Abstract
Hypertension is associated with insulin resistance (IR), metabolic syndrome (MS), and arterial stiffness. Non–insulin‐based IR indexes were developed as tools for metabolic screening. Here, we aimed to evaluate the novel non–insulin‐based Metabolic Score for IR (METS‐IR) index for the prediction of incident hypertension and arterial stiffness evaluated using pulse wave velocity (PWV) analysis, compared with other non–insulin‐based IR indexes. We evaluated two populations, a cross‐sectional evaluation of high‐risk individuals (n = 305) with a wide range of metabolic comorbidities and dyslipidemia in whom PWV measurement was performed and a 3‐year prospective cohort of normotensive individuals (N = 6850). We observed a positive correlation between METS‐IR and PWV in the cross‐sectional cohort, which was higher compared with other non–insulin‐based fasting IR indexes; furthermore, PWV values >75th percentile were associated with the upper tercile of METS‐IR values. In the prospective cohort, we observed an increased risk for incident hypertension for the upper METS‐IR tercile (METS‐IR ≥ 46.42; HR: 1.81, 95% CI: 1.41‐2.34), adjusted for known cardiovascular risk factors, and observed that METS‐IR had greater increases in the predictive capacity for hypertension along with SBP and the Framingham Hypertension Risk Prediction Model compared with other non–insulin‐based IR indexes. Therefore, METS‐IR is a novel non–insulin‐based IR index which correlates with arterial stiffness and is a predictor of incident hypertension, complementary to previously validated risk prediction models.
Keywords: cardiovascular risk, incident hypertension, insulin resistance, METS‐IR, pulse wave velocity
1. INTRODUCTION
Clinical diagnosis of insulin resistance (IR) is useful for assessment of type 2 diabetes (T2D) risk, ectopic fat accumulation, visceral adiposity, and cardiovascular risk.1 However, precise evaluation of IR requires one‐stage euglycemic‐hyperinsulinemic clamp (EHC), a method which is invasive, costly, and requires hospitalization. Therefore, surrogate insulin‐based IR markers have been developed as predictors of IR and been proven predictive of cardiovascular disease (CVD) risk.2, 3 A limitation of such indexes is the required measurement of insulin, which has a high cost and variability depending on the utilized technique.4 Recently, there has been crescent interest in developing non–insulin‐based IR indexes including the TyG index and TG/HDL‐C ratio. Components of such indices, including fasting glucose, triglycerides, and HDL‐C, have been shown predictive of hypertension and CV risk in prospective studies.5, 6, 7 A significant contribution of hypertension and CVD risk is explained by arterial stiffness, which implies degeneration of elastin fibers and deposition of collagen in arterial walls, inducing structural and functional modifications in the arterial wall.8 The TyG index and the TG/HDL‐C ratio have proven strong and consistent associations with hypertension, CVD risk, and arterial stiffness in several populations, suggesting a potential role for IR assessment in identifying arterial stiffness using non–insulin‐based IR surrogates.9, 10
The recently developed Metabolic Score for IR (METS‐IR) offers higher concordance with EHC compared with other non–insulin‐based IR indexes; furthermore, METS‐IR includes evaluation of body mass index (BMI), which has shown strong predictive capacity for CVD risk.11, 12 Overall, METS‐IR evaluates similar components to the metabolic syndrome (MS), which has been associated with age‐related structural and functional changes in arteries and increased intima‐media thickness, which confers an increased risk of hypertension and CVD.13 Here, we aimed to investigate the correlation of METS‐IR with pulse wave velocity (PWV) and other vascular health surrogates from PWV analysis. We also assessed the capacity of METS‐IR to predict incident hypertension and its complementary role for the prediction of hypertension along with blood pressure levels and risk prediction models.
