Abstract
Hypertension is considered a key risk factor for acute aortic dissection (AAD). However, there is limited evidence demonstrating if hypertension management affects AAD development. The objective of this study was to investigate the role of hypertension management in AAD development in a Chinese population. A total of 825 AAD patients and 3300 age‐ and sex‐matched controls were included. The authors analyzed data on demographics, chronic comorbidities, and hypertension management of all participants. Multiple logistic regression analysis was used to estimate the adjusted odds ratios (AOR) and 95% confidence intervals (95% CI) for the relationship between chronic comorbidities, as well as the management of hypertension and AAD risk. After adjusting for other related factors, multivariate logistic regression identified hypertension, chronic kidney disease, Marfan syndrome, history of cardiovascular surgery, and history of smoking as risk factors for AAD. Among the identified risk factors, hypertension was an important and controllable risk factor for AAD development. Thus, the authors further evaluated how hypertension management affects AAD development. A total of 848 controls and 585 AAD patients with hypertension were enrolled in this part of the study. Hypertensive patients with AAD had a longer history, higher stage, poorer medication compliance, and poor control rates of blood pressure, among which poor medication compliance (Irregular vs Regular P < 0.001; Never treated vs Regular P < 0.001) and uncontrolled hypertension (P < 0.001) significantly increased the risk of AAD development. In conclusion, uncontrolled hypertension and poor medication compliance are important precipitating and controllable factors for AAD development.
Keywords: aortic dissection, hypertension management, risk factors
1. INTRODUCTION
Hypertension, which is observed in 65%‐75% of acute aortic dissection (AAD) patients,1 is considered one of the most important risk factors for AAD development. Compared to Western countries, the diagnosis, treatment, and control of hypertension in China have been much lower and associated with significantly higher mortality.2 Although population awareness and medical compliance for treating hypertension have improved with time, more effort is still needed to appropriately manage hypertension in underdeveloped countries.3, 4 As an underdeveloped region of China, many residents in Jilin province suffer from poorly controlled hypertension due to unhealthy living habits (high dietary salt and fat intake, as well as high alcohol and cigarette consumption), lower health awareness, lack of medical compliance, and climate variability.5, 6 Poorly controlled hypertension might be attributed to AAD development.7 However, according to our research, this generally acknowledged conclusion has not been validated by population‐based evidence. Thus, it remains unknown whether management of hypertension affects AAD development. The objective of the present study was to investigate the association between hypertension management and AAD risk in age‐ and sex‐matched cohorts.
2. MATERIALS AND METHODS
2.1. Study design
This was a single‐center‐based retrospective case‐control study. Acute aortic dissection patients were consecutively enrolled from the Department of Cardiovascular Surgery in Jilin University between January 1, 2013, and December 31, 2017. Control patients were recruited from the hospital's inpatient database by matching sex, age, and year of admission with a case‐control ratio of 1:4.
2.2. Study population
2.2.1. AAD patients
Between January 1, 2013, and December 31, 2017, a total of 838 consecutive AAD patients were admitted to the Department of Cardiovascular Surgery. After exclusion of patients with AAD due to trauma or collision (n = 13), 385 patients with type A dissection and 440 patients with type B dissection were enrolled in this study. Acute aortic dissection diagnosis was based on both history and accurate imaging findings (at least one of the following imaging findings was used to confirm the AAD diagnosis: computed tomography angiography, magnetic resonance imaging, or echocardiography). The definition of type A or B dissection was based on the proximal involvement of the aorta according to the Stanford Classification.7 All enrolled patients were admitted within 7 days after disease onset.
2.2.2. Patient follow‐up
For AAD patients who survived following discharge, a telephone follow‐up was conducted each month within 3 months after discharge and every 3 months thereafter. The follow‐up information included blood pressure, current medication, recovery from in‐hospital complications, and deaths (if any, cause of death and date were recorded). Since the follow‐up data were used to calculate the awareness rate of hypertension at admission, only blood pressure and current medication after 1 month of discharge were used.
