Abstract
Over the last two decades, rapid technological advances have dramatically changed radiation delivery to children with cancer, enabling improved normal-tissue sparing. This article describes recent advances in photon and proton therapy technologies, image-guided patient positioning, motion management, and adaptive therapy that are relevant to pediatric cancer patients. For medical physicists who are at the forefront of realizing the promise of technology, challenges remain with respect to ensuring patient safety as new technologies are implemented with increasing treatment complexity. The contributions of medical physicists to meeting these challenges in daily practice, in the conduct of clinical trials, and in pediatric oncology cooperative groups are highlighted. Representing the perspective of the physics committees of the Children’s Oncology Group (COG) and the European Society for Paediatric Oncology (SIOP Europe), this paper provides recommendations regarding the safe delivery of pediatric radiotherapy. Emerging innovations are highlighted to encourage pediatric applications with a view to maximizing the therapeutic ratio.
Keywords: pediatric cancer, radiation therapy, medical physicist, COG, SIOP
1. Introduction
Over last two decades, we have seen several breakthrough technologies in radiation therapy (RT) evolve from experimental to widely adopted in the treatment of pediatric cancers. These technologies enable more conformal targeting of disease with better sparing of healthy tissues, which are often located close to the tumor, than was possible with older modalities. This sparing of normal tissues is particularly relevant to pediatric patients: As a result of the improving survival rates in that population, patients may live for decades after being treated and are, therefore, increasingly likely to experience the late effects of treatment toxicities. This article describes recent advances in RT technologies and techniques particularly relevant to pediatric cancer treatment.
Medical physicists in radiation oncology departments are at the forefront of developing and implementing new technologies for delivering RT to cancer patients, including children. In addition, they calibrate RT equipment and perform regular quality assurance (QA); provide clinical physics services and technical consultations; train resident physicians and physicists, as well as departmental staff; and conduct scientific research.1 In order to perform and supervise technical components of RT, medical physicists are normally required to undergo specialized training and obtain certification from an examination board, such as the American Board of Radiology, the Canadian College of Physicists in Medicine, or the European Federation of Organizations for Medical Physics. To increase the interdisciplinary collaboration and encourage participation from more medical physicists in optimizing the treatment of pediatric cancer, this article also highlights important roles which medical physicist play in this special field.
2. Recent advances in photon therapy that are relevant to pediatric patients
Intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) are now the standard of care for the treatment of many pediatric cancers. Applications of stereotactic body radiation therapy (SBRT) for treating bone tumors, as well as metastatic and recurrent lesions, in children are under investigation. Additionally, high dose-rate (HDR) brachytherapy is now being used in some centers to treat pediatric sarcomas.
The classic treatment for Hodgkin lymphoma (HL) with RT involved the use of a full mantle field covering a large area of the neck, chest, and axillary regions in order to encompass all the main lymph node areas in the upper half of the body. Some shielding was provided for the lungs and heart. In recent years, RT for HL has evolved to use much smaller fields targeting only the sites of disease, an approach termed involved-site radiation therapy (ISRT). The goal of ISRT is to accurately target diseased nodes while minimizing normal tissue exposure, thereby reducing toxicity and the risk of late effects.2,3 Approximately three-quarters of the patients receiving RT on the current Children’s Oncology Group (COG) high-risk HL study (AHOD1331), which incorporates the use of ISRT, have been treated with either IMRT/VMAT or proton therapy. An example is illustrated in Fig. 1. One of the credentialing requirements for the use of either IMRT/VMAT with gating/tracking methods or proton therapy on AHOD1331 is the irradiation of a tissue-equivalent lung phantom (photon or proton) available from the Imaging and Radiation Oncology Core (IROC) Houston QA Center (http://rpc.mdanderson.org/RPC/home.htm).
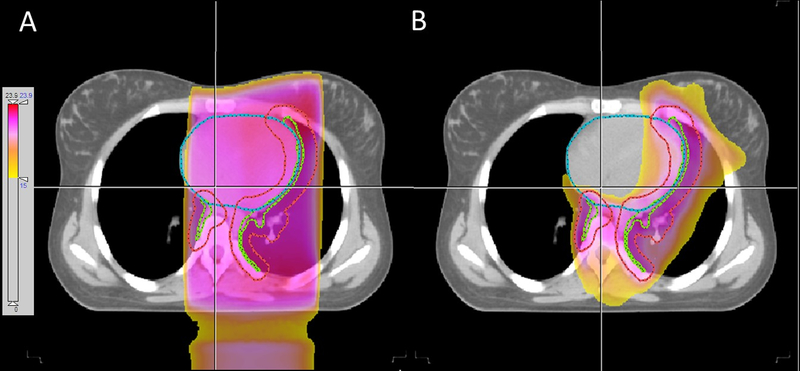
FIGURE 1.
Comparison of conventional parallel opposed field (A) and three-arc VMAT (B) plans for an adolescent female who received involved site RT for Hodgkin lymphoma. Clinical target volume (CTV) and PTV are shown in green and red, respectively. The delivered VMAT plan provided significant sparing of the heart, left lung, and left breast.
