Abstract
Stress from obstructive sleep apnea (OSA) stimulates catecholamine release consequently exacerbating hypertension. However, different studies have shown a conflicting impact of continuous positive airway pressure (CPAP) treatment in patients with OSA on catecholamine levels and blood pressure. We aimed to examine changes to catecholamine levels and blood pressure in response to CPAP treatment. We conducted a meta‐analysis of data published up to May 2020. The quality of the studies was evaluated using standard tools for assessing the risk of bias. Meta‐analysis was conducted using RevMan (v5.3) and expressed in standardized mean difference (SMD) for catecholamines and mean difference (MD) for systolic (SBP) and diastolic blood pressure (DBP). A total of 38 studies met our search criteria; they consisted of 14 randomized control trials (RCT) totaling 576 participants and 24 prospective cohort studies (PCS) of 547 participants. Mean age ranged between 41 and 62 year and body mass index between 27.2 and 35.1 kg/m2. CPAP treatment reduced 24‐hour urinary noradrenaline levels both in RCT (SMD = −1.1; 95% confidence interval (CI): −1.63 to − 0.56) and in PCS (SMD = 0.38 (CI: 0.24 to 0.53). SBP was also reduced by CPAP treatment in RCT (4.8 mmHg; CI: 2.0‐7.7) and in PCS (7.5 mmHg; CI: 3.3‐11.7). DBP was similarly reduced (3.0 mmHg; CI: 1.4‐4.6) and in PCS (5.1 mmHg; CI: 2.3‐8.0). In conclusion, CPAP treatment in patients with OSA reduces catecholamine levels and blood pressure. This suggests that sympathetic activity plays an intermediary role in hypertension associated with OSA‐related stress.
Keywords: hormones, hypertension, sympathetic activity
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition among overweight and obese individuals. 1 About half of patients with OSA have hypertension, and about half of patients with hypertension have OSA. 2 Causal link between OSA and hypertension is complex and remains debatable, but hypertension may in part arise from increased sympathetic nerve activity induced by hypoxic stress. 3 OAS is associated with a number of secondary health complications, most notably cardiovascular disease, 4 and co‐existing cardiometabolic risk factors such as dyslipidemia, endothelial dysfunction, deranged inflammatory responses, and insulin resistance 5 ; all of which are associated with obesity. 6
The main treatment for OSA is continuous positive airway pressure (CPAP). Meta‐analyses have shown CPAP slightly reduces arterial blood pressure. 7 , 8 However, studies on the impact of CPAP treatment on sympathetic activity markers such as catecholamines have drawn conflicting conclusions. 9 , 10 , 11 There is a lack of meta‐analyses on large numbers of participants recording the effects of CPAP in the same individuals on both changes in catecholamine levels and blood pressure. This information is important as it provides evidence of sympathetic activity as a mediator of OSA‐related stress and hypertension.
We therefore conducted a meta‐analysis of published data to document changes in the levels of catecholamines and their inactive metabolites [metadrenalines], as well as blood pressure in response to CPAP treatment of OSA.
2. METHODS
2.1. Search criteria
Two investigators followed PRISMA 12 and Cochrane guidelines, 13 and performed independently a literature search of MEDLINE and Google Scholar up to May 2020 using the key terms (British or US usage and abbreviations, eg, CPAP and OSA): obstructive sleep apnea, continuous positive airway pressure, urinary or plasma catecholamines, adrenaline (epinephrine), noradrenaline (norepinephrine), 3‐methoxytyramine, metanephrines, normetanephrine, metanephrine and dopamine, and hypertension. No filters for language or data were used. The Boolean operators “AND” and “OR” were used to combine search terms. Relevant studies were hand‐searched within these references.
2.2. Selection criteria
Studies examining the effect of CPAP on catecholamines in the OSA population were included irrespective of age, sex, race, comorbidities, duration of CPAP, and treatment. Studies that fit the inclusion criteria were randomized control trials (RCTs) and prospective cohort studies (PCS). Studies were excluded if they did not present numerical data for catecholamines at baseline and end point.
2.3. Outcome measures
24‐hour urinary or plasma catecholamines: dopamine, adrenaline and noradrenaline, or their products metadrenalines (metanephrines): 3‐methoxytyramine, normetadrenaline (normetanephrine) and metadrenaline (metanephrine) and blood pressure were the outcomes used for the comparison analysis.
2.4. Risk of bias
The quality of the reports was evaluated using the risk of bias assessed using Cochrane Collaboration's tool for RCTs 14 and risk of bias in non‐randomized studies of interventions (ROBINS‐I) tool for PCS. 15 The risk of bias for each report was rated independently from low, moderate, serious, or critical by two authors, and any discrepancies were resolved by reciprocal discussion.
