Abstract
The decrease in blood pressure is thought to play an important role for the renoprotective effects of sodium–glucose cotransporter 2 inhibitors in patients with diabetes mellitus. However, their influence on blood pressure at home has not been well studied. The aim of this study is to clarify how long‐term use of sodium–glucose cotransporter 2 inhibitors influence on blood pressure both at the office and at home, and the kidney function. We retrospectively analyzed 102 patients with type 2 diabetes mellitus and chronic kidney disease to whom sodium–glucose cotransporter 2 inhibitors were administered for more than 1 year, and whose blood pressure were monitored both at the office and at home. The blood pressure at the office and at home significantly decreased, and there was a significant positive correlation between both blood pressure values. Controlled, white‐coat, and sustained hypertension were observed in 9.8%, 14.7%, and 55.9% of the patients at the beginning of the treatment, which changed to 16.7%, 15.7%, and 48.0% at the time of the survey, however, the ratio of masked hypertension was not changed (19.6%). The cutoff value of mean arterial pressure at home after treatment for the improvement of urine albumin to creatinine ratio was 92.0 mm Hg, with 54.1% of sensitivity and 60.0% of specificity. Sodium–glucose cotransporter 2 inhibitors can be useful for the strict management of blood pressures both at the office and at home. The decrease in blood pressure at home by this treatment might be related to the improvement of diabetic nephropathy.
1. INTRODUCTION
Sodium–glucose cotransporter 2 inhibitors (SGLT2Is) are novel oral hypoglycemic agents that increase the urinary excretion of glucose by the inhibition of SGLT2 in the renal proximal tubules. The loss of calories in the form of urinary glucose excretion results in the loss of body weight (BW) and in pleiotropic effects, such as a decrease in blood pressure (BP) and improvements in dyslipidemia or liver function. 1 These pleiotropic effects of SGLT2Is are drawing attention for the treatment of diabetes mellitus (DM) patients. Some large‐scale trials, such as the EMPA‐REG OUTCOME trial, 2 CANVAS/CANVAS‐R program, 3 and DECLARE‐TIMI58, 4 that investigated the effects of SGLT2Is on cardiovascular outcomes revealed significant improvements in cardiovascular events and even in diabetic nephropathy in patients with type 2 DM. In our previous study, we confirmed the beneficial renal effects of SGLT2 inhibitors in Japanese patients with type 2 DM and CKD. 5 , 6 In a Japanese prospective study, dapagliflozin significantly improved albuminuria levels and the home BP profile in T2DM and diabetic nephropathy patients, and the improved morning home systolic BP was associated with an albuminuria reduction. 7 From these studies, SGLT2Is are thought to be suitable for the treatment of diabetic patients with CKD.
The BP lowering effect is one of the most important pleiotropic effects of SGLT2Is because strict BP management is required in patients with DM, especially in those with a history of cardiovascular events or CKD. According to the current Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014 guidelines), 8 home BP monitoring is recommended because it has a stronger mortality predictive power than BP measurements at the office, and the target BP is <130/80 mm Hg at the office and <125/75 mm Hg at home in patients with hypertension and DM. However, only a few reports have evaluated the relationship between the renal effects of SGLT2Is and their impact on home BP monitoring.
Here, we retrospectively studied the influence of SGLT2Is on BP, both at the office and at home in the early morning in Japanese patients with type 2 DM and CKD.
2. MATERIALS AND METHODS
2.1. Study design and study population
This study is a sub‐analysis of the studies that we previously reported, therefore, the method of the collection of the data used in this study has already been described in detail in our previous report. 6 In short, the main study subjects were 797 Type 2 DM patients who visited the clinics of the Kanagawa Physicians Association for clinical consultation (16 diabetologists, 7 nephrologists, 7 cardiologists, and 6 miscellaneous specialties) between October and December 2018. The inclusion criteria were as follows: T2DM patients: (a) commenced treatment with SGLT2I for the first time more than 1 year before enrollment; (b) CKD, as defined by the Kidney Disease Outcome Quality Initiativ (K/DOQI) clinical practice guidelines for CKD 9 ; and (c) more than 20 years of age. The following exclusion criteria were also applied: (a) type 1 DM; (b) undergoing chronic dialysis; (c) severe liver dysfunction, such as liver cirrhosis or severe infection; (d) terminal stage malignancy; (e) pregnancy; (f) irregular use of SGLT2Is; and (g) individuals who showed an intention to opt‐out. Based on the above criteria, 34 patients were excluded from the study.
Thus, the main study included data from 763 patients. Among them, we analyzed BP both at the office and at home in the early morning in 102 patients who performed the measurement of home BP monitoring in this sub‐analysis study. The following parameters were recorded at both the time of the initiation of SGLT2I treatment and at the time of the survey: age, sex, BW, BP (both systolic, SBP; and diastolic, DBP), serum Cr, hemoglobinA1c(HbA1c), and ACR or qualitative proteinuria. The values of eGFR were calculated using the following formula: eGFR (ml/min/1.73 m2) = 194 × age–0.287 × serum creatinine–1.094 (×0.739 for women). 10
2.2. Ethical approval
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the special ethics committee of Kanagawa Medical Association in Japan (Krec304401, 6 March 2018).