2.2.3. Control patients
Control patients were enrolled from the inpatient database of the Second Hospital of Jilin University during the same period as AAD patient enrollment. A total of 419 603 patients without AAD were identified. After matching for sex, age, and year of admission, a total of 3300 control patients were enrolled in this study (1540 controls for type A dissection and 1760 controls for type B dissection). In the first part of our AAD risk factor analysis, a 1:4 case‐control cohort was generated by matching sex, age, and year of admission for type A and type B dissection, respectively. To analyze the effect of hypertension management on AAD development, patients with hypertension in the same cohorts were identified and subjected to a second analysis. The number of enrolled patients and matching procedures is shown in Figure 1.
Figure 1.
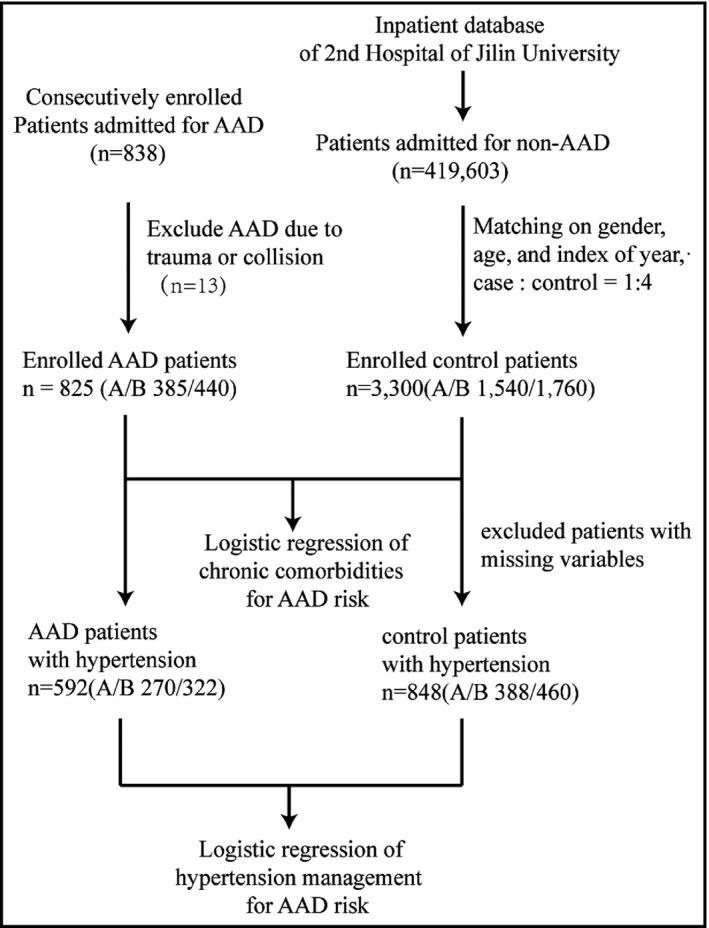
Flowchart of the present study
2.3. Definitions of medication compliance
Medication compliance is a complex problem that can be evaluated in many ways, but currently, there is no “best” measurement strategy.8 We were unable to determine an accurate description of medication compliance because medication compliance was retrospectively self‐reported by the patients rather than prospectively recorded on a daily basis. After interviewing several patients in this study, medication‐taking behavior was used to define medication compliance.9 We used “vacant day” to measure the levels of medication‐taking behavior, which was defined as any missed use of an anti‐hypertensive medicine within a calendar day. Patients were categorized into three levels of hypertension management according to their self‐reported adherence: (a) patients on regular medication, who had at most one vacant day within 1 week; (b) patients on irregular medication, who had two or more vacant days within 1 week; and (c) patients who had never been treated, who took no prescribed medication for hypertension.