Two recent COG sarcoma studies (AEWS1221 and ARST1431) have incorporated SBRT for treating bone metastases. Building on the success of SBRT in treating metastatic tumors of the spine, lung, and liver, the rationale for its use in these sarcoma studies relies on the radioresistant histology of sarcomas and the promise of lower toxicity because of the rapid dose fall-off surrounding the target lesions.4 The proper use of SBRT requires strict immobilization of the patient, methods to account for or limit the motion of internal organs during treatment, and daily image guidance. It is the responsibility of the medical physicist to ensure the accurate delivery of SBRT and the completion of required credentialing tests, often by irradiating appropriate phantoms as specified in the clinical trial protocols.
New techniques for craniospinal irradiation are being used to treat medulloblastoma in children with the aim of minimizing late effects by reducing the dose to normal tissues.5,6 In a recent COG study (ACNS0332), one-quarter of the patients receiving RT were treated with either helical tomotherapy or proton therapy for precisely this purpose. The improved dose conformity achievable with these techniques can markedly decrease the radiation dose to the heart, lungs, and vertebral bodies.7 However, it is believed that a high dose gradient within vertebrae in children who have not completed the growth spurt may lead to an increased risk of radiation-induced kyphoscoliosis. A recent expert consensus from the European Society for Paediatric Oncology (SIOP Europe) radiotherapy working group recommended homogeneous dose in the left-right and posterior-anterior vertebral dimensions if possible.8 Because literature reports lack detailed analysis of dose distributions over the vertebrae, research based on reliable data is needed to validate or update this practice recommendation.
The use of IMRT has been proposed to improve cardiac sparing in the delivery of whole-lung irradiation (WLI), which is used in the management of pulmonary metastases from various childhood cancers.9 A recent multi-institutional feasibility study has demonstrated the feasibility of using IMRT for WLI and has confirmed its advantages, including superior cardiac protection and superior dose coverage of four-dimensional (4D) lung volumes.10 This is a fairly novel application of IMRT, and medical physicists need to be involved in all steps of the planning process, including IMRT credentialing, treatment planning, and quality assurance before treatment.
When bone marrow transplantation is used to treat childhood leukemia, one step in the preparatory regimen may be total-body irradiation (TBI). There are several techniques for delivering TBI, and the choice of technique for a particular institution can depend on various factors, including the age of the patient, the size of the treatment room, and the need for anesthesia in the case of young children. The implementation of the chosen technique requires the participation of the medical physicist. Most institutions deliver TBI with a relatively standard treatment-field setup, but several more elaborate techniques have been developed that can facilitate sparing of normal tissues, especially the lungs. An earlier innovation is the modulated arc technique,11 which uses multiple static fields in an arc formation. Inverse optimization is used to optimize the relative weights of the fields. Tomotherapy has also been used to deliver both TBI and total marrow irradiation with excellent sparing of normal tissue.12 More recently, several institutions reported successful experience in implementing linac-based VMAT for TBI.13–15
Although HDR brachytherapy is less common than external-beam RT in treating pediatric cancers, outcomes were encouraging when used to treat pediatric soft tissue sarcomas.16 The shifting trend from low dose-rate to HDR brachytherapy is likely due to the advantages of minimal radiation exposure to caregivers and better patient compliance.17,18 The use of pulsed dose-rate brachytherapy has been reported for treating pediatric rhabdomyosarcoma but is not widely adopted compared to HDR brachytherapy.
3. Recent advances in proton therapy that are relevant to pediatric patients
Proton therapy is the most widely available version of particle therapy. Recently, pencil-beam scanning (PBS) techniques have started to replace conventional delivery methods for proton therapy, such as passive scattering, and are now considered to represent the state of the art for this treatment modality. PBS enables increased conformity and flexibility for treating complex tumors with novel techniques that are simply not achievable with conventional proton or photon therapy. PBS planning and delivery systems are capable of producing single-field uniform dose (SFUD) treatment fields, which are generally considered to be more robust to treatment uncertainties19,20, as well as the more complex, highly modulated, but also highly conformal, distributions resulting from intensity-modulated proton therapy (IMPT)21. In particular, the flexibility of IMPT has allowed for the introduction of novel techniques in pediatric radiation oncology, such as IMPT-enabled craniospinal irradiations without match lines (field junction), and treatments that can significantly reduce the doses to the anterior organs of the patient. Among the many planning techniques used to achieve this goal, the split-target gradient technique is attractive because it enables soft transitions between fields, making the plan less sensitive to field overlaps due to setup errors.22,23 Fig. 2 shows the actual delivered plan of an example patient.
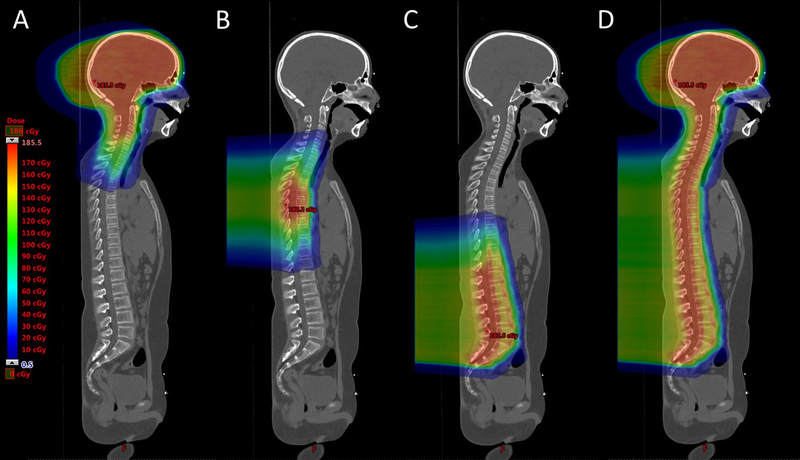
FIGURE 2.