2. METHODS
2.1. Cross‐sectional cohort
We evaluated subjects with high‐cardiovascular risk conditions including obesity (BMI > 30kg/m2), carbohydrate intolerance or prediabetes (2‐hour glucose challenge ≥140 mg/dL but <200 mg/dL), and primary dyslipidemias including familial hypercholesterolemia and familial hypertriglyceridemia. Participants were instructed to not consume caffeinated beverages refrain from smoking ≤48 hours before evaluation. Upon evaluation, subjects were placed in a supine position for 10 minutes, and baseline supine brachial artery blood pressure (BP) and heart rate (HR) were recorded using a semiautomated cuff‐based device (SphygmoCor XCEL, AtCor Medical Pty Ltd, USA). PWV measurements were taken after achieving hemodynamic stability, defined as two readings within systolic BP (SBP) of ±9 mm Hg, diastolic BP (DBP) ±6 mm Hg and HR ±8 beats/min. To assess PWV, carotid pulse waves were measured by applanation tonometry and femoral pulse waves were simultaneously obtained by a partially inflated cuff over the femoral artery at the leg midway between hip and knee. PWV was determined by calculating the ratio of corrected distance between pulse measuring sites to time delay between carotid and femoral pulse waves. Distance was measured with a nonstretchable tape from the suprasternal notch to the carotid site, from the femoral artery at the inguinal ligament to the proximal edge of the thigh cuff from the suprasternal notch to the proximal edge of the thigh cuff. Distances 1 and 2 were subtracted from distance 3 and used in the calculation of PWV.
2.2. Metabolic syndrome cohort
The prospective MS cohort was developed to evaluate risk of MS components in incident T2D, hypertension, and cardiovascular mortality in an urban population living in 9 different cities in Mexico.14 Inclusion criteria considered subjects aged 25‐69 years, BMI ≥23 kg/m2, without T2D, hypertension or other significant cardiovascular comorbidities, and obese individuals (BMI ≥ 30 kg/m2) with at least one of the following conditions: BP ≥140/90 mm Hg, fasting glucose >100 mg/dL, total cholesterol >200 mg/dL, and triglyceride levels >150 mg/dL. Individuals with diagnosed T2D, coronary artery disease, cerebral vascular disease, alcoholism, taking corticosteroids, with liver disease, kidney dysfunction, or life‐threatening diseases that would prevent follow‐up were excluded. Subjects were interviewed to obtain medical history, sociodemographic information, dietary and physical activity habits, and anthropometric measurements. BP measurement was also performed using a manual sphygmomanometer after subjects remained seated ≥5 minutes and refrained from consuming caffeine before measurements. We obtained a 20mL blood sample after 9‐ to 12‐hour fast for biochemical measures. These same evaluations were carried out after ≥2 years follow‐up. Incident hypertension was defined as a construct of previous medical diagnosis of hypertension, taking antihypertensive medication and/or blood BP at levels consistent with any‐degree of hypertension according to current ESC/ESH guidelines. Time to follow‐up was estimated from recruitment up to last follow‐up or hypertension diagnosis, whichever occurred first. We also used the Framingham Hypertension Risk Prediction Model to estimate the risk of incident hypertension.15
2.3. Biochemical and anthropometric evaluations
In both evaluated cohorts, we obtained from all subjects a complete medical and family history, including use of medications. Subjects were weighed on calibrated scales, and height was determined with a floor scale stadiometer; BMI was calculated as weight in kg divided by the squared product of height in meters. Blood was obtained between 08:00 and 09:00 hours after 8‐ to 12‐hour fast. Plasma glucose concentration was measured by an automated glucose analyzer (Yellow Springs Instruments Co.), serum insulin concentration was measured by using a chemiluminescent immunoassay (Beckman Coulter Access 2), and A1c levels were measured by using high‐performance liquid chromatography (HPLC) (Variant II Turbo, BIORAD). Lipid concentrations (cholesterol, triglycerides, and HDL cholesterol), apo A, apo B, uric acid, creatinine, and hepatic enzymes were measured using colorimetric assays (Unicel DxC 600 Synchron Clinical System Beckman Coulter). LDL‐cholesterol was calculated with the Friedewald equation when triglycerides were <250 mg/dL. METS‐IR was calculated using the formula (LN((2*G0) + TG0))*BMI/(LN(HDL‐C)), where G0 and TG0 were fasting glucose and triglycerides, respectively.
2.4. Statistical analysis
To evaluate inter‐group differences, we used Student's t test and Mann‐Whitney U test, where appropriate. Frequency distribution of categorical variables is reported as frequencies and percentages and was compared between groups using chi‐squared tests. For measurements in follow‐up studies, we used Student's paired t test and Wilcoxon's rank‐sign tests, where appropriate. Logarithmic and inverse transformations were applied to approximate normality in variables showing nonparametric distribution. Data are presented as mean ± SD or as median and interquartile range.