2.5. Statistical analysis
Meta‐analysis was performed using Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). The standardized mean difference (SMD) was used to determine the effect size on catecholamines to accommodate for a variety of ways they were measured. The SMD expresses the size of the intervention effect in each study relative to the variability observed in that study. The mean difference (MD) used on the original scale of measurement to determine the effect size on blood pressure. Pooled estimates of each outcome for each treatment were obtained via the DerSimonian and Laird method using a random‐effects model. 16 Statistical significance threshold was accepted as P < .05. The I 2 statistic was used to assess heterogeneity of trial results used to construct pooled estimates of effect. 17
RESULTS
A total of 38 studies met the above search criteria: 14 RCT 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 including a total of 576 participants (295 in treatment groups and 281 controls) and 24 PCS, 32 , 33 , 34 , 35 totaling 547 participants (Figure 1). The mean age ranged between 41 and 62 yr and body mass index between 27.2 and 35.1 kg/m2 (Table 1). The remaining baseline parameters including heart rate, sleep study characteristics, and noradrenaline levels are shown in Table S1. The duration of CPAP treatment ranged from one day to eight months in RCTs and one day to a year in PCS. Most studies used 24‐hour urinary noradrenaline as outcome measure, including nine RCTs 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 and 13 PCS. 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 A fewer RCTs and PCS, respectively, reported 24‐hour urinary adrenaline (n = 6 and 7), normetadrenaline (n = 3 and 0) and metadrenaline (n = 2 and 0), or plasma noradrenaline (n = 5 and 13) and adrenaline (n = 3 and 6) (Table 2). Subsequently, data from studies on 24‐hour urinary noradrenaline are presented herein while the remaining data on other methods of measurement of catecholamines and their metabolites are shown in Figures S1 and S2.
FIGURE 1.
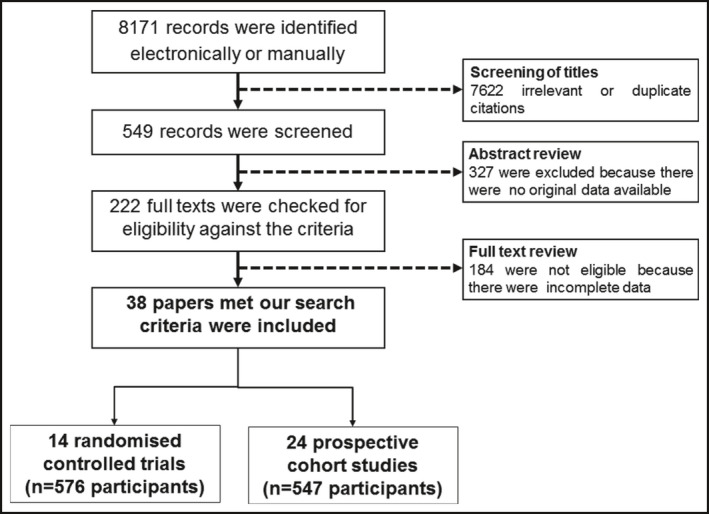
QUOROM (quality of reporting of meta‐analyses) flow chart of literature search
TABLE 1.
Baseline characteristics of participants
| Sex (M/F) | Mean ± SD (or range where indicated) | CPAP duration | ||||
|---|---|---|---|---|---|---|
| Age (years) | BMI (kg/m2) | SBP (mmHg) | DBP (mmHg) | |||
| RCT (CPAP group) | ||||||
| Arias et al (2008) a , 18 | 30/0 | 52 ± 13 | 30.5 ± 4.0 | 121.5 ± 11.4 | 74.5 ± 7.8 | 3 mo |
| Casitas et al (2017) a , 19 | 26/6 | 56 ± 11.2 | 29.2 ± 5.6 | 131.5 ± 12.0 | 78.8 ± 8.5 | 12 wk |
| Comondore et al (2008) a , 20 | 9/4 | 55 ± 7.1 | 31.1 | 138.4 | 83.8 | 4 wk |
| de Araújo et al (2013) 21 | 8 (Both) | 43 ± 12 | 28 ± 4 | 112 ± 12 | 67 ± 8 | 1 night |
| Drager et al (2007) 27 | 12/0 | 44 ± 7 | 29.9 ± 3.0 | 123 ± 12 | 73 ± 10 | 4 mo |