An example of a robust optimized IMPT plan for craniospinal irradiation of a 12-year-old male with medulloblastoma. No junction changes were required during the RT course. Dose distributions, from left to right, are for the brain treatment fields (A), the upper spine field (B), the lower spine field (C), and the sum of all fields (D).
An important consideration for proton therapy is the increase in linear energy transfer (LET) in the Bragg peak and the resulting variation of LET across a given proton field. LET is defined as the energy deposited per micron of a proton’s path, which in turn can be directly related to the complexity of DNA and biological damage. Although the clinical relevance of this is not clear, it would be prudent to also evaluate the dose-averaged LET distribution together with the physical dose distribution for each patient to avoid the treatment plan unknowingly placing high dose-weighted LET inside critical organs such as pediatric brainstem, potentially causing severe toxicity. LET can be further incorporated into the plan optimization, such that potential variations in the biological effect can be better managed or even exploited by focusing regions of high LET into the tumor.24 To exploit the biological variation of a proton beam is currently an active area of medical physics research.
Lateral penumbra and secondary neutron dose are physical aspects of proton therapy that have specific impact on pediatric patients. First, lateral penumbra is the sharpness of the dose fall-off orthogonal to the field direction. Often, shallower targets have larger penumbras because of larger pencil beam spot sizes at lower proton energies. Many pediatric treatments, such as for retinoblastoma as well as orbital and extremity sarcomas, could benefit from a sharper treatment field with an aperture collimator, even though the use of collimation is not well supported in current PBS technology. This is an area where great advances are being made by medical physicists for improving pediatric PBS proton therapy. Second, neutron dose, particularly with passive scattering systems, was a matter of concern and controversy25 as neutrons have a high biological effectiveness. Because PBS is widely available now and a much more efficient delivery method which reduces neutron contamination by between 2–3 orders of magnitude in comparison to passive scattering, the previous concern for pediatric neutron exposure have subsided. Currently, a neutron background approximately equal to the neutron level from irradiation with photons with energies above 10MV can be expected. If collimation is used however, the additional neutron component in the beam resulting from proton interactions in the collimator should not be overlooked, even if the levels will still be considerably less than from passively scatter techniques.
Due to the well-defined range of protons, proton therapy is also assumed to be more sensitive to delivery uncertainties than conventional radiotherapy with photons, resulting in the development of sophisticated tools for evaluating and optimizing the robustness of proton plans to set-up and range uncertainties.26–30 These tools are now being increasingly employed in the clinic, leading to new concepts of uncertainty management in proton therapy that move away from the conventional planning target volume (PTV) concept. Additional parameters that account for factors such as anatomical variations and/or respiratory motion may also need to be considered to best exploit the advantages of proton therapy for pediatric cases, as will be discussed in the following section.
In parallel with management of uncertainties at the planning stage, it is also important to reduce uncertainties as much as possible by directly addressing their sources. Several techniques for reducing range uncertainty, for example, dual-energy computed tomography (CT) and in vivo range verification, have been proposed and deployed in clinical environments. One source of range uncertainty derives from the calibration of the CT number to proton stopping-power ratio. Dual-energy CT improves the accuracy of the range calculation by differentiating between mass density and chemical composition changes, which is not possible with conventional single-energy CT. This improvement can decrease the range uncertainty and normal tissue exposure.31,32 In addition to dosimetric improvement, dual-energy CT can improve tissue contrast and artifact management, which are also sources of uncertainty. Moreover, in vivo range verification techniques, such as prompt gamma or protoacoustic detection and in-treatment positron-emission tomography, have shown promise for directly measuring the range of a given proton beam in the patient, thereby allowing patient-specific adjustments in order to avoid irradiating normal tissues or improve tumor coverage.33
Alternatively, the calibration to proton stopping power can, in principle, be avoided completely with the use of proton-CT, i.e. the direct use of high energy protons for tomographic imaging of patients 34,35. Although still in its infancy, this technique shows some promise. Indeed, despite many practical issues, not least of which is the need for proton energies high enough to penetrate through all regions of the patient, proton-CT could find its first applications in pediatric proton therapy. With maximum ranges of around 30–35cm, many current proton facilities, have sufficient energy to penetrate, and therefore image, all anatomical regions of younger pediatric patients, thus allowing for proton based, volumetric imaging of all pediatric tumor sites. In addition, the much reduced imaging dose resulting from proton-CT would have obvious advantages for pediatric patients.
From the clinical point of view, the main advantage of protons over conventional therapy for pediatric treatments is in the reduction of the volumes of normal tissue receiving mid-to-low doses in relation to the tumor dose. These reductions in turn result in significant reductions in the integral dose delivered to all non-target healthy tissues, an extremely important goal in the irradiation of pediatric patients. Depending on the position and volume of the tumor and the beam angle and selection, normal tissue integral doses can be reduced substantially.36,37 Such an approach can be seen in Fig. 2, showing the PBS proton irradiation of a craniospinal axis using all posterior fields for the spinal treatment volume, which clearly minimize the path length to the spinal cord and completely spare all abdominal organs and structures.