2.4.1. Prediction of incident hypertension using METS‐IR
To evaluate the association of METS‐IR and incident hypertension in the MS cohort, we performed survival analysis comparing across METS‐IR terciles, quartiles, and cutoff value ≥50, using Kaplan‐Meier curves compared with log‐rank tests. Cox proportional risk regression analyses were used to evaluate the risk of incident hypertension across METS‐IR percentiles, adjusted for age, sex, cholesterol levels (TC), waist circumference (WC), SBP, DBP, and smoking status. To evaluate increases in predictive capacity for hypertension risk using METS‐IR, we estimated the Framingham Hypertension Risk Prediction Model and assessed predictive improvements with an omnibus test of model coefficients for changes across predictive models (X 2) and changes in c‐statistic.
2.4.2. Correlation of METS‐IR with PWV and BP
In our cross‐sectional PWV cohort, we tested METS‐IR scores using trend analysis and linear regression against quartiles of PWV, SBP, and DBP, adjusted for age, sex, and smoking status. Finally, we evaluated whether METS‐IR would predict PWV values >75th percentiles for this population adjusted for age, sex, smoking, and hypertension. Model diagnostics were conducted using the Hosmer‐Lemeshow test. Statistical analyses were performed using R software version 3.4.3, Statistical Package for Social Science (SPSS) version 21.0 and GraphPad Prism, version 7.0.
3. RESULTS
3.1. Correlation between METS‐IR values, arterial stiffness, and PWV
In the PWV cohort, we included 305 subjects, predominantly female (68.9%), with mean age of 49.86 ± 13.09, BMI of 29.01 ± 5.80, and SBP and DBP of 122.9 ± 15.19 and 72.47 ± 9.61, respectively. One hundred and sixteen subjects had prediabetes (38.0%), 75 had familial hypercholesterolemia under statin therapy (24.6%), 64 moderate‐to‐severe hypertriglyceridemia (21.0%), and 50 were metabolically healthy (16.4%); ninety were active smokers (29.5%), and 57 had treatment for hypertension (18.7%). Their biochemical values included the following: median fasting glucose of 94.0 mg/dL (IQR: 86‐104), fasting triglycerides of 130 mg/dL (IQR: 92.0‐180.5), total cholesterol of 206 mg/dL (IQR: 173‐247), and HDL‐C of 44 mg/dL (IQR: 38‐54).
We observed a significant correlation between METS‐IR and PWV that increased after adjustments for age, sex, and smoking; we also observed a trend of increasing PWV, SBP, and DBP values with increasing METS‐IR terciles (Figure 1). Using linear regression, we observed that METS‐IR predicts 34.0% of the variability in PWV measures (β = 0.290, P < 0.001), adjusted for sex, age, treatment for hypertension, and smoking status. When evaluating PWV >75th percentile using multiple logistic regression analyses, we observed an association with both METS‐IR scores (OR 1.03 95% CI 1.01‐1.06) and the upper METS‐IR tercile (OR 2.49 95% CI 1.19‐5.23) adjusted for age, sex, and smoking. Finally, we contrasted those observations evaluating the same parameters against the TG/HDL and TyG indexes and observed that METS‐IR had the highest correlation compared with other indexes even after adjustment (Table 1).
Figure 1.
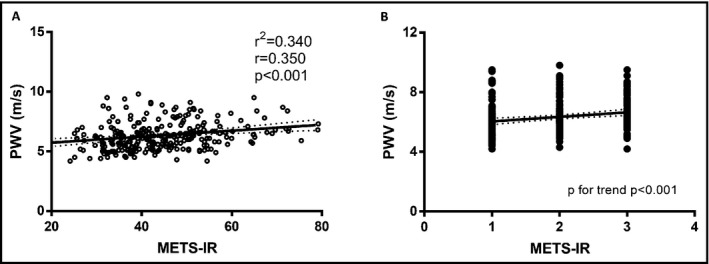
Correlation and linear regression between METS‐IR and pulse wave velocity (A) and trend analyses for increasing METS‐IR terciles (B) adjusted for age, sex, treatment for hypertension, and waist circumference
Table 1.