| Kohler et al (2008) 28 | 51/0 | 48.1 ± 9.5 | 35.8 ± 7.3 | 131.3 ± 13.9 | 83.9 ± 9.3 | 4 wk |
| Lam et al (2010) 22 | 31/0 | 46.5 ± 10.8 | 27.8 ± 3.7 | 130.8 ± 14.7 | 80.1 ± 10.8 | 4 wk |
| Mansfield et al (2004) 23 | 28/0 | 57.2 ± 9 | 33.5 ± 4.8 | 99 ± 15.9 b | 105 ± 15.9 b | 3 mo |
| Mills et al (2006) 24 | 15/2 | 47.6 ± 10.7 | 31.7 ± 5.8 | 155.2 ± 18.6 | 84.2 ± 10.7 | 2 wk |
| Phillips et al (2011) a , 25 | 35/3 | 49 ± 13 | 32.1 ± 4.3 | – | – | 2 mo |
| Rubinsztajn et al (2006) 29 | 15/0 | 50.6 ± 10.0 | 31.5 ± 6.3 | 130.1 ± 17.8 | 87.3 ± 13.5 | 8 mo |
| Ruzicka et al (2020) 30 | 7/0 | 59 (58‐67) c | 33 (31‐35) c | 140 (136‐165) c | 73 (66‐85) c | 6 wk |
| Ryan et al (2005) 26 | 9/1 | 57.6 ± 7 | 28.3 ± 4.1 | 120.7 ± 17.1 | 64.6 ± 9.5 | 1 mo |
| Thunstrom et al (2016) 31 | 15/9 | 58 ± 6.7 | 27.7 ± 3.2 | 164.9 ± 16.2 | 96.5 ± 10.9 | 6 wk |
| RCT (control group) | ||||||
| Arias et al (2008) a , 18 | 30/0 | 52 ± 13 | 30.5 ± 4.0 | 121.5 ± 11.4 | 74.5 ± 7.8 | 3 mo |
| Casitas et al (2017) a , 19 | 26/6 | 56 ± 11.2 | 29.2 ± 5.6 | 131.5 ± 12.0 | 78.8 ± 8.5 | 12 wk |
| Comondore et al (2009) a , 20 | 9/4 | 55 ± 7.1 | 31.1 | 138.4 | 83.8 | 4 wk |
| de Araújo et al (2013) 21 | 8 (Both) | 43 ± 12 | 28 ± 4 | 112 ± 12 | 67 ± 8 | 1 night |
| Drager et al (2007) 27 | 12/0 | 47 ± 6 | 29.7 ± 2.9 | 123 ± 12 | 73 ± 10 | 4 mo |
| Kohler et al (2008) 28 | 51/0 | 48.7 ± 10.6 | 34.5 ± 5.0 | 138.9 ± 20.8 | 88.3 ± 8.1 | 4 wk |
| Lam et al (2010) 22 | 30/0 | 46.1 ± 9.8 | 27.2 ± 3.7 | 129.5 ± 16.5 | 82.0 ± 11.6 | 4 wk |
| Mansfield et al (2004) 23 | 24/3 | 57.5 ± 8.3 | 34.6 ± 6.2 | 99 ± 15.9 b | 105 ± 15.9 | 3 mo |
| Mills et al (2006) 24 | 13/3 | 49 ± 10.4 | 32.2 ± 6.8 | 149 ± 23.2 | 83.6 ± 13.6 | 2 wk |
| Phillips et al (2011) a , 25 | 35/3 | 49 ± 13 | 32.1 ± 4.3 | – | – | 2 mo |
| Rubinsztajn et al (2006) 29 | 10/0 | 45.4 ± 16.5 | 27.6 ± 3.1 | 126.7 ± 12.3 | 84.2 ± 10.0 | 8 mo |
| Ruzicka et al (2020) 30 | 6/0 | 63 (55‐71) c | 34 (33‐36) c | 138 (127‐148) c | 71 (62‐81.5) c | 6 wk |
| Ryan et al (2005) 26 | 7/1 | 60.3 ± 11.6 | 35.1 ± 10.5 | 139 ± 15.6 | 69.9 ± 12.2 | 1 mo |
| Thunstrom et al (2016) 31 | 17/6 | 59 ± 3.7 | 27.6 ± 4.1 | 164.9 ± 16.2 | 96.5 ± 10.9 | 6 wk |
| Prospective cohort studies | ||||||
| Baruzzi et al (1991) 32 | 6/0 | 41.3 ± 12.9 | 36 ± 6 | – | – | 1 night |
| Bischof et al (2019) 57 | 18/0 | 55.8 ± 9.5 | 35.5 ± 3.8 | 133.2 ± 14.1 | 80.2 ± 10.6 | 6 mo |
| Bratel et al (1999) 33 | 16/0 | 51.3 ± 10.8 | 32.0 ± 5.6 | 143.8 ± 17.2 | 87.5 ± 10 | 7 mo |
| Burioka et al (2008) 58 | 8/0 | 45.9 ± 12.2 | 25.9 ± 1.7 | – | – | 3 mo |
| Castro‐Grattoni et al (2017) 34 | 48/12 | 52.3 ± 9.56 | 30.7 ± 4.2 | 122.7 ± 9.9 | 77.2 ± 7.7 | 6 mo |
| Donadio et al (2007) 45 | 10/0 | 50 ± 9.5 | 32 ± 6.3 | 144 ± 6.3 | 98 ± 3.2 | 6 mo |
| Feres et al (2014) 35 | 6/3 | 56.0 ± 15.6 | – | – | – | 1 y |
| Ferrier et al (2008) 36 | 16/3 | 58.5 ± 11.2 | 30.2 ± 6.7 | 132 ± 16 | 80 ± 9 | 6 mo |
