arterial stiffness in Chinese population. J Clin Hypertens. 2020;22:1674–1681. 10.1111/jch.13979
Funding information
This work was supported by National Natural Science Foundation of China [Grand NO. 81873537], Startup Fund for scientific research, Fujian Medical University [Grant NO. 2017XQ2039 and 2018QH2034].
REFERENCES
- 1. Mule G, Castiglia A, Cusumano C et al Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol. 2017;956:279‐306. [DOI] [PubMed] [Google Scholar]
- 2. Swoboda PP, McDiarmid AK, Erhayiem B et al Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6:e005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konno S, Munakata M. Moderately increased albuminuria is an independent risk factor of cardiovascular events in the general Japanese population under 75 years of age: the Watari study. PLoS One. 2015;10:e0123893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inker LA, Astor BC, Fox CH et al KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713‐735. [DOI] [PubMed] [Google Scholar]
- 5. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, Jensen T, Kofoed‐Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219‐226. [DOI] [PubMed] [Google Scholar]
- 6. Wang D, Wang Z, Zhang L, Wang Y. Roles of cells from the arterial vessel wall in atherosclerosis. Mediators Inflamm. 2017;2017:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka F, Komi R, Makita S et al Low‐grade albuminuria and incidence of cardiovascular disease and all‐cause mortality in nondiabetic and normotensive individuals. J Hypertens. 2016;34(3):506‐512; discussion 512. [DOI] [PubMed] [Google Scholar]
- 8. Wang T, Zhong H, Lian G et al Low‐grade albuminuria is associated with left ventricular hypertrophy and diastolic dysfunction in patients with hypertension. Kidney Blood Press Res. 2019;44:590‐603. [DOI] [PubMed] [Google Scholar]
- 9. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou T, Huang X, Cai X, Xie L. Combined treatment of irbesartan and diltiazem ameliorates endothelium dependent vasodilatation in hypertensives. Clin Exp Hypertens. 2017;39:612‐618. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell GF, Hwang SJ, Vasan RS et al Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Bortel LM, Laurent S, Boutouyrie P et al Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 13. Huang L, Yang L, Zhang S, Liu D, Yan X, Yan S. Low‐grade albuminuria associated with brachial‐ankle pulse wave velocity in young adults with type 2 diabetes mellitus in China. Diabetes Metab Res Rev. 2015;31:262‐268. [DOI] [PubMed] [Google Scholar]
- 14. Liu JJ, Tavintharan S, Yeoh LY et al High normal albuminuria is independently associated with aortic stiffness in patients with Type 2 diabetes. Diabet Med. 2014;31:1199‐1204. [DOI] [PubMed] [Google Scholar]
- 15. Schutte R, Schutte AE, Huisman HW et al Arterial stiffness, ambulatory blood pressure and low‐grade albuminuria in non‐diabetic African and Caucasian men: the SABPA study. Hypertens Res. 2011;34:862‐868. [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W et al 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes A. Standards of medical care in diabetes–2014. Diabetes care. 2014;37(Suppl 1):S14‐S80. [DOI] [PubMed] [Google Scholar]
- 18. Gong J, Xie Q, Han Y et al Relationship between components of metabolic syndrome and arterial stiffness in Chinese hypertensives. Clin Exp Hypertens. 2020;42:146‐152. [DOI] [PubMed] [Google Scholar]
- 19. Wang T, Xu G, Cai X, Gong J, Xie Q, Xie L. Association of homocysteine with carotid‐femoral pulse wave velocity in a southern Chinese population. Aging (Albany NY). 2019;11:9709‐9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanna DB, Xu S, Melamed ML et al Association of albuminuria with cardiac dysfunction in US hispanics/latinos. Am J Cardiol. 2017;119:2073‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lehmann ED. Clinical value of aortic pulse‐wave velocity measurement. Lancet. 1999;354:528‐529. [DOI] [PubMed] [Google Scholar]
- 22. Spronck B, Heusinkveld MH, Vanmolkot FH et al Pressure‐dependence of arterial stiffness: potential clinical implications. J Hypertens. 2015;33:330‐338. [DOI] [PubMed] [Google Scholar]
- 23. Kimura T, Ueno T, Doi S et al High‐normal albuminuria is associated with subclinical atherosclerosis in male population with estimated glomerular filtration rate >/=60 mL/min/1.73 m2: A cross‐sectional study. PLoS One. 2019;14:e0218290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y, Chen Y, Xu M et al Low‐grade albuminuria is associated with carotid intima‐media thickness in Chinese type 2 diabetic patients. J Clin Endocrinol Metab. 2010;95:5122‐5128. [DOI] [PubMed] [Google Scholar]
- 25. Huang X, Cai X, Zheng W, Shen Y, Xie L. Relationship between uric acid and endothelial function in hypertensive patients with metabolic syndrome. J Clin Exp Cardiolog. 2016;07:416‐422. [Google Scholar]
- 26. Kong X, Jia X, Wei Y et al Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima‐media in elderly patients with normal renal function. BMC Nephrol. 2012;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma H, Lin H, Hofman A et al Low‐grade albuminuria is associated with carotid atherosclerosis in normotensive and euglycemic Chinese middle‐aged and elderly adults: the Shanghai Changfeng Study. Atherosclerosis. 2013;228:237‐242. [DOI] [PubMed] [Google Scholar]
- 28. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388‐400. [DOI] [PubMed] [Google Scholar]
- 29. Gu X, Zhao L, Zhu J et al Serum Mimecan Is Associated With Arterial Stiffness in Hypertensive Patients. J Am Heart Assoc. 2015;4:e002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palatini P, Rosei EA, Casiglia E et al Management of the hypertensive patient with elevated heart rate: Statement of the Second Consensus Conference endorsed by the European Society of Hypertension. J Hypertens. 2016;34:813‐821. [DOI] [PubMed] [Google Scholar]
- 31. Tomlinson B, Sritara P, Lopez E, Dalal J, Erwinanto E, Pancholia AK. Hypertension and elevated heart rate: focus on the Asia Pacific region. J Hypertens. 2016;34:2330‐2332. [DOI] [PubMed] [Google Scholar]
- 32. Su HM, Lee KT, Chu CS et al Effects of heart rate on brachial‐ankle pulse wave velocity and ankle‐brachial pressure index in patients without significant organic heart disease. Angiology. 2007;58:67‐74. [DOI] [PubMed] [Google Scholar]
- 33. Park BJ, Lee HR, Shim JY, Lee JH, Jung DH, Lee YJ. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis. 2010;103:246‐252. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Liu L, Zhou Y et al Increased fasting glucose and the prevalence of arterial stiffness: a cross‐sectional study in Chinese adults. Neurol Res. 2014;36:427‐433. [DOI] [PubMed] [Google Scholar]
- 35. Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high‐normal glucose levels and in subjects with pre‐diabetes. Cardiovasc Diabetol. 2011;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation ‐ a mini‐review. Gerontology. 2012;58:227‐237. [DOI] [PubMed] [Google Scholar]
- 37. van Eupen MG, Schram MT, van Sloten TT et al Skin autofluorescence and pentosidine are associated with aortic stiffening: the maastricht study. Hypertension. 2016;68:956‐963. [DOI] [PubMed] [Google Scholar]
- 38. Nemcsik J, Cseprekál O, Tislér A. Measurement of arterial stiffness: a novel tool of risk stratification in hypertension. Adv Exp Med Biol. 2017;956:475‐488. [DOI] [PubMed] [Google Scholar]