2.3. Data collection
The median duration from the initiation of SGLT2Is treatment to the survey was 30.0 months (range from 12 to 55 months). The changes in clinical findings at the time of the initiation of SGLT2Is treatment and the time of the survey were compared. To analyze the data depending on the level of albuminuria, 83 patients were divided into three groups: normoalbuminuria (ACR <30 mg/gCr), micro‐albuminuria (30 ≤ACR <300 mg/gCr), and macroalbuminuria (ACR ≦300 mg/gCr).
2.4. BP measurements at the office and at home
The methods used for BP measurements were described in our previous report. 11 We collected data based on the observations of the general practitioners (GPs), and BP at home was measured according to the JSH 2014 guidelines. 8 Patients were instructed to measure their BP at home in a sitting position in an appropriate environment after resting for 1–2 min, with their legs not crossed, and with the position of the upper arm cuffs maintained at the level of the heart. Patients were instructed to measure their home BP once or twice in the morning ( this means within 1 hour after wake‐up according JSH 2014, 8 and there are no restrictions on when to wake‐up), and the average data of several days were collected.
Based on the JSH 2014 guidelines, 8 oscillometric devices with upper arm cuffs are widely used in Japan for home BP monitoring. Because we did not insist on a single device for this retrospective multi‐facility study, different cuff sizes might have been used. BP measurements at the office were performed at each institution using their own validated cuff oscillometric devices. According to the JSH 2014 guidelines 8 (11), BP at the office was measured in a quiet environment after rest for a few minutes in the seated position on a chair with the legs not crossed. When two consecutive measurements were taken 1–2 min apart, the average of the two measurements was defined as BP at the office. To analyze BP distribution, patients were divided into 4 groups based on the target BP both at the office and at home: controlled hypertension group, BP at the office <130/80 and at home <125/75 mm Hg; masked hypertension group, BP at the office <130/80 and at home ≧ 125/75 mm Hg; white‐coat hypertension group, BP at the office ≧ 130/80 and at home <125/75 mm Hg; and sustained hypertension group, BP at the office ≥ 130/80 and at home ≧ 125/75 mm Hg.
2.5. Statistical analysis
SPSS Statistics version 25.0 (IBM Inc) was used for statistical analyses. The data were analyzed for parametric distribution (normal distribution and equal variance). If the data were parametrically distributed, significance tests would be performed utilizing parametric procedures; however, if the data were not parametric, non‐parametric procedures would be applied. Comparisons between the two groups of data were performed according to the paired t‐test for parametric data and Wilcoxon signed‐rank test for non‐parametric data. We performed a chi‐square test to compare the distribution of BP control status divided into four groups; controlled, masked, white‐coat, and sustained hypertension, before and after SGLT2I treatment. A p‐value lower than .05 was considered significant.
The correlation between two continuous variables was analyzed using Spearman's rank correlation coefficient test. The chi‐square test was performed for the evaluation of the distribution of the four groups based on the target BP, both at the office and at home.
The overall prediction accuracy of BP at office before SGLT2I treatment was examined by the analysis of the Receiver operating characteristic (ROC) curve and the improvement of BP. The cutoff value of BP at office before treatment for further analysis was determined from the results of the ROC analysis. The patients were divided into two groups: patients with BP at office before treatment of more than the cutoff and those with BP at office before treatment less than the cut‐off value determined by ROC analysis.
Further, the overall prediction accuracy of BP at home after SGLT2I treatment was examined by the analysis of the ROC curve and the improvement of ACR. The cutoff value of BP at home after treatment for further analysis was determined from the results of the ROC analysis. The patients were divided into two groups: patients with BP at home after treatment of more than the cutoff and those with BP at home after treatment less than the cutoff value determined by ROC analysis.
Multiple linear regression analysis was also performed to evaluate independent predictors of the change in MAP at the office.
We chose the clinical variables that might correlated with ΔMAP; age, gender, administration periods, MAP at baseline, BW at baseline, ΔBW, eGFR at baseline, ΔeGFR, LNACR at baseline, ΔLNACR, HbA1c at baseline, ΔHbA1c, concomitant antihypertensive agents, hypoglycemic agents and statins. Then, we inputted these variables into a stepwise multiple linear regression to establish the significant determination of ΔMAP. Variance inflation factors (VIF) were also used to assess multicollinearity with the data, and the VIF exceeding 10 is regarded as indication serious multicollinearity, and values > 4.0 may be a cause for concern.