| Grimpen et al (2000) 59 | 26/3 | 56.9 ± 8.6 | 29.5 ± 3.8 | 98.4 ± 2.7 b | 98.4 ± 2.7 b | 14 mo d |
| Heitmann et al (2000) 60 | 18 (Both) | 50.0 ± 10.4 | 29.7 ± 3.7 | 136.8 ± 15.7 | 84.9 ± 12.5 | 42 d d |
| Jennum et al (1989) 46 | 13/1 | 42 (36‐66) c | 26.13 ± 3.5 | 147.5 ± 5.2 | 122.4 ± 4.3 | 1 wk |
| Kita et al (1998) 37 | 12/2 | 53 ± 14.5 | 29.9 ± 4.9 | 127.6 ± 19.8 | 77.8 ± 11.6 | 1 night |
| Krieger et al (1989) 38 | 20/1 | 51 ± 10.1 | 32.0 ± 1.3 | – | – | 1 night |
| Lemmer et al (2016) 47 | 17/0 | 60.5 ± 8.1 | 35.0 ± 4.7 | 138 ± 15.2 | 83.3 ± 10.2 | 8 wk |
| Minemura et al (1998) 39 | 26/0 | 47.8 ± 11.1 | 30.6 ± 5.1 | 125 ± 15 | 80 ± 10.9 | 1 night |
| Mokhlesi et al (2017) 61 | 6/6 | 54.6 ± 10.2 | 37.7 ± 8.7 | – | – | 1 wk |
| Myhill et al (2012) 40 | 27/17 | 66.1 ± 8.8 | 33.6 ± 5.5 | 149 ± 23 | 80 ± 12 | 3 mo |
| Nakamura et al (2001) 62 | 18/0 | 49.9.3 | 29.9 ± 5.1 | 119.9 ± 16.1 | 84 ± 11.9 | 1 night |
| Nicholl et al (2018) 48 | 17/8 | 49 ± 10 | 33.5 ± 6.5 | 127 ± 10 | 79 ± 10 | 4 wk |
| Pinto et al (2013) 41 | 67/0 | 49.4 ± 8.8 | 31.8 ± 5.3 | 124.7 ± 12.6 | 77.8 ± 9.2 | 1 mo |
| Rodenstein et al (1992) 63 | 11/1 | 50.0 ± 9.0 | 36.9 ± 8.6 | – | – | 2‐3 nights |
| Sukegawa et al (2005) 42 | 17/0 | 53.1 ± 13.5 | 26.7 ± 4.8 | – | – | 1 night |
| Tachikawa et al (2016) 43 | 51/12 | 60.6 ± 10.0 | 27.9 ± 3.8 | – | – | 3 mo |
| Unterberg et al (2005) 44 | 9/1 | 61 (50‐69) c | 33 (27‐46) c | – | – | 3 nights |
Abbreviations: BMI, body mass index; CPAP, continuous positive airway pressure; M/F, male/female; RCT, randomized controlled trial; SBP and DBP, systolic and diastolic blood pressure.
Crossover study.
Mean arterial pressure.
Range.
Mean values.
TABLE 2.
Number of studies and participants reporting 24‐hurinary or plasma tests to determine catecholamines or their metabolites, and blood pressure
| Studies (n) | Participants (n) | |
|---|---|---|
| Randomized controlled trials (n = 14) | Case/control | |
| 24‐h urinary noradrenaline | 9 | 186/180 |
| 24‐h urinary adrenaline | 6 | 140/135 |
| 24‐h urinary normetadrenaline | 3 | 92/87 |
| 24‐h urinary metadrenaline | 2 | 41/36 |
| Plasma noradrenaline | 5 | 24/23 |
| Plasma adrenaline | 3 | 46/39 |
| Blood pressure | 10 | 208/199 |
| Prospective cohort studies (n = 24) | ||
| 24‐h urinary noradrenaline | 13 | 367 |
| 24‐h urinary adrenaline | 7 | 173 |
| 24‐h urinary normetadrenaline | – | – |
| 24‐h urinary metadrenaline | – | – |
| Plasma noradrenaline | 13 | 269 |
| Plasma adrenaline | 6 | 85 |
| Blood pressure | 10 | 297 |
NB: Some studies reported more than one test.
CPAP treatment reduced the levels of 24‐hour urinary noradrenaline levels both in RCT: SMD = −1.1 (95%CI = −1.63 to − 0.56) (Figure 2A) and in PCS: SMD = 0.38 (95%CI = 0.24 to 0.53) (Figure 2B). Inter‐study heterogeneity was high among RCT (I 2 = 81%) but low among PCS (I 2 = 0%).
FIGURE 2.