4. Image-guided patient positioning, motion management, and adaptive therapy for pediatric patients
The importance and adoption of image-guided patient positioning have increased over the last two decades as pediatric RT has become more conformal and less forgiving of patient positioning inaccuracy. Based on a 2017 COG survey of pediatric image-guided radiotherapy (IGRT) practice patterns, daily image guidance was favored for various conformal RT treatments of brain and body tumors by a high percentage (45%–74%) of the surveyed radiation oncologists.38 However, only approximately one third would prescribe volumetric CT or cone-beam CT (CBCT) for image-guided verification. Similarly, the 2015 survey of 40 international member institutions of the Paediatric Radiation Oncology Society reported that only 32% performed either kilovoltage or megavoltage daily volumetric IGRT for pediatric patients.39 The reluctance to fully embrace volumetric image guidance techniques despite their advantages may be a consequence of the longer room time required and the concern over increased secondary cancer risk in children with higher radiation exposure from image guidance. While awaiting evidence from large consortium studies, such as the international pediatric CT scan study (EPI-CT),40 it is recommended that institutions, particularly medical physicists, examine and optimize their pediatric image guidance protocols to promote a culture and practice of gentle IGRT.41 The COG Radiation Oncology Discipline is preparing expert recommendations on managing the imaging dose for pediatric patients. Vendors have already developed limited-arc CBCT acquisition and iterative reconstruction methods to reduce radiation exposure without sacrificing image quality. Physicists also determine the feasibility of reducing the safety margin with frequent volumetric image guidance, which could decrease the volume of tissues receiving high doses and potentially offer a better tradeoff than a larger margin with less frequent imaging. Surface imaging 42 and magnetic resonance (MR) guidance 43,44 could also be viable non-ionizing IGRT alternatives for pediatric patients, having attractive capabilities to monitor patient movement and/or track internal tumor motion.
In the last few years, several clinics have begun to perform MR-only treatment planning for prostate and gynecologic cancers,45,46 a planning technique that had traditionally been limited to brain stereotactic radiosurgery. MR planning eliminates the systematic uncertainties arising from CT-MR image registration and the radiation exposure to patients from the CT simulation procedure. These advantages are particularly relevant to pediatric patients for whom the radiation oncology community strives to minimize the risk of radiation-induced complications. Although such practice is still rare for pediatric patients, the feasibility has been demonstrated to generate synthetic CT from magnetic resonance imaging (MRI) for photon and proton therapy of pediatric brain and abdominal tumors.47,48
Large respiratory motion can result in significant declines in target coverage and dose homogeneity if delivered without additional motion mitigation. This is particularly relevant when treating pediatric patients with neuroblastoma, Hodgkin lymphoma, hepatoblastoma, Wilms tumor, chest wall sarcoma, or tumors near the diaphragm. Various 4D CT, 4D MRI, and CBCT techniques have been used to measure pediatric tumor and organ motion.49–52 As commercial 4D CT was designed primarily for adult patients with lung cancer, the scan parameters and pressure bellows need to be modified for children. Studies have found that the extent of respiration-induced motion is smaller in younger children than in older individuals but that this motion can approach or exceed 1 cm for the diaphragm, spleen, kidney, and some tumors of adolescents.53 Because of the large individual variations in adolescents, population motion data may not be predictive; therefore, patient-based assessment to define an appropriate internal target volume (ITV) and regular monitoring during the treatment course are recommended.54 An example to illustrate the patient-specific assessment of target motion with 4D MRI is given in Fig. 3. For photon therapy, spirometry-assisted breath-hold and deep inspiration breath-hold techniques have proved feasible in the pediatric setting.55–57 For PBS proton therapy, re-scanning (the multiple application of all Bragg peaks within a field) is recommended in order to smooth out motion induced dose homogeneities, and where possible, combining re-scanning with gating or breath-hold techniques in order also to reduce the magnitude of motion.
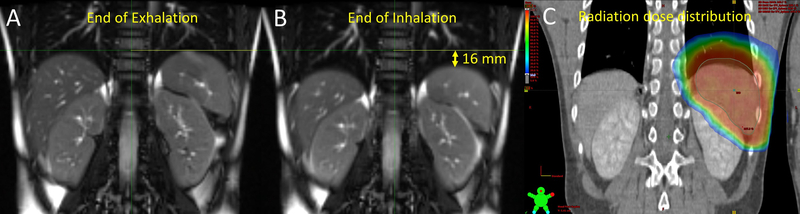
FIGURE 3.
An 11-year-old male with Hodgkin lymphoma who underwent 4D MRI to define the ITV for consolidative RT to the spleen. The spleen position differed by 16 mm between the end of exhalation (A) and the end of inhalation (B). The radiation dose distribution of the photon therapy plan is shown at right (C).
The practice of adaptive therapy is common when treating children with RT. Seventy-three percent of physicist respondents in the COG survey reported that adaptive re-planning was routinely performed in their institutions. This replanning was performed offline rather than online which would have time and resource constraints if the patient was waiting on the treatment table. Adaptive replanning could be triggered by repeat CT, by image guidance with CBCT, or by MR imaging performed during the RT course. It may benefit pediatric patients with steroid-related weight changes, cyst expansion, rapid tumor response, tumor progression, or tumor position shifts due to hydrocephalus or epidural fluid changes.58–60 Despite the progress in biology-guided adaptive therapy for adults,61 pediatric adaptive therapy continues to be anatomy based. The optimal timing for on-treatment surveillance imaging has yet to be determined for pediatric malignant neoplasms.