Data were expressed as means and standard deviations in the cases of parametric data, and as medians and [25%, 75%] interquartile ranges (IQR) in the cases of non‐parametric data.
3. RESULTS
3.1. Patient demographics
The basic demographic data of the patients are shown in Table 1. The mean age was 63.2 ± 11.2 years (range from 29 to 89 years), with 69 males and 33 females. The median ACR value was 26.8 [12.2, 58.5] mg/gCr. Normo‐, micro‐, and macro‐albuminuria were observed in 44 (53%), 33 (40%), and 6 (7%) patients, respectively. In this study, the history of DM was not surveyed.
TABLE 1.
Background data of patients and SGLT2Is, along with concomitant medications
| n = 102 | ||
|---|---|---|
| Age (years) | 63.2 ± 11.2 | |
| Sex; males | 69 (67.6%) | |
| SGLT2I | n a | Daily dosage |
| Ipragliflozin | 9 | 25 mg, n = 3; 50 mg, n = 6 |
| Dapagliflozin | 26 | 25 mg, n = 17; 10 mg, n = 9 |
| Tofogliflozin | 13 | 10 mg, n = 1; 20 mg, n = 12 |
| Luseogliflozin | 10 | 2.5 mg, n = 7; 5 mg, n = 3 |
| Canagliflozin | 12 | all 100 mg |
| Empagliflozin | 24 | 10 mg; n = 21, 25 mg, n = 3 |
| Administration periods of SGLT2I (months) | 30.0 [22, 40] | |
| Concomitant medications at survey | ||
| Glucose‐lowering agents | ||
| SGLT2I alone | 20 (19.6%) | |
| DPP4 inhibitors | 55 (53.9%) | |
| Sulphonylureas | 20 (19.6%) | |
| Metformin | 37 (36.3%) | |
| Insulin | 24 (23.5%) | |
| GLP‐1 receptor agonists | 11 (10.8%) | |
| Pioglitazone | 15 (14.7%) | |
| Others (αGI, glinides etc) | 18 (17.6%) | |
| Antihypertensive agents | ||
| None | 22 (21.6%) | |
| RAS inhibitors | 58 (56.9%) | |
| Ca channel blockers | 58 (56.9%) | |
| Aldosterone blockers | 22 (21.6%) | |
| Diuretics(thiazides) | 6 (5.9%) | |
| Diuretics(lupus) | 1 (1.0%) | |
| β‐blocker | 19 (18.6%) | |
| Others (α‐blockers, etc) | 4 (3.9%) | |
| Statins | 73 (71.6%) | |
Data; mean ± SD, n (%), or medians [25% IQR,75% IQR].
Abbreviations: αGI, alpha‐glucosidase inhibitor, DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; IQR, interquartile ranges; RAS, renin–angiotensin system; SGLT2I, sodium–glucose cotransporter 2 inhibitor.
The SGLT2I was changed during the administration period in 8 cases.
In Japan, six SGLT2Is are currently approved and their usage is also shown in Table 1. In eight cases, SGLT2I was changed to another therapeutic agent during the study period. Concomitant medications during the survey are shown in Table 1. Twenty patients (19.6%) were prescribed SGLT2Is alone, while additional glucose‐lowering agents were administered to all the remaining patients, resulting in an average of 1.9 drugs per patient. No antihypertensive agent was prescribed to 22 patients (21.6%), while at least one agent was prescribed to all the remaining patients (average, 1.6 drugs per patient).
3.2. Comparison of clinical findings
Comparison of clinical findings between the time of the initiation of SGLT2I treatment and the time of the survey is shown in Table 2. The value of HbA1c and BW decreased significantly (p < .001, respectively). Systolic BP both at the office and at home, diastolic BP at home, and mean arterial pressure (MAP) at home were significantly decreased (p < .05, p < .005, p < .01, and p < .01, respectively). There were no significant changes in ACR or logarithmic value of ACR (LNACR), however, eGFR significantly decreased from 74.7 ± 24.1 to 70.7 ± 22.0 ml/min/1.73 m2 (p < .01), as did creatinine clearance from 106.1 ± 49.5 to 99.2 ± 45.7 ml/min (p < .01).
TABLE 2.