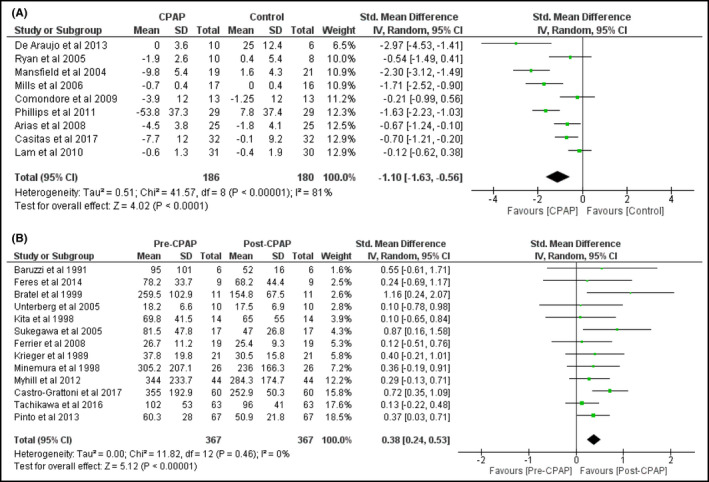
Changes in 24‐hour urinary noradrenaline levels by CPAP treatment in RCT (A) and in PCS (B)
Blood pressure as study outcome measure was reported in ten RCTs totaling 407 participants 18 , 19 , 20 , 21 , 22 , 30 and ten PCS containing 297 participants 34 , 36 , 39 , 40 , 41 , 45 , 46 , 47 , 48 (Table 2). CPAP treatment led to a blood pressure reduction. With RCT, mean reductions of SBP were 4.8 mmHg (95%CI = 2.0‐7.7 mmHg) (Figure 3A) and of DBP were 3.0 mmHg (95%CI = 1.4‐4.6 mmHg) (Figure 3B). With PCS, mean reductions of SBP were 7.5 mmHg (95%CI = 3.3 to 11.7 mmHg) (Figure 3C) and of DBP were 5.1 mmHg (95%CI = 2.3‐8.0 mmHg) (Figure 3D). There was evidence of substantial inter‐study heterogeneity both in RCTs (I 2 = 84% and 62%, respectively, in SBP and DBP analyses) and in PCS (I 2 = 71% and 64%, respectively, in SBP and DBP analyses).
FIGURE 3.
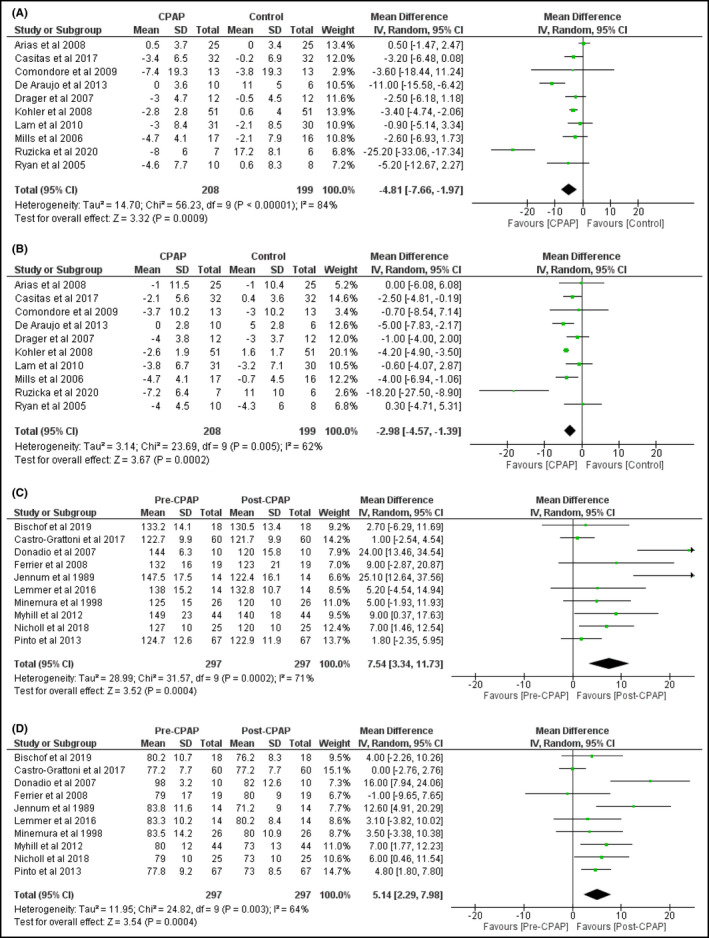
Changes in systolic (A) and diastolic (B) blood pressure in RCT and systolic (C) and diastolic (D) blood pressure in PCS by CPAP treatment
We have also observed that CPAP had similar impact on the reduction of other catecholamines and their metabolites (Results not shown).
Risk of bias for the RCT assessed by random sequence generation (Figure 4A) showed a high risk in three studies since they were not double‐blinded. 20 , 21 , 23 All of the studies used intention‐to‐treat analysis or did not have any dropouts, to minimize risk of incomplete outcome data. All of the studies lacked information of selective reporting bias as they did not mention the study protocol. There were confounding factors in one study 21 due to all of the patients undergoing surgery and in another 23 where patients were undergoing heart failure treatment.
FIGURE 4.