Given the higher dose conformity provided by the proton Bragg peak, adaptive therapy is arguably even more important in proton therapy. For instance, in addition to changes in tumor volume, changes in the normal tissues and anatomies surrounding the tumor can substantially affect the range, and therefore accuracy, of proton irradiations62. This can be particularly a problem for very young pediatric patients, where growth rates can be substantial, or for patients on concomitant chemo-therapy where substantial loss of weight throughout the treatment period is not uncommon. Thus, regular monitoring of patient anatomy is required, with possibly frequent adaptation of the treatment to account for any changes. As such, an efficient workflow for quickly generating new treatment plans in order to react to anatomical and tumor changes would be of great advantage.
5. Quality assurance and patient safety
To ensure that pediatric patients are treated safely, a QA program and safety measures need to be established at each institution. For confirming constancy and accuracy, medical physicists perform regular QA tests and measurements on treatment planning systems, RT equipment, imaging equipment for RT simulation and patient positioning, record and verify systems for RT delivery, and patient-specific dosimetry. Although QA tests do not typically differ for pediatric and adult treatments, there are unique aspects that medical physicists consider when treating children.
Because many pediatric patients are treated on clinical trials and specific guidelines must be followed, physicists and dosimetrists must be attentive to the differences in normal tissue constraints for adults and children. Before participating in cooperative group trials, physicists should carefully read the trial requirements and communicate with trial QA centers for site qualification and benchmark credentialing requirements to enable timely enrollment. Pediatric anthropomorphic phantoms are being increasingly used for end-to-end tests and credentialing. Examples include a pediatric TBI phantom 63 and a pediatric spine phantom 64, along with a pediatric CT dose index and anthropomorphic phantoms for dosimetry verification.
Superficial or small treatment fields are not unique to pediatric RT but are commonly used for younger patients and for those with extremity or head and neck (including orbital) sarcomas or metastases and/or for those undergoing re-irradiation. The use of a small calculation grid size, in vivo dosimetry for the surface dose, Monte Carlo calculations, and improved small-volume detectors for small-field dosimetry are some of the methods that have been recommended to medical physicists.65
Strategies to enhance patient safety for children undergoing RT have not been reported beyond sedation and anesthesia procedures.66 Institutions are encouraged to participate in and read reports from the Radiation Oncology Incident Learning System (RO-ILS),67 which are generally applicable to pediatric RT. We provide the following recommendations regarding the safe delivery of pediatric RT:
Avoid rushed planning and treatments. When a tight schedule is unavoidable because of emergencies or timing constraints due to concurrent chemotherapy specified in clinical trials, pretreatment plan checks and patient-based QA should still be carefully performed.
Multimodality image registration is common in pediatric RT planning because of the large percentage of brain and soft tissue tumors in children. To prevent the use of outdated or incorrect patient scans, care should be taken when importing images acquired in outside departments that use different naming systems.
Some organs are more radiosensitive in children than in adults. Be aware of normal tissue constraints that are not typically monitored for adult patients such as musculoskeletal system and out-of-field doses to thyroid, breasts, and gonads. Evidence-based radiation dose-volume guidelines for normal tissue constraints in treatment planning from the PENTEC group are forthcoming.68
Effective handoff communication is important, particularly for treatments with isocenter shifts or multiple isocenters, such as craniospinal irradiation.
Robust pretreatment verification and time-out procedures should be strictly followed. This is particularly important for sedated or anesthetized children who cannot communicate.
Use proper immobilization techniques and surveillance cameras to minimize and monitor patient movement during treatment.
Understand your image guidance doses to children and modify adult scan protocols for pediatric patients.
Understand and resolve institutional deviations and violations in pediatric clinical trials.
6. The roles of medical physicists in clinical trials cooperative groups for pediatric cancers
Medical physicists actively contribute to pediatric oncology cooperative groups and professional societies. Within the COG Radiation Oncology Discipline, the Physics Committee collaborates with the Disease Committees and the IROC to develop RT protocol guidelines, design benchmarks for evaluating institutional capability, conduct surveys on practice patterns, study radiation dosimetry and clinical outcomes, communicate with physics representatives from National Clinical Trials Network (NCTN) groups, and make practice recommendations. Medical physicists are also on staff at IROC Rhode Island and IROC Houston, two clinical trial QA centers that support COG trials with credentialing services and the review of radiation therapy treatment data. COG and SIOP offer medical physicists excellent opportunities to participate in all phases of clinical trials, obtain access to large datasets that would otherwise be unavailable at a single institution, and make clinical impacts at the international level.
7. Outlook
In addition to the activities and advances described above, many exciting technological innovations in RT are being evaluated at selected institutions. Most of them are led by medical physicists and are expected to exert a substantial impact on efficiency, safety, and novel applications of RT. These innovations include automation (organ segmentation, knowledge-based planning, plan QA), the application of machine learning, knowledge-guided prescription, improved biological modeling and optimization, fast and low-dose image guidance procedures, dynamic trajectory optimization of beam delivery, ultrahigh dose rate delivery, online adaptive therapy, and big data analytics. Although the pediatric applications are currently limited, the impact of these innovations is expected to increase over the next decade. Future opportunities are abundant, but the close collaboration of physicists with other members of the radiation oncology team is required to expedite clinical adoption and achieve the ultimate goal of curing cancers while minimizing toxicity.