Comparison of clinical findings at the time of the initiation of SGLT2I treatment and at the time of the survey
| At baseline | At survey | p‐value | |
|---|---|---|---|
| BP at the office (mm Hg) | |||
| Systolic | 136.7 ± 16.1 | 132.9 ± 17.8 | <.05 |
| Diastolic | 77.1 ± 12.7 | 76.6 ± 12.3 | n.s. |
| MAP | 97.0 ± 12.1 | 95.4 ± 12.1 | n.s. |
| BP at home (mm Hg) | |||
| Systolic | 128.7 ± 11.8 | 126.3 ± 9.1 | <.05 |
| Diastolic | 76.6 ± 10.1 | 74.2 ± 9.1 | <.01 |
| MAP | 94.0 ± 9.9 | 91.5 ± 7.9 | <.01 |
| ACR (mg/gCr) | 26.8 [12.2, 58.5] | 26.9 [10.3, 68.0] | n.s. |
| Logarithmic value of ACR | 1.50 ± 0.59 | 1.44 ± 0.63 | n.s. |
| eGFR (ml/min/1.73 m2) | 74.7 ± 24.1 | 70.7 ± 22.0 | <.01 |
| HbA1c (mmol/mol (%)) | 58.7 ± 13.2(7.5 ± 1.2) | 53.0 ± 10.7(7.0 ± 1.0%) | <.01 |
| BW (kg) | 76.8 ± 14.5 | 74.2 ± 13.7 | <.01 |
| BMI | 28.4 ± 4.4 | 27.5 ± 4.2 | <.01 |
Data; mean ± SD or medians [25% IQR,75% IQR].
Abbreviations: ACR, urine albumin to creatinine ratio; BP, blood pressure; BW, body weight; IQR, interquartile ranges; MAP, mean arterial pressure; n.s., not significant; SGLT2I, sodium–glucose cotransporter 2 inhibitor.
3.3. Distribution of BP categories at the initiation of SGLT2I treatment and at the time of the survey
Figure 1 shows that the number of patients with continuous hypertension was significantly decreased and that of those with controlled hypertension significantly increased (p < .01). In contrast, the number of those with masked or white‐coat hypertension did not change.
FIGURE 1.
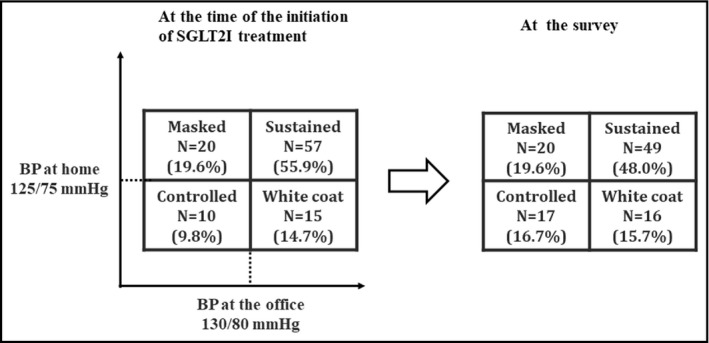
Distribution of BP categories at the initiation of SGLT2I treatment and at the survey. BP, blood pressure; Controlled, controlled hypertension group (BP at the office <130/80 and BP at home <125/75); Masked, masked hypertension group (BP at the office <130/80 and BP at home ≥125/75); SGLT2I, Sodium–glucose cotransporter 2 inhibitor; Sustained, sustained hypertension group (BP at the office ≥130/80 and BP at home ≥125/75); White coat, white‐coat hypertension group (BP at the office ≥130/80 and BP at home <125/75)
3.4. Changes in MAP at the office and at home with SGLT2 inhibitor treatment depending on BP status
Figure 2 and Table S1 show the change in MAP at the office and at home induced by SGLT2I treatment, depending on the BP status at baseline. MAP both at the office and at home significantly decreased after SGLT2I treatment in patients with sustained hypertension at baseline (p < .001). MAP at the office significantly increased in patients with masked hypertension after SGLT2I treatment (p = .01). There were no significant changes at the office or at home in patients with controlled or white‐coat hypertension.
FIGURE 2.
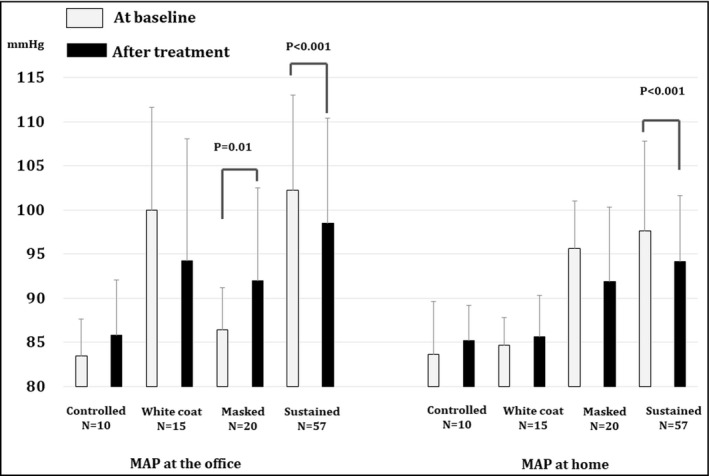
Changes in MAP at the office and at home with SGLT2 inhibitor treatment depending on BP status. BP, blood pressure; Controlled, controlled hypertension group (BP at the office <130/80 and BP at home <125/75); Masked, masked hypertension group (BP at the office <130/80 and BP at home ≥125/75); SGLT2I, Sodium–glucose cotransporter 2 inhibitor; Sustained, sustained hypertension group (BP at the office ≥130/80 and BP at home ≥125/75); White coat, white‐coat hypertension group (BP at the office ≥130/80 and BP at home <125/75)
3.5. Correlation of changes between BP at the office and at home
The correlation between BP at the office and at home is shown in Figure 3. There was a significant positive correlation between the BP at the office and at home (r = 0.28, p < .01).