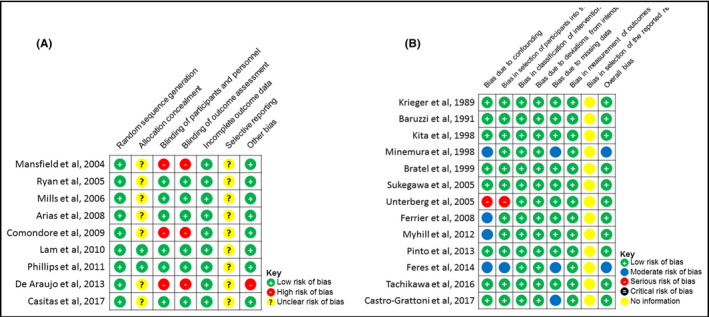
Risk of bias of RCTs evaluated by Cochrane Collaboration's tool (A) and risk of bias of PCS evaluated by ROBINS‐I tool (B)
Risk of bias for PCS was evaluated using the risk of bias in non‐randomized studies of interventions (ROBINS‐I) tool (Figure 4B). Bias due to confounding factors was seen in five studies, 36 , 39 , 44 but in only three was there a moderate risk of overall bias. 35 , 39 , 44 Patients in one study 44 had previously been receiving CPAP therapy for at least three months. Bias in selection of participants was seen in one study: There was no reimbursement for CPAP usage in Brazil. 35 Missing data were assessed to cause a moderate risk of bias in three studies. 34 , 35 , 39 None of the studies had bias in measurement of outcomes. There was insufficient information from any of the studies for assessing bias in selection of the reported result.
3. DISCUSSION
In this meta‐analysis of data from over a thousand patients with OSA, it was observed that CPAP treatment significantly reduced urinary or plasma catecholamines, and their metabolites, as well as blood pressure. This suggests that a reduction of OSA‐related stress by CPAP decreases sympathetic activity (catecholamines) and consequently blood pressure. These findings lend further support for the intermediary role of sympathetic activity in the relationship between OSA‐related stress and hypertension.
The impact of CPAP both on catecholamines and blood pressure has previously been debatable due to inconsistent findings. Forest plots in this analysis revealed high inter‐study heterogeneity which may be explained by a variation in study designs and patient characteristics. For example, inclusion criteria of baseline blood pressure or severity of OSA may differ and other factors such as antihypertensive medications and duration of CPAP treatment are also likely to vary between studies.
The reduction in blood pressure observed in this analysis is consistent with findings from previous meta‐analyses on CPAP and blood pressure. 7 , 8 , 49 , 50 Although the reduction of systolic blood pressure is relatively small (about 5‐7.5 mmHg), this is clinically relevant in reducing stroke incidence. 51
We found studies from existing literature reported a variety of methods of measurements, either urinary or plasma and catecholamines or their metabolites, but the majority reported urinary noradrenaline. The application of urinary catecholamines and metadrenalines or plasma metadrenaline depends on the degree of risk of an individual to have catecholamine‐secreting tumor; urinary method which has high specificity (98%) is suggested for testing low‐risk patients, while plasma method (high sensitivity: 97%) is suggested for high‐risk patients. 52 Albeit, we found that CPAP had very similar effects on the reduction of all catecholamines and their metabolites.
In this study, we analyzed both RCTs and PCS and observed CPAP to have overall effects both on the reduction of catecholamine levels and blood pressure, which is consistent with observations made by Benson and Hartz. 53 We observed that reductions in blood pressure appear to be higher in PCS than those in RCTs but not able to clarify the underlying reasons for these differences, but bias in selection of participants and CPAP treatment regimen may contribute.
It would be of interest to examine the effects of CPAP on heart rate in response to the reduction of catecholamines. However, only 15 studies reported heart rate at baseline (Appendix S1) and only one studied changes in heart rate in relation to CPAP treatment. 21 Heart rate was therefore not included as an outcome measure in our study.
There are certain limitations identified in this study, as expected for a meta‐analysis. These include different methods of measuring catecholamines and their metabolites.
There were also varying methods applied to control groups in the RCTs, and some received no treatment while other received sham CPAP treatment. This may introduce a risk of bias since sham CPAP treatment has been shown to have greater influences on the results than non‐treatment, 54 which may underestimate the effect of CPAP on catecholamines and blood pressure. 55 Further bias may also arise from the inability to disguise sham CPAP from patients in RCTs; about two‐thirds of patients are able to determine whether they were receiving sham CPAP or therapeutic CPAP. The numbers of participants also vary widely between studies, while CPAP treatment duration ranges from one night to one year which would contribute to significant inter‐study heterogeneity. There was a low representation of female participants relative to the overall prevalence of women with OSA 56 ; thus, the findings from this study should be interpreted cautiously in the female population.
4. CONCLUSIONS
CPAP treatment in patients with OSA reduces catecholamines levels and blood pressure suggesting sympathetic activity plays an intermediary role in the relationship between OSA‐related stress and hypertension.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTION
TSH created the study concept and design. TSH and GK‐D reviewed the literature. MG performed data collection and data analysis under the guidance of TSH and GK‐D. TSH wrote the first draft of the manuscript and edited subsequent versions. DF, CS, PS, and CHF commented on the manuscript. All authors checked, interpreted the results, and approved the final manuscript.