Correlations between non–insulin‐based IR indexes and PWV, SPB, and DBP. Age, sex, hypertension treatment, and smoking status were considered in the adjusted value
| Index | METS‐IR (ρ, 95% CI) | TG/HDL‐C index (ρ, 95% CI) | TyG index (ρ, 95% CI) | |||
|---|---|---|---|---|---|---|
| Parameter | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| PWV | 0.253* (0.135‐0.366) | 0.350* (0.204‐0.418) | 0.301* (0.199‐0.396) | 0.289* (0.197‐0.382) | 0.260* (0.147‐0.361) | 0.238* (0.137‐0.382) |
| Central SBP | 0.219* (0.124‐0.318) | 0.263* (0.163‐0.360) | 0.062 (−0.034 to 0.161) | 0.114* (0.000‐0.212) | 0.064 (0.029‐0.167) | 0.079 (−0.016 to 0.170) |
| Peripheral SBP | 0.219* (0.120‐0.315) | 0.267* (0.169‐0.373) | 0.044 (−0.043 to 0.145) | 0.076 (−0.037 to 0.181) | 0.041 (−0.062 to 0.159) | 0.062 (−0.035 to 0.161) |
| Peripheral DBP | 0.316* (0.226‐0.412) | 0.309* (0.225‐0.397) | 0.152* (0.067‐0.250) | 0.138* (0.036‐0.247) | 0.161* (0.067‐0.271) | 0.133* (0.044‐0.227) |
Abbreviation: DPB, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
P < 0.05.
3.2. Prediction of incident hypertension using METS‐IR in the MS cohort
For prospective evaluation, we included 6850 normotensive subjects from the MS cohort at baseline, from which 3974 subjects completed follow‐up. We observed 592 cases of incident hypertension over 9549 accumulated persons‐years, yielding an incidence rate of 61.99 cases per 1000 person‐years or 14.9% in an average of 2.4 years of follow‐up. Subjects who developed hypertension were older, had higher fasting glucose, insulin, LDL‐C, apolipoprotein B and BMI, and lower HDL‐C at baseline and follow‐up (Table S1). Individuals who developed hypertension had significantly higher METS‐IR scores at baseline and after follow‐up in comparison with those who did not (P < 0.001). Both groups had an increase in METS‐IR scores between visits, which remained larger in subjects who developed hypertension. We observed a low but significant correlation between METS‐IR and baseline SBP (ρ = 0.095) and DBP (ρ = 0.056) adjusted for age, sex, smoking status, WC, TC, and family history of hypertension. The correlation was higher for baseline METS‐IR and follow‐up SBP (ρ = 0.138) and DBP (ρ = 0.126) and was particularly stronger for individuals with incident hypertension (ρ = 0.180 and ρ = 0.139, respectively).
We observed the highest rate of incident hypertension for the upper METS‐IR tercile compared with lower terciles (log‐rank test P < 0.001). This observation was confirmed in Cox proportional risk regression analysis, which showed progressively higher risk of incident hypertension for the upper (METS‐IR ≥ 46.42) and middle METS‐IR terciles (39.15 ≤ METS‐IR < 46.42) in comparison with the lowest, adjusted for age, sex, smoking status, TC, WC, and family history of hypertension (Figure 2A, Table 2). Given the known role of diabetes and dysglycemia in increasing arterial stiffness and hypertension risk, we performed further adjustments by baseline hyperglycemia (fasting glucose ≥ 100mg/dL), which did not modify the strength of the association for the upper (HR 1.312 95% CI 1.060‐1.624) or middle terciles (HR 1.817 95% CI 1.428‐2.311). We also observed that subjects with METS‐IR ≥50 had a higher adjusted risk to develop hypertension (HR: 1.44, 95% CI 1.17‐1.78, Figure 2B). Next, we assessed which of the components of the score provided better predictive capacity for incident hypertension; the higher predictive accuracy was driven by BMI (HR 1.014 95% CI 1.007‐1.021) and glucose (HR 1.043 95% CI 1.022‐1.064). When comparing the information provided by the sum of the individual components of METS‐IR compared to METS‐IR alone, using METS‐IR had a larger decrease using Akaike's information criterion (AIC) compared with individual components (9381.72 vs 9465.081). When assessing the insulin‐based HOMA‐IR index, it also proved predictive of hypertension (HR 1.067 95% CI 1.028‐1.107) adjusted for age, sex, family history of hypertension, smoking, and blood pressure levels, but both the c‐statistic (c‐statistic = 0.635 vs 0.633) and the AIC (9381.72 vs 9487.50) demonstrated better predictive performance for METS‐IR.