FIGURE 3.
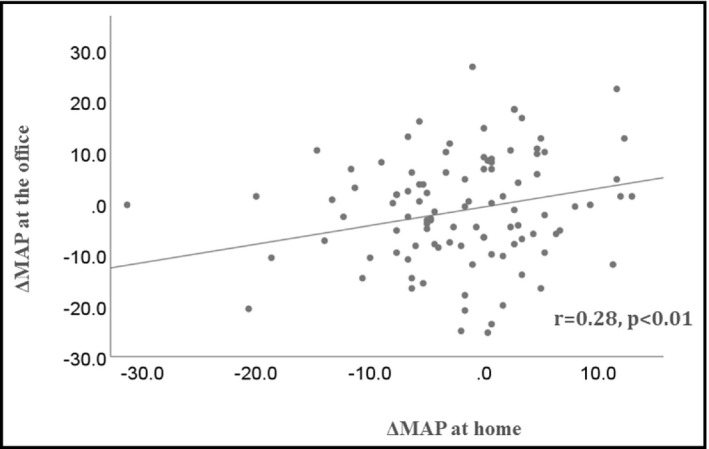
Correlation of BP changes at the office and BP at home. BP, blood pressure; ΔMAP; change in mean arterial pressure
3.6. The ROC curve to determine the possibility of improvement of MAP at office
The ROC curve showed that the area under the curve was 0.69 [0.59, 0.80] which was equivalent to a high accuracy, and the cutoff value of MAP at office before SGLT2I treatment for the improvement of MAP was 96.3 mm Hg, with 68.1% of sensitivity and 72.7% of specificity (Figure 4). The patients were divided into 2 groups; MAP at office before treatment <96.3 mm Hg (n = 47), and ≥96.3 mm Hg (n = 55). MAP at office was significantly decreased in patients with MAP at office before treatment ≥96.3 mm Hg (from 105.9 ± 8.3 to 100.0 ± 12.1 mm Hg, p < .001), however, it was significantly increased in patients with MAP at office before treatment <96.3 mm Hg (from 86.5 ± 5.7 to 90.0 ± 9.8 mm Hg, p = .011).
FIGURE 4.
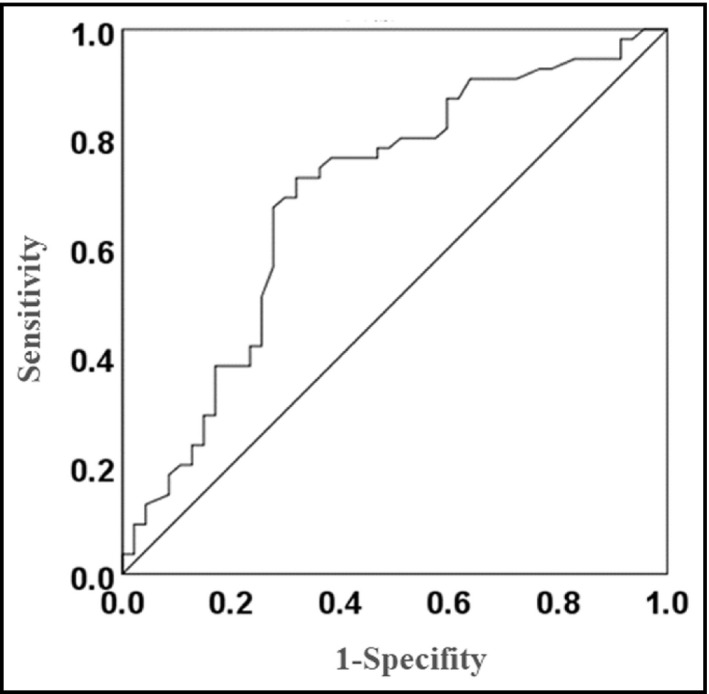
The ROC curve to determine the possibility of improvement of MAP at office. MAP, mean arterial pressure; ROC, Receiver operating characteristic
3.7. The ROC curve to determine the possibility of improvement of ACR
The ROC curve showed that the area under the curve was 0.52 [0.39, 0.65] which was equivalent to a soft accuracy, and the cutoff value of MAP at home after SGLT2I treatment for the improvement of ACR was 92.0 mm Hg, with 54.1% of sensitivity and 60.0% of specificity (Figure 4). The patients were divided into 2 groups; MAP at home after treatment <92 mm Hg (n = 45), and ≥92 mm Hg (n = 38). LNACR was significantly decreased only in patients with MAP at home after treatment <92 mm Hg (p = .04, Table 3). ΔLNACR in patients with MAP at home after treatment <92 mm Hg seemed larger than the rest (−0.10 ± 0.33 [95% confidence interval [CI]; −0.20, 0.00] vs. 0.01 ± 0.34 [95%CI; −0.11, 0.12]), however, the significant difference could not be observed (Table 3). Regarding the cutoff value of MAP at the office after treatment, significant cutoff value was not found in this study.