Supporting information
Appendix S1
Green M, Ken‐Dror G, Fluck D, et al. Meta‐analysis of changes in the levels of catecholamines and blood pressure with continuous positive airway pressure therapy in obstructive sleep apnea. J Clin Hypertens. 2021;23:12–20. 10.1111/jch.14061
REFERENCES
- 1. Jehan S, Zizi F, Pandi‐Perumal SR, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):pii: 00019. [PMC free article] [PubMed] [Google Scholar]
- 2. Silverberg DF, Iaina A, Oksenberg A. Treating obstructive sleep apnea improves essential hypertension and quality of life. Am Fam Physician. 2002;65:229‐236. [PubMed] [Google Scholar]
- 3. Khayat R, Patt B, Hayes D. Obstructive sleep apnea: the new cardiovascular disease. Part I: obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev. 2009;14:143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046‐1053. [DOI] [PubMed] [Google Scholar]
- 5. Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C‐reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med. 2011;6:120‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoyos CM, Drager LF, Patel SR. OSA and cardiometabolic risk: What's the bottom line? Respirology. 2017;22:420‐429. [DOI] [PubMed] [Google Scholar]
- 7. Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta‐analysis of randomized controlled trials. Lung. 2007;185:67‐72. [DOI] [PubMed] [Google Scholar]
- 8. Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417‐423. [DOI] [PubMed] [Google Scholar]
- 9. Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19:774‐782. [DOI] [PubMed] [Google Scholar]
- 10. Ziegler MG, Mills PJ, Loredo JS, Ancoli‐Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887‐893. [DOI] [PubMed] [Google Scholar]
- 11. Phillips CL, Yang Q, Williams A, et al. The effect of short‐term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217‐225. [DOI] [PubMed] [Google Scholar]
- 12. http://www.prisma‐statement.org/ [Accessed 15 April 2020].
- 13. https://training.cochrane.org/handbook/current/chapter‐04 [Accessed 15 April 2020].
- 14. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, et al. (Eds). Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. Chichester: John Wiley & Sons; 2019. [Google Scholar]
- 17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 18. Arias MA, García‐Río F, Alonso‐Fernández A, et al. CPAP decreases plasma levels of soluble tumour necrosis factor‐α receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32:1009‐1015. [DOI] [PubMed] [Google Scholar]
- 19. Casitas R, Martinez‐Ceron E, Galera R, et al. The effect of treatment for sleep apnoea on determinants of blood pressure control. Eur Respir J. 2017;50(5):1701261. [DOI] [PubMed] [Google Scholar]
- 20. Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung. 2008;187:17‐22. [DOI] [PubMed] [Google Scholar]
- 21. de Araújo MT, Bissoli NS, Gouvêa SA, et al. CPAP therapy prevents increase in blood pressure after upper airway surgery for obstructive sleep apnoea. Sleep Breath. 2013;17:1289‐1299. [DOI] [PubMed] [Google Scholar]
- 22. Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138‐145. [DOI] [PubMed] [Google Scholar]
- 23. Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361‐366. [DOI] [PubMed] [Google Scholar]
- 24. Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343‐348. [DOI] [PubMed] [Google Scholar]
- 25. Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo‐controlled crossover trial. Am J Respir Crit Care Med. 2011;184:355‐361. [DOI] [PubMed] [Google Scholar]
- 26. Ryan CM. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi‐Filho G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706‐712. [DOI] [PubMed] [Google Scholar]
- 28. Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32:1488‐1496. [DOI] [PubMed] [Google Scholar]
- 29. Rubinsztajn R, Kumor M, Byśkiniewicz K, Bielicki P, Chazan R. The influence of 8 months therapy with nasal continues positive airway The influence of 8 months therapy with nasal continues positive airway pressure (nCPAP) on sympathetic activity and leptin serum concentration in patients with obstructive sleep apnea syndrome. Sen. 2006;6:64‐73. [Google Scholar]
- 30. Ruzicka M, Knoll G, Leenen FH, Leech J, Aaron SD, Hiremath S. . Effects of CPAP on blood pressure and sympathetic activity in patients with diabetes mellitus, chronic kidney disease, and resistant hypertension. CJC Open. 2020;2(4):258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thunström E, Manhem K, Yucel‐Lindberg T, Rosengren A, Lindberg C, Peker Y. Neuroendocrine and inflammatory responses to losartan and continuous positive airway pressure in patients with hypertension and obstructive sleep apnea. A randomized controlled trial. Ann Am Thorac Soc. 2016;13:2002‐2011. [DOI] [PubMed] [Google Scholar]
- 32. Baruzzi A, Riva R, Cirignotta F, Zucconi M, Cappelli M, Lugaresi E. Atrial natriuretic peptide and catecholamines in obstructive sleep apnea syndrome. Sleep. 1991;14:83‐86. [DOI] [PubMed] [Google Scholar]
- 33. Bratel T, Wennlund A, Carlström K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP). Respir Med. 1999;93:1‐7. [DOI] [PubMed] [Google Scholar]
- 34. Castro‐Grattoni AL, Torres G, Martínez‐Alonso M, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24‐h ambulatory blood pressure monitoring. Eur Respir J. 2017;50(4):1700651. [DOI] [PubMed] [Google Scholar]