Figure 2.
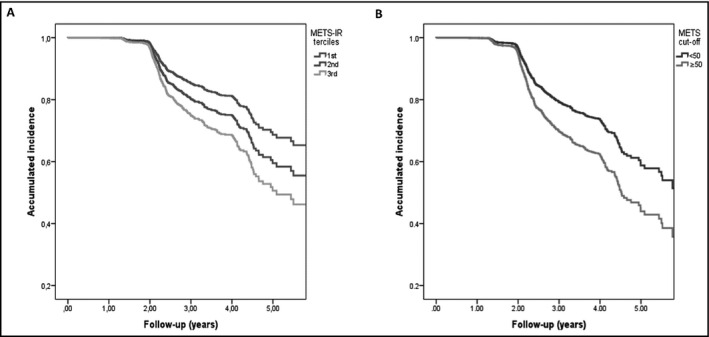
Incidence of hypertension comparing across METS‐IR terciles (A) and divided by the METS‐IR cutoff point (≥50, B), adjusted for age, sex, smoking status, cholesterol at baseline, abdominal circumference, and family history of hypertension
Table 2.
Cox proportional hazard regression for risk of incident hypertension defined by ESC/ESH guidelines across METS‐IR terciles and quartiles, adjusted for age, sex, smoking status, systolic and diastolic blood pressure, family history of hypertension, waist circumference, and total cholesterol
| Model | METS‐IR Percentile | β | Wald | HR | P‐value | 95% CI |
|---|---|---|---|---|---|---|
| 1 | Q2 | 0.073 | 0.271 | 1.076 | 0.603 | 0.816‐1.419 |
| Q3 | 0.332 | 5.554 | 1.393 | 0.018 | 1.057‐1.385 | |
| Q4 | 0.593 | 15.304 | 1.810 | <0.001 | 1.344‐2.436 | |
| 2 | T2 | 0.296 | 6.939 | 1.345 | 0.008 | 1.079‐1.677 |
| T3 | 0.554 | 18.172 | 1.740 | <0.001 | 1.329‐2.245 |
Finally, we evaluated the complementary use of METS‐IR to predict hypertension in comparison with BP levels and the previously validated Framingham Hypertension Risk Prediction Model. When we included METS‐IR at baseline along with SBP levels, we observed a significant change in predictive capacity for incident hypertension (X 2 = 8.74, P = 0.003); furthermore, we observed a significant increase when including METS‐IR at baseline along with the Framingham Hypertension Risk Prediction Model (X 2 = 10.70, P = <0.001). The changes in c‐statistic (AUC) were also superior for the combination of METS‐IR and Framingham or SBP compared with either alone, and METS‐IR had superior combined predictive performance in comparison with other non–insulin‐based IR indexes and compared to the insulin‐based HOMA‐IR index as assessed by the AIC (Table 3).
Table 3.
Predictive performance of combined regression models comprising non–insulin‐based fasting insulin resistance indexes complementary to Framingham hypertension risk equation in prediction of incident hypertension using c‐statistics
| IR index + Hypertension risk score | c‐statistic | AIC | |
|---|---|---|---|
| METS‐IR | Index | 0.579 | 8695.89 |
| Index + BP | 0.599 | 8674.47 | |
| Index + Framingham | 0.643 | 8584.60 | |
| TyG | Index | 0.530 | 8828.20 |
| Index + BP | 0.573 | 8805.33 | |
| Index + Framingham | 0.640 | 8695.84 | |
| TG/HDL‐C | Index | 0.518 | 8838.24 |
| Index + BP | 0.594 | 8812.70 | |
| Index + Framingham | 0.643 | 8699.18 | |
| HOMA‐IR | Index | 0.571 | 8813.83 |
| Index + BP | 0.596 | 8789.66 | |
| Index + Framingham | 0.647 | 8685.43 |
Abbreviations: AIC, Akaike's information criterion; HOMA‐IR, Homeostasis model assessment for insulin resistance; METS‐IR, Metabolic Score for Insulin Resistance; TG/HDL‐C, triglyceride‐high‐density lipoprotein cholesterol; TyG, triglycerides‐glucose product.