2.4. Diagnosis of hypertension
For AAD patients, the diagnosis of hypertension was made if the patient was diagnosed as having hypertension by a physician and had at least 2 records of elevated blood pressure (more than 140/90 mm Hg) before admission, and/or the patient was currently taking anti‐hypertensive medication for blood pressure control. Acute aortic dissection patients without a hypertension history when admitted and who survived after discharge were followed up, and they could be diagnosed as having hypertension according to the former criteria after at least 1 month during the follow‐up period. For control patients, hypertension history was based on the case history in the inpatient database. Hypertension and classification of hypertension stages were based on JNC 6.10
2.5. Data collection
An original questionnaire consisting of demographic, clinical characteristics, chronic comorbidities, and hypertension management was developed before data collection. The questionnaire inquired about sex, age, history of smoking, alcohol consumption, Marfan syndrome, bicuspid aortic valve (BAV), hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), treatment of hemodialysis, cerebral infarction, cerebral hemorrhage, coronary artery disease (CAD), connective tissue disease, liver cirrhosis, length of hypertension history, stage and control of hypertension, type of anti‐hypertensive medications, medication compliance, and control of blood pressure. Acute aortic dissection patient data were collected before discharge, and data for the control patients were collected by retrospectively reviewing hospital records (demographic and chronic comorbidities) and phone interviews (management of hypertension). The definition of hypertension and classification of hypertension stages were based on the JNC 6 guidelines.10 Data forms were simultaneously completed by two physicians, and any ambiguous or conflicting variables were resolved by discussion with another physician.
2.5.1. Processing of missing variables
There were no missing data for comorbidities. Only patients with complete data with regards to characteristics of hypertension were enrolled in analysis. The percentages of missing variables are reported in Table S4.
2.6. Ethics statement
All patients admitted to the hospital for AAD signed informed consent, and the study protocol was approved by the Ethics Committee of the Second Hospital of Jilin University. Demographic and chronic comorbidities data of control patients in this project were retrospectively collected from hospital records, and data from phone interviews were analyzed only after verbal consent was provided. All data were analyzed anonymously. All aspects of this study complied with the Declaration of Helsinki of the World Medical Association.
2.7. Statistical analysis
Continuous variables or categorical variables are shown as mean (standard deviation; SD), median (interquartile range; IQR), or number (percentage; %). Comparisons between groups for continuous variables were performed using a two‐sample t test or Mann‐Whitney U test where appropriate. Categorical variables were compared using the chi‐square test, with or without Yate's continuity correction, or the Fisher's exact test. A forward‐and‐backward stepwise multivariate logistic regression model was fitted with R function “glm” (family = binomial (link = “logit”)) and “step” (direction = “both”, test = “Chisq”). The model with a minimum Akaike information criterion (AIC) was selected to calculate the adjusted odds ratios (AOR) and 95% confidence intervals (95% CI) of the variables. The significance of AOR was tested by the Wald chi‐square test. Group matching was performed with R function “matchit” with “Nearest Neighbor Matching” provided by R package “MatchIt”.11 A two‐tailed P‐value of <0.05 was considered statistically significant. All data in this study were analyzed using R version 3.4.0.12
3. RESULTS
3.1. Common comorbidities and AAD risk
The baseline characteristics of the AAD and the control group are shown in Table 1. The mean age of the study patients was 55.0 ± 12.0 years for both the AAD and control groups. Males constituted 68.5% of the population in each group. There were no significant differences in age and sex between the two groups, indicating that the frequency matching was adequate. Acute aortic dissection patients had a higher frequency of chronic comorbidities, including history of smoking, hypertension, Marfan syndrome, history of cardiac surgery, BAV, and CKD, while control patients had a higher frequency of a history of diabetes and CAD (Table 1). Multivariate logistic regression analysis is shown in Table 2, which revealed that some chronic comorbidities were positively associated with AAD risk after adjusting for other related factors, including hypertension (AOR 6.95, 95% CI: 5.82‐8.32), CKD (AOR 21.24, 95% CI: 9.67‐51.55), Marfan syndrome (AOR 36.18, 95% CI: 11.43‐159.92), and history of smoking (AOR 1.37, 95% CI: 1.15‐1.63). A negative association was found between AD risk and diabetes (AOR 0.14, 95% CI: 0.09‐0.21), CAD (AOR 0.35, 95% CI: 0.22‐0.53), and hemodialysis (AOR 0.09, 95% CI: 0.02‐0.46). Univariate analysis of logistic regression was performed, and the results are shown in Table S1.