- 35. Feres MC, Cintra FD, Rizzi CF,, et al. Evaluation and validation of a method for determining platelet catecholamine in patients with obstructive sleep apnea and arterial hypertension. PLoS One. 2014;9:e98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrier KA, Neill AM, O’Meeghan T, Richards M, Campbell AJ. Continuous positive airway pressure in heart failure patients with obstructive sleep apnoea. Int Med J. 2008;38:829‐836. [DOI] [PubMed] [Google Scholar]
- 37. Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7:199‐207. [DOI] [PubMed] [Google Scholar]
- 38. Krieger J, Schmidt M, Sforza E, et al. Urinary excretion of guanosine 3′: 5′‐cyclic monophosphate during sleep in obstructive sleep apnoea patients with and without nasal continuous positive airway pressure treatment. Clin Sci (Lond). 1989;76:31‐37. [DOI] [PubMed] [Google Scholar]
- 39. Minemura H, Akashiba T, Yamamoto H, Akahoshi T, Kosaka N, Horie T. Acute effects of nasal continuous positive airway pressure on 24‐hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med. 1998;37:1009‐1013. [DOI] [PubMed] [Google Scholar]
- 40. Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab. 2012;97:4212‐4218. [DOI] [PubMed] [Google Scholar]
- 41. Pinto P, Bárbara C, Montserrat JM, et al. Effects of CPAP on nitrate and norepinephrine levels in severe and mild‐moderate sleep apnea. BMC Pulm Med. 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sukegawa M, Noda A, Sugiura T, et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24‐hour urinary catecholamines. Clin Cardiol. 2005;28:519‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tachikawa R, Ikeda K, Minami T, et al. Changes in energy metabolism after continuous positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:729‐738. [DOI] [PubMed] [Google Scholar]
- 44. Unterberg C, Lüthje L, Szych J, Vollmann D, Hasenfuß G, Andreas S. Atrial overdrive pacing compared to CPAP in patients with obstructive sleep apnoea syndrome. Eur Heart J. 2005;26:2568‐2575. [DOI] [PubMed] [Google Scholar]
- 45. Donadio V, Liguori R, Vetrugno R, et al. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res. 2007;16:327‐332. [DOI] [PubMed] [Google Scholar]
- 46. Jennum P, Wildschiødtz G, Christensen NJ, Schwartz T. Blood pressure, catecholamines, and pancreatic polypeptide in obstructive sleep apnea with and without nasal continuous positive airway pressure (nCPAP) treatment. Am J Hypertens. 1989;2(11 Pt 1):847‐852. [DOI] [PubMed] [Google Scholar]
- 47. Lemmer B, Scholtze J, Schmitt J. Circadian rhythms in blood pressure, heart rate, hormones, and on polysomnographic parameters in severe obstructive sleep apnea syndrome patients. Blood Press Monit. 2016;21:136‐143. [DOI] [PubMed] [Google Scholar]
- 48. Nicholl DD, Hanly PJ, Zalucky AA, et al. CPAP therapy delays cardiovagal reactivation and decreases arterial renin‐angiotensin system activity in humans with obstructive sleep apnea. Journal of clinical sleep medicine. J Clin Sleep Med. 2018;14:1509‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta‐analysis of placebo‐controlled randomized trials. Arch Intern Med. 2007;167:757‐764. [DOI] [PubMed] [Google Scholar]
- 50. Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta‐analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turnbull F. Effects of different blood‐pressure lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527‐1535. [DOI] [PubMed] [Google Scholar]
- 52. Sawka AM, Jaeschke R, Singh RJ, Young WF Jr. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24‐hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553‐558. [DOI] [PubMed] [Google Scholar]
- 53. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878‐1886. [DOI] [PubMed] [Google Scholar]
- 54. Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham‐CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long‐term Efficacy Study (APPLES). Sleep. 2012;35:1593‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Franklin K, Lindberg E. Obstructive sleep apnea is a common disorder in the population ‐ a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bischof F, Egresits J, Schulz R, et al. Effects of continuous positive airway pressure therapy on daytime and nighttime arterial blood pressure in patients with severe obstructive sleep apnea and endothelial dysfunction. Sleep Breath. 2020;24(3):941‐951. [DOI] [PubMed] [Google Scholar]
- 58. Burioka N, Koyanagi S, Endo M, et al. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2008;32:105‐112. [DOI] [PubMed] [Google Scholar]
- 59. Grimpen F, Kanne P, Schulz E, Hagenah G, Hasenfuss G, Andreas S. Endothelin‐1 plasma levels are not elevated in patients with obstructive sleep apnoea. Eur Respir J. 2000;15:320‐325. [DOI] [PubMed] [Google Scholar]
- 60. Heitmann J, Ehlenz K, Penzel T, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J. 2000;15:320‐325. [DOI] [PubMed] [Google Scholar]
- 61. Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24‐hour profiles of glucose, insulin and counter‐regulatory hormones in type 2 diabetes. Diabet Obesity Metabol. 2017;19:452‐456. [DOI] [PubMed] [Google Scholar]
- 62. Nakamura T, Chin K, Shimizu K, et al. Acute effect of nasal continuous positive airway pressure therapy on the systemic immunity of patients with obstructive sleep apnea syndrome. Sleep. 2001;24:545‐553. [DOI] [PubMed] [Google Scholar]
- 63. Rodenstein DO, d'Odemont JP, Pieters T, Aubert‐Tulkens G. Diurnal and nocturnal diuresis and natriuresis in obstructive sleep apnea: effects of nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1992;145:1367‐1371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