TABLE 3.
Comparison of change in LNACR between the two groups: the group of patients with MAP at home after SGLT2I treatment <92.0 mm Hg and the group of patients with MAP at home after SGLT2I treatment ≥92.0 mm Hg
| At baseline | At the survey | Change from baseline to survey | Comparison of at baseline vs. survey (paired t‐test) |
Comparison at baseline between the two groups (unpaired t‐test) |
Comparison for MAP at home at survey (unpaired t‐test) |
|
|---|---|---|---|---|---|---|
|
MAP at home after SGLT2I treatment <92.0 mm Hg n = 45 |
1.57 ± 0.64 | 1.46 ± 0.66 |
−0.10 ± 0.33 [95%CI; −0.20, 0.00] |
P = .04 | n.s. (p = .25) | n.s. (p = .76) |
|
MAP at home after SGLT2I treatment ≥92.0 mm Hg n = 38 |
1.41 ± 0.53 | 1.42 ± 0.60 |
0.01 ± 0.34 [95%CI; −0.11, 0.12] |
n.s. (P = .90) |
Data; mean ± SD.
[95%CI; −0.18, 0.02] vs. −0.00 ± 0.34.
3.8. Independent predictors of ΔMAP at the office by using multiple linear regression analysis
Multiple linear regression analysis was performed in 763 patients, whose clinical backgrounds were described in our previous study. 6 The independent predictors of ΔMAP were identified (r = 0.68, p < .001), and baseline MAP at the office, the change in the LNACR (ΔLNACR), age, baseline BW, use of loop diuretics, baseline LNACR, use of insulin, administration periods of SGLT2I, and use of Ca channel blockers were independent factors of ΔMAP at the office with regression coefficient values (p‐value, [95% CI]) of −0.69 (p < .001, [−0.75, −0.63]), 4.44 (p < .001, [2.77, 6.11]), −0.11 (p = .002, [−0.19, −0.04]), 0.08 (p = .003, [0.03, 0.13]), −7.79 (p = .011, [−13.78, −1.80]), 1.49 (p = .013, [0.32, 2.67]), −2.05 (p = .013, [−3.68, −0.43]), −0.08 (p = .016, [−0.15, −0.02]), and 1.72 (p = .025, [0.22, 3.22]), respectively. Evidence for multicollinearity was absent because the VIF for independent variables in the models was <4.0.
4. DISCUSSION
The Steno‐2 12 , 13 and J‐DOIT3 studies 14 showed that strict plasma glucose control and multi‐systemic management, including BP and lipid control, were effective in preventing the progression of microvascular complications. Reboldi reported that a low risk of stroke events was observed in patients with DM with a lower BP. 15 In Japan, where the incidence of stroke is higher than in the USA or Europe, 16 it is considered reasonable that the target BP in patients with DM is strictly defined as <130/80 mm Hg at the office and <125/75 mm Hg at home, in the early morning. 8
Ambulatory blood pressure monitoring (ABPM) is also an excellent measurement method for investigating dipper, non‐dipper, or riser patterns of nocturnal hypertension. However, it has some limitations: it needs to be performed frequently, cannot be used in regular practice, or may cause sleep disturbance that affects BP. 17 In fact, Kobayashi et al 18 reported that only 20.2% of patients were found to own ABPM devices and 10.1% used it in regular practice by a questionnaire survey in GPs in Kanagawa prefecture in Japan. From the results of trials using ABPM, empagliflozin decrease daytime BP more than nighttime BP, 19 , 20 but the 24‐hours BP pattern was not sufficiently discussed about the other SGLT2Is. In this study, BP was measured only at two points; at home in the early morning and at office. As shown in Figure 3, each ΔMAP showed a gradual correlation, and it might rather show that the BP decreased uniformly over 24 hours in a day. We collected more than 100 cases from multiple medical facilities and showed that systolic BP at the office, diastolic BP at home, and the mean BPs both at the office and at home were significantly decreased using SGLT2I treatment. According to the IDACO database, no antihypertensive medications resulted in a higher prevalence of masked hypertension in DM patients who were normotensive at the office (29.3%) than in those without DM (18.8%). 21 Masked hypertension is believed to be a risk factor for cardiovascular disease. Fagard et al performed a meta‐analysis of seven studies involving two studies in which home BP monitoring was used instead of ABPM to define masked hypertension. 22 They reported that the adjusted hazard ratio of cardiovascular events in patients with masked hypertension was 2.00 (95% CI: 1.58–2.52) compared with normotensive patients, and the elevated hazard ratios of masked hypertension were similar to those of sustained hypertension. 22 In the present study, the number of patients with sustained hypertension tended to decrease following the administration of SGLT2Is, and the prevalence of masked or white‐coat hypertension did not seem to change.