8. Conclusion
The overall trend in pediatric radiation oncology is toward delivering highly conformal RT with IMRT, VMAT, and PBS proton therapy for significant normal tissue sparing, even in patients with diseases traditionally treated with parallel opposed beams, such as Hodgkin lymphoma and neuroblastoma. Faced with increased treatment complexity, the RT team should foster a culture of patient safety and embrace the challenges that such a culture presents in order to advance pediatric cancer therapy. Medical physicists will continue to play essential roles in cancer patient treatment and in pediatric clinical trials.
Acknowledgements
We acknowledge the support by NCI/NIH grant U24 CA180803 to the Imaging and Radiation Oncology Core. The authors thank Dr. John Kalapurakal, COG Radiation Oncology Discipline Chair, and Dr. Geert O. Janssens, SIOP Europe Radiotherapy Group Chair, for their leadership and for their valuable feedback on the manuscript. We also thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript.
ABBREVIATIONS KEY
- 4D
four-dimensional
- CBCT
cone-beam computed tomography
- COG
Children’s Oncology Group
- CT
computed tomography
- CTV
clinical target volume
- HDR
high dose rate
- HL
Hodgkin lymphoma
- IGRT
image guided radiation therapy
- IMPT
intensity-modulated proton therapy
- IMRT
intensity-modulated radiation therapy
- IROC
Imaging and Radiation Oncology Core
- ISRT
involved-site radiation therapy
- ITV
internal target volume
- LET
linear energy transfer
- MRI
magnetic resonance imaging
- NCTN
National Clinical Trials Network
- PBS
pencil-beam scanning
- PTV
planning target volume
- QA
quality assurance
- RT
radiation therapy
- SBRT
stereotactic body radiation therapy
- SFUD
single-field uniform dose
- SIOP
International Society of Paediatric Oncology
- TBI
total-body irradiation
- VMAT
volumetric modulated arc therapy
- WLI
whole-lung irradiation
Footnotes
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.American Association of Physicists in Medicine. What do medical physicists do? https://www.aapm.org/medical_physicist/default.asp. Accessed July 3, 2019.
- 2.Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson DC, Dieckmann K, Terezakis S, et al. Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol. 2015;5(2):85–92. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, Bastasch M, Saha D, et al. Stereotactic body radiation therapy: normal tissue and tumor control effects with large dose per fraction. Front Radiat Ther Oncol. 2011;43:382–394. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Drodge S, Jacques A, et al. A comparative planning analysis and integral dose of volumetric modulated arc therapy, helical tomotherapy, and three-dimensional conformal craniospinal irradiation for pediatric medulloblastoma. J Med Imag Radiat Sci. 2015;46(2):134–140. [DOI] [PubMed] [Google Scholar]
- 6.Brower JV, Gans S, Hartsell WF, et al. Proton therapy and helical tomotherapy result dose deposition to the pancreas in the setting of cranio-spinal irradiation for medulloblastoma: implications for reduced risk of diabetes mellitus in long-term survivors. Acta Oncol. 2015;54(4):563–566. [DOI] [PubMed] [Google Scholar]
- 7.Seravalli E, Bosman M, Lassen-Ramshad Y, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy working group). Acta Oncol. 2018;57(9):1240–1249. [DOI] [PubMed] [Google Scholar]
- 8.Hoeben BA, Carrie C, Timmermann B, et al. Management of vertebral radiotherapy dose in paediatric patients with cancer: consensus recommendations from the SIOPE radiotherapy working group. Lancet Oncol. 2019:20(3):e155–e166. [DOI] [PubMed] [Google Scholar]
- 9.Kalapurakal JA, Zhang Y, Kepka A, et al. Cardiac-sparing whole lung IMRT in children with lung metastasis. Int J Radiat Oncol Biol Phys. 2013;85(3):761–767. [DOI] [PubMed] [Google Scholar]
- 10.Kalapurakal JA, Gopalakrishnan M, Walterhouse DO, et al. Cardiac-sparing whole lung IMRT in patients with pediatric tumors and lung metastasis: final report of a prospective multicenter clinical trial. Int J Radiat Oncol Biol. 2019;103(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby N, Held M, Morin O, et al. Inverse-planned modulated-arc total-body irradiation. Med Phys. 2012;39(5):2761–2764. [DOI] [PubMed] [Google Scholar]
- 12.Wong JYC, Rosenthal J, Liu A, et al. Image guided total marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73(1):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Springer A, Hammer J, Winkler E, et al. Total body irradiation with volumetric modulated arc therapy: dosimetric data and first clinical experience. Radiat Oncol. 2016;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang L, Folkerts M, Zhang Y, et al. Volumetric modulated arc therapy based total body irradiation: Workflow and clinical experience with an indexed rotational immobilization system. Phys Imag Radiat Oncol. 2017:4:22–25. [Google Scholar]
- 15.Tas B, Furmus IF, Okumus A, et al. Total-body irradiation using linac-based volumetric modulated arc therapy: Its clinical accuracy, feasibility and reliability. Radiat Oncol. 2018:527–533. [DOI] [PubMed] [Google Scholar]
- 16.Tinkle CL, Lucas JT. The non-rhabdomyosarcoma soft tissue sarcomas, desmoid tumor and osteosarcoma. In: Merchant TE, Kortmann RD. (eds) Pediatric Radiation Oncology. Pediatric Oncology. New York: Springer; 2018. 45–85 p. [Google Scholar]