Table 1.
Baseline clinical characteristics of AD and control patients
| Total | Type A | Type B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | AD | P value | Control | AD | P value | Control | AD | P value | |
| Patients, n | 3300 | 825 | 1540 | 385 | 1760 | 440 | |||
| Age, mean (SD) | 55.0 (12.0) | 55.0 (12.0) | 0.98 | 53.7 (12.0) | 53.7 (12.0) | 0.99 | 56.1 (11.9) | 56.1 (11.9) | 0.99 |
| Male, n (%) | 2260 (68.5) | 565 (68.5) | 1 | 968 (62.9) | 242 (62.9) | 1 | 1292 (73.4) | 323 (73.4) | 1 |
| Ever smoker, n (%) | 1012 (30.7) | 314 (38.1) | <0.001 | 459 (29.8) | 136 (35.3) | 0.042 | 553 (31.4) | 178 (40.5) | <0.001 |
| Alcohol, n (%) | 578 (17.5) | 145 (17.6) | 1 | 251 (16.3) | 60 (15.6) | 0.792 | 327 (18.6) | 85 (19.3) | 0.774 |
| Hypertension, n (%) | 1035 (31.4) | 592 (71.8) | <0.001 | 464 (30.1) | 270 (70.1) | <0.001 | 571 (32.4) | 322 (73.2) | <0.001 |
| Diabetes, n (%) | 430 (13) | 32 (3.9) | <0.001 | 193 (12.5) | 8 (2.1) | <0.001 | 237 (13.5) | 24 (5.5) | <0.001 |
| Cerebral infarction, n (%) | 223 (6.8) | 61 (7.4) | 0.569 | 90 (5.8) | 27 (7) | 0.46 | 133 (7.6) | 34 (7.7) | 0.984 |
| Coronary artery disease | 206 (6.2) | 30 (3.6) | 0.005 | 83 (5.4) | 12 (3.1) | 0.087 | 123 (7) | 18 (4.1) | 0.035 |
| Marfan syndrome, n (%) | 3 (0.1) | 17 (2.1) | <0.001a | 1 (0.1) | 11 (2.9) | <0.001a | 2 (0.1) | 6 (1.4) | 0.001a |
| History of cardiovascular surgery, n (%) | 10 (0.3) | 16 (1.9) | <0.001 | 4 (0.3) | 11 (2.9) | <0.001a | 6 (0.3) | 5 (1.1) | 0.082a |
| BAV, n (%) | 0 (0) | 3 (0.3) | 0.008b | 0 (0) | 2 (0.5) | 0.04b | 0 (0) | 1 (0.2) | 0.2b |
| Cerebral hemorrhage, n (%) | 37 (1.1) | 16 (1.9) | 0.09 | 16 (1) | 14 (3.6) | 0.001 | 21 (1.2) | 2 (0.5) | 0.271a |
| CKD, n (%) | 16 (0.5) | 40 (4.8) | <0.001 | 6 (0.4) | 27 (7.0) | <0.001 | 10 (0.6) | 13 (3.0) | <0.001a |
| Hemodialysis, n (%) | 7 (0.2) | 5 (0.6) | 0.129a | 3 (0.2) | 2 (0.5) | 0.576a | 4 (0.2) | 3 (0.7) | 0.298a |
| COPD, n (%) | 39 (1.2) | 3 (0.4) | 0.057 | 18 (1.2) | 2 (0.5) | 0.399a | 21 (1.2) | 1 (0.2) | 0.12a |
| CTD, n (%) | 17 (0.5) | 7 (0.8) | 0.384 | 10 (0.6) | 3 (0.8) | 1a | 7 (0.4) | 4 (0.9) | 0.326a |
| Liver cirrhosis, n (%) | 15 (0.5) | 2 (0.2) | 0.584a | 8 (0.5) | 1 (0.3) | 0.802a | 7 (0.4) | 1 (0.2) | 0.929a |
Continuous variables were compared with a two‐sample t test. Categorical variables were compared with a chi‐square test if no designation was marked.