The difference between patients with sustained hypertension and those with masked hypertension is the level of BP at office. In this study, MAP at office significantly decreased in patients with sustained hypertension, however it significantly increased in patients with masked hypertension. The results of multiple linear regression analysis showed that baseline MAP at office was an independent factor for ΔMAP after SGLT2I treatment and the influence on ΔMAP by this treatment was thought to little in patients with masked hypertension at baseline. In this study, the cutoff value of baseline MAP at office for its improvement of was 96.3 mm Hg, that is approximately 130/80 mm Hg, and MAP significantly increased after SGLT2I treatment in patients with basal MAP at office <96.3 mm Hg, including 20 patients with masked hypertension. There is a possibility that some patients stopped antihypertensive agents or their doses were decreased because of their well BP control at home during SGLT2I; however, we could not confirm it because the information about concomitant agents was collected only at the survey. Though this study could not clarify the reason for this increase in BP, it might also indicate that SGLT2I treatment prevent excessive decrease in BP in patients. To date, unfortunately there are no studies to determine the effects of SGLT2I on blood pressure in patients with T2DM even accompanied by masked hypertension. Further studies should be required to clarify the effects of SGLT2I on this population and the differences between sustained hypertension and masked hypertension accompanied by T2DM.
In this study, we could not observe the significant decrease in ACR, however, the patients with lower level of MAP at home, not at the office, after SGLT2I treatment had tendency to improve the ACR from the analysis of ROC curve. From the result of sin95% CI value of ΔLNACR between two groups, significant results might be obtained by increasing the number of cases. The fact that BP at home was more related in ACR improvement than BP at the office was consistent with the previous study. 7 The study might show the importance of control of BP at home to exert a renoprotective effect by SGLT2I treatment. Further large‐scale prospective studies should be conducted to investigate BP monitoring at home as an indicator of ΔLNACR.
In the present study, the target BP at the office and at home in the early morning were achieved in 26.9% and 25.3%, and in 34.6% and 34.3% of patients, respectively, at the time of the initiation of SGLT2I treatment.
Despite the fact SGLT2I treatment was useful for BP control, the average number of concomitant antihypertensive drugs was 1.6, especially, more than half of patients were administrated with renin–angiotensin system inhibitors or Ca channel blockers. It should be noted that multiple concomitant treatment is required in many cases for BP management in T2DM patients. Although the target BP for patients with DM is slightly different in each country, this study revealed that the achievement rate of target BP was still insufficient, despite with SGLT2Is treatment in Japanese patients with DM and CKD.
This study has some serious limitations. This is a retrospective observational study without control group, then, large‐scale prospective studies are required to prove the results obtained in the present study. In addition, the number of institutions participating in this study was too small, and it is impossible to deny the variations among the patient groups. Furthermore, the confirmation of concomitant agents was done only at the time of the survey, and we did not have any information of the concomitant agents at baseline. Then we could not accurately clarify the influence on BP by concomitant antihypertensive agents with SGLT2Is treatment. Further studies with higher precision are warranted.
5. CONCLUSION
Sodium–glucose cotransporter 2 inhibitors can be useful for the strict management of BP both at the office and at home. The decrease in BP at home by SGLT2I might be related to the improvement of diabetic nephropathy.
CONFLICT OF INTEREST
All authors declare that there are no conflicts of interest associated with this study.
AUTHOR CONTRIBUTIONS
TF and MT contributed in the concept and design, the data acquisition, data analysis, drafting and final approval, and agreed for all aspects of the work. KK and NH contributed in t the concept and design, the data acquisition, data analysis, statistical analysis, drafting and final approval, and agreed for all aspects of the work. HS, KS, MM, and AK contributed in the concept and design, the data acquisition, drafting, and final approval and agreed for all aspects of the work.
6.
FIGURE 5.