- 17.Viani GA, Novaes PE, Jacinto AA, et al. High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol. 2008;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskar S, Pilar A, Khanna N, et al. Interstitial brachytherapy for pediatric soft tissue sarcoma: evolving practice over three decades and long‐term outcomes. Pediatr Blood Cancer. 2018;65(9):227112. [DOI] [PubMed] [Google Scholar]
- 19.Stuschke M, Kaiser A, Pöttgen C, et al. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother Oncol. 2012;104(1):45–51. [DOI] [PubMed] [Google Scholar]
- 20.Boria AJ, Uh J, Pirlepesov F, et al. Interplay effect of target motion and pencil beam scanning in proton therapy for pediatric patients. Int J Part Ther. 2018;5(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safai S, Trofimov A, Adams JA, et al. The rationale for intensity-modulated proton therapy in geometrically challenging cases. Phys Med Biol. 2013;58(18):6337–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seravalli E, Bosman M, Lassen-Ramshad Y, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy working group). Acta Oncol. 2018;57(9):1240–1249. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Ding X, Kirk M, et al. Supine craniospinal irradiation using proton pencil beam scanning technique without match line changes for field junctions. Int J Radiat Oncol Biol Phys. 2014;90(1):71–78. [DOI] [PubMed] [Google Scholar]
- 24.Bai X, Lim G, Grosshans D, et al. Robust optimization to reduce the impact of biological effect variation from physical uncertainties in intensity-modulated proton therapy. Phys Med Biol. 2019;64(2):025004. [DOI] [PubMed] [Google Scholar]
- 25.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1–7. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Zhang X, Li Y, et al. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39(2):1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe M, Aitkenhead A, Albertini F, et al. A robust optimization approach accounting for the effects of fractionation on setup uncertainties. Phys Med Biol. 2017;62:8178–8196. [DOI] [PubMed] [Google Scholar]
- 28.Wedenberg M, Beltran C, Mairani A, Alber M. Advanced treatment planning. Med Phys. 2018;45(11):e1011–e1023. [DOI] [PubMed] [Google Scholar]
- 29.Unkelbach J, Alber M, Bangert M, et al. Robust radiotherapy planning. Phys Med Biol. 2018;63(22):22TR02. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Frank SJ, Li X, et al. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys. 2013;40(5):051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Elmpt W, Landry G, Das M, et al. Dual energy CT in radiotherapy: current applications and future outlook. Radiother Oncol. 2016;119(1):137–44. [DOI] [PubMed] [Google Scholar]
- 32.Bär E, Lalonde A, Royle G, et al. The potential of dual-energy CT to reduce proton beam range uncertainties. Med Phys. 2017;44(6):2332–2344. [DOI] [PubMed] [Google Scholar]
- 33.Parodi K, Polf JC. In vivo range verification in particle therapy. Med Phys. 2018;45(11):e1036–e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poludniowski G, Allinson NM, Evans PM. Proton radiography and tomography with application to proton therapy. Br J Radiol. 2015;88(1053):20150134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedes G, Johnson RP, Pankuch M, et al. Experimental fluence-modulated proton computed tomography by pencil beam scanning. Med Phys. 2018;5(7):3287–3296. [DOI] [PubMed] [Google Scholar]
- 36.Lomax AJ, Bortfeld T, Goitein G, et al. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51(3):257–71. [DOI] [PubMed] [Google Scholar]
- 37.Schneider U, Lomax AJ, Timmermann B. Second cancers in children treated with modern radiotherapy techniques. Radiother Oncol. 2008;89(2):135–140. [DOI] [PubMed] [Google Scholar]
- 38.Hua C, Vern-Gross TZ, Hess CB, et al. Practice patterns of pediatric image-guided radiotherapy (IGRT): a Children’s Oncology Group report. Int J Radiat Oncol Biol Phys. 2019;105(1):S187. [Google Scholar]
- 39.Seiersen K. Result of large international medical physics survey on paediatric radiation therapy. Presentation PD-119. 48th Congress of the International Society of Paediatric Oncology, Dublin, Ireland, October 19–22, 2016. [Google Scholar]
- 40.Bosch de Basea M, Pearce MS, Kesminiene A, et al. EPI-CT: design, challenges and epidemiological methods of an international study on cancer risk after paediatric and young adult CT. J Radiol Prot. 2015;35:611–628. [DOI] [PubMed] [Google Scholar]
- 41.Hess CB, Thompson HM, Benedict SH, et al. Exposure risks among children undergoing radiation therapy: considerations in the era of image guided radiation therapy. Int J Radiat Oncol Biol Phys. 2016;94(5):978–992. [DOI] [PubMed] [Google Scholar]
- 42.Sueyoshi M, Olch AJ, Liu KX, et al. Eliminating daily shifts, tattoos, and skin marks: streamlining isocenter localization with treatment plan embedded couch values for external beam radiation therapy. Prac Radiat Oncol. 2019;9(1):e110–e117. [DOI] [PubMed] [Google Scholar]
- 43.Acharya S, Fischer-Valuck BW, Kashani R, et al. Online MR image-guided radiotherapy: first clinical applications. Int J Radiat Oncol Biol Phys. 2016;94(2):394–403. [DOI] [PubMed] [Google Scholar]