Abbreviations: AD, aortic dissection; BAV, bicuspid aortic valve; CKD, chronic kidney disease; CTD, connective tissue disease.
chi‐square test with Yate's continuity correction.
Fisher's exact test
Table 2.
Multivariate logistic regression analysis of chronic comorbidities as risk factors of AAD patients after adjustment for other factors
| Total | Type A | Type B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | P value | AOR | 95% CI | P value | AOR | 95% CI | P value | |
| Hypertension | 6.95 | 5.82‐8.32 | <0.001 | 6.87 | 5.29‐8.98 | <0.001 | 7.04 | 5.53‐9.01 | <0.001 |
| Diabetes | 0.14 | 0.09‐0.21 | <0.001 | 0.07 | 0.03‐0.14 | <0.001 | 0.21 | 0.13‐0.32 | <0.001 |
| CKD | 21.24 | 9.67‐51.55 | <0.001 | 52.11 | 15.39‐259.29 | <0.001 | 8.76 | 2.83‐29.36 | <0.001 |
| Marfan syndrome | 36.18 | 11.43‐159.92 | <0.001 | 65.97 | 11.35‐1253.54 | <0.001 | 22.53 | 4.89‐157.99 | <0.001 |
| Cardiovascular surgery | 8.34 | 3.39‐21.51 | <0.001 | 18.88 | 5.14‐85.7 | <0.001 | 3.99 | 1.0‐15.6 | 0.045 |
| Coronary artery disease | 0.35 | 0.22‐0.53 | <0.001 | 0.38 | 0.18‐0.73 | 0.06 | 8.76 | 0.18‐0.58 | <0.001 |
| Hemodialysis | 0.09 | 0.02‐0.46 | 0.004 | 0.03 | 0‐0.52 | 0.012 | 0.19 | 0.02‐1.66 | 0.134 |
| Cerebral hemorrhage | NI | 2.88 | 1.22‐6.83 | 0.015 | 0.23 | 0.04‐0.84 | 0.055 | ||
| Ever smoker | 1.37 | 1.15‐1.63 | <0.001 | 1.37 | 1.04‐1.79 | 0.024 | 1.4 | 1.10‐1.77 | 0.005 |
| COPD | 0.36 | 0.09‐1.07 | 0.105 | NI | 0.2 | 0.01‐1.02 | 0.123 | ||
A bidirectional eliminated stepwise multivariate logistic regression model was fitted with R function “glm,” model with the minimum Akaike information criterion (AIC) was used to calculate the adjusted OR (AOR) and 95% CI, and coefficient of variables was tested by Wald chi‐square test.
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NI, not included in the model.
3.2. Management of hypertension and AAD risk
There were 1035 and 592 hypertensive patients in the control group and AAD group, respectively. After exclusion of patients with incomplete or unavailable data regarding hypertension management, 848 control and 585 AAD patients with hypertension were included in this part of the analysis. The mean ages of patients in the control and AAD groups were 56.8 ± 11.3 and 57.3 ± 12.0 years (P = 0.379), respectively, with males accounting for 56.8% and 57.3% (P = 0.125) of the study group, respectively. The length of hypertension history was longer in AAD patients compared to controls (10 [5‐12] vs 8 [4‐10], P < 0.001; Table 3). The percentages of patients at stage 1, stage 2, and stage 3 hypertension were 9.4%, 25.7%, and 64.9% in the control group, and 6.8%, 20.9%, and 72.1% in the AAD group, respectively. Wilcoxon rank test analysis showed a significant difference in the stage of hypertension between the control and AAD group (P < 0.001). There were more patients with stage 3 hypertension in the AAD group compared to the control group (64.9% vs 72.1%, P = 0.004; Table S3). Patients with stage 3 hypertension had an increased risk of AAD (AOR 1.33, 95% CI: 1.01‐1.75, P = 0.045) compared to those at stage 2. However, compared to stage 1, stage 2 did not significantly increase the risk of AAD (AOR 1.07, 95% CI: 0.68‐1.72, P = 0.762; Table 4).