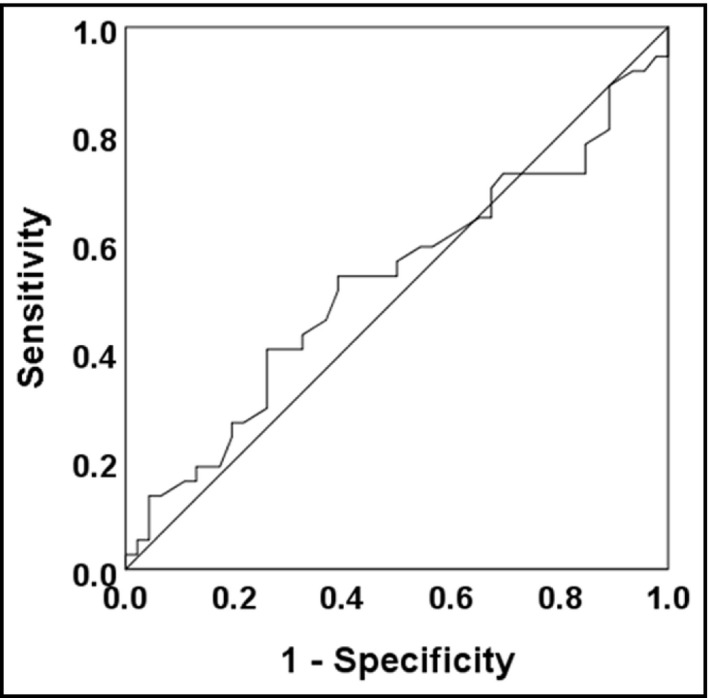
The ROC curve to determine the possibility of improvement of ACR. ACR, urine albumin to creatinine ratio; ROC, Receiver operating characteristic
Supporting information
Tab S1
ACKNOWLEDGMENTS
We are grateful to all the participants who took part in this study and we thank Editage (www.editage.jp) for English‐language editing.
Furuki T, Kobayashi K, Toyoda M, et al. The influence of long‐term administration of SGLT2 inhibitors on blood pressure at the office and at home in patients with type 2 diabetes mellitus and chronic kidney disease. J Clin Hypertens. 2020;22:2306–2314. 10.1111/jch.14084
All authors met the criteria for the authorship in accordance with the ICMJE recommendations outlined below: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and Drafting the work or revising it critically for important intellectual content; and Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1. DeFronzo RA, Norton L, Abdul‐Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13(1):11‐26. [DOI] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 5. Kobayashi K, Toyoda M, Kimura M, et al. Retrospective analysis of effects of sodium‐glucose co‐transporter 2 inhibitor in Japanese type 2 diabetes mellitus patients with chronic kidney disease. Diab Vasc Dis Res. 2019;16(1):103‐107. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi K, Toyoda M, Hatori N, et al. Retrospective analysis of the renoprotective effects of long‐term use of six types of sodium‐glucose cotransporter 2 inhibitors in Japanese patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Technol Ther. 2020. 10.1089/dia.2020.0165 [DOI] [PubMed] [Google Scholar]
- 7. Kinguchi S, Wakui H, Ito Y, et al. Improved home BP profile with dapagliflozin is associated with amelioration of albuminuria in Japanese patients with diabetic nephropathy: the Yokohama add‐on inhibitory efficacy of dapagliflozin on albuminuria in Japanese patients with type 2 diabetes st. Cardiovasc Diabetol. 2019;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimamoto K, Ando K, Fujita T, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253‐390. [DOI] [PubMed] [Google Scholar]
- 9. K/DOQI clinical practice guidelines for chronic kidney disease . evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1‐S266. [PubMed] [Google Scholar]
- 10. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982‐992. [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi K, Toyoda M, Kimura M, et al. Renal effects of sodium glucose co‐transporter 2 inhibitors in Japanese type 2 diabetes mellitus patients with home blood pressure monitoring. Clin Exp Hypertens. 2019;41(7):637‐644. [DOI] [PubMed] [Google Scholar]
- 12. Gæde P, Vedel P, Larsen N, Jensen GVH, Parving H‐H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383‐393. [DOI] [PubMed] [Google Scholar]
- 13. Gæde P, Lund‐Andersen H, Parving H‐H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 14. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951‐964. [DOI] [PubMed] [Google Scholar]
- 15. Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta‐analysis in 73 913 patients. J Hypertens. 2011;29(7):1253‐1269. [DOI] [PubMed] [Google Scholar]
- 16. Kitamura A, Sato S, Kiyama M, et al. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: the Akita‐Osaka study. J Am Coll Cardiol. 2008;52(1):71‐79. [DOI] [PubMed] [Google Scholar]
- 17. Shin J, Kario K, Chia YC, et al. Current status of ambulatory blood pressure monitoring in Asian countries: a report from the HOPE Asia network. J Clin Hypertens. 2020;22(3):384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi K, Sato K, Hatori N, Miyakawa M. A questionnaire survey of general practitioners in Japan's Kanagawa Prefecture for the Japanese society of hypertension guidelines for the management of hypertension 2014. Clin Exp Hypertens. 2017;39(8):705‐710. [DOI] [PubMed] [Google Scholar]
- 19. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420‐428. [DOI] [PubMed] [Google Scholar]
- 20. Kario K, Okada K, Kato M, et al. Twenty‐four-hour blood pressure‐lowering effect of a sodium‐glucose cotransporter 2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension. Circulation. 2019;139(18):2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61(5):964‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white‐coat, masked and sustained hypertension versus true normotension: a meta‐analysis. J Hypertens. 2007;25(11):2193‐2198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1