- 44.Guerreiro F, Servalli E, Janssens GO, et al. Potential benefit of MRI-guided IMRT for flank irradiation in pediatric patients with Wilms’ tumor. Acta Oncologica. 2018;58(2):243–250. [DOI] [PubMed] [Google Scholar]
- 45.Edmund JM and Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenhunen M, Korhonen J, Kapanen M, et al. MRI-only based radiation therapy of prostate cancer: workflow and early clinical experience. Acta Oncol. 2018;57(7):902–907. [DOI] [PubMed] [Google Scholar]
- 47.Uh J, Merchant TE, Li Y, et al. MRI-based treatment planning with pseudo CT generated through atlas registration. Med Phys. 2014;41(5):051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerreiro F, Koivula L, Seravalli E, et al. Feasibility of MRI-only photon and proton dose calculations for pediatric patients with abdominal tumors. Phys Med Biol. 2019;64(5):055010. [DOI] [PubMed] [Google Scholar]
- 49.Pai Panandiker AS, Sharma S, Naik M, et al. Novel assessment of renal motion in children as measured via 4-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2012;82(5):1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uh J, Khan MA, Hua C. Four-dimensional MRI using internal respiratory surrogate derived by dimensionality reduction. Phys Med Biol. 2016;61(21):7812–7832. [DOI] [PubMed] [Google Scholar]
- 51.Guerreiro F, Servalli E, Janssens GO, et al. Intra-and inter-fraction uncertainties during IGRT for Wilms’ tumor. Acta Oncologica. 2018;57(7):941–949. [DOI] [PubMed] [Google Scholar]
- 52.Huijskens SC, van Dijk IWEM, Visser J, et al. Abdominal organ position variation in children during image-guided radiotherapy. Radiat Oncol. 2018;13(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uh J, Krasin MJ, Li Y, et al. Quantification of pediatric abdominal organ motion with a 4-dimensional magnetic resonance imaging method. Int J Radiat Oncol Biol Phys. 2017;99(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huijskens SC, van Dijk IWEM, Visser J, et al. Predictive value of pediatric respiratory-induced diaphragm motion quantified using pre-treatment 4DCT and CBCTs. Radiat Oncol. 2018;13(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Claude L, Malet C, Pommier P, et al. Active breathing control for Hodgkin’s disease in childhood and adolescence: feasibility, advantages, and limits. Int J Radiat Oncol Biol Phys. 2007;67(5):1470–1475. [DOI] [PubMed] [Google Scholar]
- 56.Demoor-Goldschmidt C, Chiavassa S, Josset S, et al. Respiratory-gated bilateral pulmonary radiotherapy for Ewing’s sarcoma and nephroblastoma in children and young adults: dosimetric and clinical feasibility studies. Cancer Radiother. 2017;21(2):124–129. [DOI] [PubMed] [Google Scholar]
- 57.Lundgaard AY, Hjalgrim LL, Rechner LA, et al. TEDDI: radiotherapy delivery in deep inspiration for pediatric patients—a NOPHO feasibility study. Radiat Oncol. 2018;13(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkfield KM, Linsenmeier C, Yock TI, et al. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73(3):716–721. [DOI] [PubMed] [Google Scholar]
- 59.Laskar S, Pandit P, Mallik S, et al. Adaptive radiation therapy for pediatric head and neck malignancies: dosimetric implications. Pract Radiat Oncol. 2015;5(2):e87–e94. [DOI] [PubMed] [Google Scholar]
- 60.Hua C, Uh J, Krasin MJ, et al. Clinical implementation of magnetic resonance imaging systems for simulation and planning of pediatric radiation therapy. J Med Imag Radiat Sci. 2018;49(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grau C, Overgaard J, Høyer M, et al. Biology-guided adaptive radiotherapy (BiGART) is progressing towards clinical reality. Acta Oncol. 2015;54:1245–1250. [DOI] [PubMed] [Google Scholar]
- 62.Cubillos-Mesías M, Troost EGC, Lohaus F, et al. Including anatomical variations in robust optimization for head and neck proton therapy can reduce need of adaption. Radiother Oncol. 2019;131:127–134. [DOI] [PubMed] [Google Scholar]
- 63.Molineu A, Mehrens H, Followill D. IROC-Houston’s Pediatric TBI phantom credentialing program. Presentation SU-E-108–5. AAPM 59th Meeting and Exhibition, Denver, CO, July 30–August 3, 2017. [Google Scholar]
- 64.Lewis DJ, Taylor PA, Followill DS, et al. A new anthropomorphic pediatric spine phantom for proton therapy clinical trial credentialing. Int J Part Ther. 2018;4(4):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das IJ, Ding GX, Ahnesjö A. Small fields: nonequilibrium radiation dosimetry. Med Phys. 2008;35:206–215. [DOI] [PubMed] [Google Scholar]
- 66.Anghelescu DL, Burgoyne LL, Liu W, et al. Safe anesthesia for radiation therapy in pediatric oncology: the St. Jude Children’s Research Hospital experience, 2004–2006. Int J Radiat Oncol Biol Phys. 2008;71(2):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radiation Oncology Incident Learning System. https://www.astro.org/Patient-Care-and-Research/Patient-Safety/RO-ILS. Accessed July 3, 2019.
- 68.Constine LS, Ronckers CM, Hua C- H, et al. Pediatric normal tissue effects in clinic (PENTEC): an international collaboration to analyze normal tissue radiation dose–volume response relationships for pediatric cancer patients. Clin Oncol (R Coll Radiol). 2019;32(3):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]