4. DISCUSSION
Metabolic Score for IR is a useful tool to identify cases with increased risk of incident arterial hypertension and arterial stiffness. This finding is in agreement with the well‐known contribution of IR to the pathogenesis of atherogenesis, vascular changes, and hypertension.16 First, we observed a linear correlation between METS‐IR and PW along with BP measurements in a cohort of high‐risk individuals. METS‐IR was also shown to be predictive of incident arterial hypertension, and we observed that the correlation between METS‐IR and BP measures is higher in subjects with hypertension. Furthermore, METS‐IR increased the predictive capacity for incident hypertension when combined with SBP/DBP and the Framingham Hypertension Risk Prediction Model and was superior to other previously validated non–insulin‐based IR measures.
The correlation between METS‐IR, arterial stiffness, and incident hypertension is supported by pathophysiological evidence. The most accepted hypothesis linking IR and arterial hypertension includes overstimulation of the sympathetic nervous system, increasing peripheral vascular resistance, and cardiac output leading to increases in systemic BP.17 Decreased insulin action, glucotoxicity, and MS stimulate activity of the renin‐angiotensin‐aldosterone system, increasing tubular Na+ reabsorption leading to volume expansion and BP changes.18 Impaired insulin signaling also causes endothelial dysfunction and a decrease in activity of nitric oxide synthase, leading to systemic vasoconstriction.19 Visceral adiposity and ectopic fat accumulation have also been shown to be predicted by METS‐IR and are recognized risk factors for the development of CV disease and hypertension20; adjustments of the observed association for surrogates of abdominal obesity did not attenuate the observed and confirm an independent role for METS‐IR in its prediction.
When we included METS‐IR along with the Framingham Hypertension Risk Prediction Model, we observed significant increases in predictive capacity for incident hypertension. The Framingham Hypertension Risk Prediction Model has been validated in several population cohorts, and its predictive capacity for incident hypertension, morphological heart changes, and altered vascular function has not been shown to be superior to SBP alone.21, 22 Since components of the metabolic syndrome and IR also modify the prediction of incident hypertension, it is expected that METS‐IR evaluation would be helpful to predict short‐term hypertension risk.23 Overall, this confirms that prediction of hypertension risk using METS‐IR could be explained by the increased cardiovascular risk associated with both IR and MS. Individual components of the METS‐IR score have also been linked independently to incident hypertension, including triglycerides and HDL‐C as well as BMI as a marker of whole‐body fat content.24, 25 As demonstrated by our results, besides BMI, glucose levels are also highly predictive of arterial stiffness and incident arterial hypertension; despite this, the better model assessed by decreased in AIC was comprised by METS‐IR and not by its individual components. This is significant, since it confirms that METS‐IR is useful as a complementary metabolic evaluation tool when assessing risk of arterial hypertension.
The relationship between increased risk of incident hypertension and vascular health explained by METS‐IR is further strengthened by our observation of increased correlation with PWV. PWV is a surrogate marker of arterial stiffness, which has also been previously associated with HOMA‐IR, the TyG index, and TG/HDL‐C ratio26, 27; in our study, we were able to demonstrate a superior correlation using PWV analysis for METS‐IR in comparison with other non–insulin‐based IR indexes in a cohort of high‐risk individuals. The relevance of evaluating this association was demonstrated in a previous study, which showed that whereas endothelial function increases with or without IR, arterial stiffness increases only in relation to IR, especially in individuals with family history of T2D.28 The mechanisms underlying this association are related to IR and hyperglycemia, which lead to nonenzymatic glycation of matrix proteins causing subendothelial accumulation of advanced end glycation products and arterial stiffening, leading to altered vessel hemodynamics.29 The inclusion of individuals at high‐cardiovascular risk allows us to extrapolate results to high‐risk populations beyond hyperglycemia, but since statin therapy is known to have rheological impacts and reduce PWV,30 additional evaluations of the predictive capacity of MET‐IR for vascular health in untreated populations are warranted. In other populations, PWV has been shown to be a predictor of incident cardiovascular events and arterial calcification, which indicates that METS‐IR could be a potential predictor of both and should be evaluated in future studies.31 Although PWV is not a routine evaluation in primary care, our results show that METS‐IR might be treated as subrogate of arterial stiffness and a predictor of incident hypertension.
Our study had some strengths and limitations. First, we evaluated a large cohort of normotensive but at‐risk individuals, which allowed power for predictive modeling of incident hypertension and represents an adequate setting for validating the role of METS‐IR to predict incident hypertension. We also had and noninvasive surrogate to assess arterial stiffness which provides pathophysiological evidence to complement our epidemiological observations. Limitations to be acknowledged are the inclusion of high‐risk subjects in the PWV cohort, which limits extrapolation of such results to the general population; furthermore, the sample of subjects included in the MS cohort was already at a higher risk compared with general population, which calls for further studies in lower risk populations to assess the utility of METS‐IR in this subset of patients. Finally, all analyses were controlled for age, sex, and cardiovascular risk factors and there remains a possibility of residual confounding, particularly since the association between arterial stiffness and BP values is modified by age.32
In conclusion, METS‐IR is a novel non–insulin‐based IR index which predicts incident hypertension and its complementary risk prediction with SBP and the Framingham Hypertension Risk Prediction Model is stronger compared with other non–insulin‐based IR indexes and HOMA‐IR. Furthermore, METS‐IR is correlated with PWV, SBP, and DBP in high‐risk patients and is predictive of arterial stiffness. METS‐IR can be used to evaluate cardio‐metabolic risk complementary to routine evaluation and identify subjects at an increased risk of hypertension, which makes it useful in primary care practice as a screening tool to evaluate metabolic heath in at‐risk individuals.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
OYBC, NEAV, AVV, AJM and CAAS: developed the conceptualization of the study. AJM, RM, OAC, DGV, PAV, MAMH, ICB, LMH, LEG, JJGG, UA, YOY, RCR, LSR, MEGS, JMMH, LMGR, FJGP, RR, and MTTL: conducted the studies in the different cohort centers, recruited patients, and processed biological samples. OYBC, NEAV, AVV, and CAAS: performed and interpreted statistical analyses and developed predictive models. OYBC, NEAV, and CAAS: also participated in manuscript drafting and processing. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors read and approved the final version of this manuscript.
Supporting information
ACKNOWLEDGMENTS
All authors would like to thank the staff of the Endocrinology and Metabolism Department for their support, particularly Maria Del Carmen Moreno‐Villatoro, Guadalupe Lopez, and Adriana Cruz‐Lopez. We are thankful to the study volunteers for all their work and support throughout the realization of the study. Neftali Eduardo Antonio Villa, Arsenio Vargas‐Vázquez, and Omar Yaxmehen Bello‐Chavolla are enrolled at the PECEM program of the Faculty of Medicine at UNAM. Arsenio Vargas‐Vázquez and Omar Yaxmehen Bello‐Chavolla are supported by CONACyT.
Bello‐Chavolla OY, Antonio‐Villa NE, Vargas‐Vázquez A, et al. Prediction of incident hypertension and arterial stiffness using the non–insulin‐based metabolic score for insulin resistance (METS‐IR) index. J Clin Hypertens. 2019;21:1063–1070. 10.1111/jch.13614
O. Y. Bello‐Chavolla and N. E. Antonio‐Villa contributed equally to the drafting of this paper.
Funding information
The project was supported by a grant from the “Consejo Nacional de Ciencia y Tecnología (CONACyT)” (S0008‐2009‐1‐115250) and research grant by Sanofi. The sponsors had no role in the design, development, and analysis of writing of the present project.
REFERENCES
- 1. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131‐1141. [DOI] [PubMed] [Google Scholar]
- 2. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214‐E223. [DOI] [PubMed] [Google Scholar]
- 3. Sarafidis PA, Lasaridis AN, Nilsson PM, et al. Validity and reproducibility of HOMA‐IR, 1/HOMA‐IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21(9):709‐716. [DOI] [PubMed] [Google Scholar]
- 4. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta‐analysis. PLoS ONE. 2012;7(12):e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budoff M. Triglycerides and triglyceride‐rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118(1):138‐145. [DOI] [PubMed] [Google Scholar]
- 6. Yi SW, Park S, Lee YH, Park HJ, Balkau B, Yi JJ. Association between fasting glucose and all‐cause mortality according to sex and age: a prospective cohort study. Sci Rep. 2017;7(1):8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8(4):222‐232. [DOI] [PubMed] [Google Scholar]
- 8. Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55(1):9‐14. [DOI] [PubMed] [Google Scholar]
- 9. Chung TH, Shim JY, Kwon YJ, Lee YJ. High triglyceride to high‐density lipoprotein cholesterol ratio and arterial stiffness in postmenopausal Korean women. J Clin Hypertens (Greenwich). 2019;21(3):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambrinoudaki I, Kazani MV, Armeni E, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 11. Bello‐Chavolla OY, Almeda‐Valdes P, Gomez‐Velasco D, et al. METS‐IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533‐544. [DOI] [PubMed] [Google Scholar]
- 12. Eeg‐Olofsson K, Gudbjörnsdottir S, Eliasson B, Zethelius B, Cederholm J, NDR.. The triglycerides‐to‐HDL‐cholesterol ratio and cardiovascular disease risk in obese patients with type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Res Clin Pract. 2014;106(1):136‐144. [DOI] [PubMed] [Google Scholar]
- 13. Scuteri A, Franco OH, Majiid AlGhatrif, et al. The relationship between the metabolic syndrome and arterial wall thickness: a mosaic still to be interpreted. Atherosclerosis. 2016;255:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arellano‐Campos O, Gómez‐Velasco DV, Bello‐Chavolla OY, et al. Development and validation of a predictive model for incident type 2 diabetes in middle‐aged Mexican adults: the metabolic syndrome cohort. BMC Endocr Disord. 2019;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near‐term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148(2):102‐110. [DOI] [PubMed] [Google Scholar]
- 16. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293‐302. [DOI] [PubMed] [Google Scholar]
- 17. Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14(11 Pt 2):304S‐309S. [DOI] [PubMed] [Google Scholar]
- 18. Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int. 2015;87(3):497‐499. [DOI] [PubMed] [Google Scholar]
- 19. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888‐1904. [DOI] [PubMed] [Google Scholar]
- 20. Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izzo JL Jr. Brachial vs. central systolic pressure and pulse wave transmission indicators: a critical analysis. Am J Hypertens. 2014;27(12):1433‐1442. [DOI] [PubMed] [Google Scholar]
- 22. Muntner P, Woodward M, Mann DM, et al. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the multi‐ethnic study of atherosclerosis. Hypertension. 2010;55(6):1339‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang T, Zhang H, Li S, et al. Impact of adiposity on incident hypertension is modified by insulin resistance in adults: longitudinal observation from the Bogalusa heart study. Hypertension. 2016;67(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sánchez‐Íñigo L, Navarro‐González D, Pastrana‐Delgado J, Fernández‐Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34(7):1257‐1265. [DOI] [PubMed] [Google Scholar]
- 25. Jiang J, Deng S, Chen YI, et al. Comparison of visceral and body fat indices and anthropometric measures in relation to untreated hypertension by age and gender among Chinese. Int J Cardiol. 2016;219:204‐211. [DOI] [PubMed] [Google Scholar]
- 26. Webb DR, Khunti K, Silverman R, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia. 2010;53(6):1190‐1198. [DOI] [PubMed] [Google Scholar]
- 27. Lee SB, Ahn CW, Lee BK, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scuteri A, Tesauro M, Rizza S, et al. Endothelial function and arterial stiffness in normotensive normoglycemic first‐degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18(5):349‐356. [DOI] [PubMed] [Google Scholar]
- 29. Aronson D. Cross‐linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 30. Dilaveris P, Giannopoulos G, Riga M, Synetos A, Stefanadis C. Beneficial effects of statins on endothelial dysfunction and vascular stiffness. Curr Vasc Pharmacol. 2007;5(3):227‐237. [DOI] [PubMed] [Google Scholar]
- 31. Tsao CW, Pencina KM, Massaro JM, et al. Cross‐sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34(11):2495‐2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scuteri A, Morrell CH, Orrù M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64(6):1219‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