Table 3.
Management of hypertension in AAD and control patients
| Total | Type A | Type B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | AAD | P value | Control | AAD | P value | Control | AAD | P value | |
| Patients | 848 | 585 | 388 | 265 | 460 | 320 | |||
| Male, n (%) | 578 (68.2) | 376 (64.3) | 0.125 | 261 (67.3) | 171 (64.5) | 0.468 | 317 (68.9) | 205 (64.1) | 0.157 |
| Age, mean (SD) | 56.8 (11.3) | 57.3 (12.0) | 0.379 | 57.1 (11.2) | 57.4 (12.5) | 0.748 | 56.6 (11.) | 57.3 (11.7) | 0.365 |
| Year of history, median (IQR) | 8 (4‐10) | 10 (5‐12) | <0.001 | 8 (4‐10) | 10 (5‐12) | 0.015 | 8 (4‐10) | 10 (5‐12) | 0.014 |
| Stage of hypertension* | |||||||||
| Stage 1 | 80 (9.4) | 40 (6.8) | 40 (10.3) | 19 (7.2) | 40 (8.7) | 21 (6.6) | |||
| Stage 2 | 218 (25.7) | 122 (20.9)a | 95 (24.5) | 52 (19.6) | 123 (26.7) | 71 (22.2) | |||
| Stage 3 | 550 (64.9) | 422 (72.1)a | <0.001b | 253 (65.2) | 194 (73.2)a | 0.028b | 297 (64.6) | 228 (71.3) | 0.048b |
| Management of hypertension | |||||||||
| Regular treated | 598 (70.5) | 278 (47.5)a | 279 (71.9) | 126 (47.5)a | 319 (69.3) | 152 (47.5)a | |||
| Irregular treated | 179 (21.1) | 231 (39.4)a | 79 (20.4) | 103 (38.9)a | 100 (21.7) | 128 (40)a | |||
| Never treated | 71 (8.4) | 76 (13.0)a | <0.001b | 30 (7.7) | 36 (13.6)a | <0.001b | 41 (8.9) | 40 (12.5) | <0.001b |
| Uncontrolled hypertension | 393 (46.3) | 422 (72.1) | <0.001 | 179 (46.1) | 194 (73.2) | <0.001 | 214 (46.5) | 228 (71.3) | <0.001 |
Continuous variables were compared with a two‐sample t test or Wilcoxon signed‐rank test. Categorical variables were compared with the chi‐square test. The ordinal categorical variable was compared with the Kruskal‐Wallis test. Wilcoxon signed‐rank test was used if a difference between groups was observed. Uncontrolled hypertension, hypertensive patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg).
The stage of hypertension was based on JNC6.
P < 0.05 comparing with the control group, for exact P value, please refer to Table S3.
Wilcoxon signed‐rank test.
Table 4.
Multivariate logistic regression analysis of hypertension management as risk factors of AAD patients after adjusting for other factors
| Total | Type A | Type B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | P value | AOR | 95% CI | P value | AOR | 95% CI | P value | |
| Male sex | 0.81 | 0.64‐1.03 | 0.083 | NI | 0.75 | 0.55‐1.04 | 0.083 | ||
| Years of history, per year | 1.02 | 1.01‐1.04 | 0.001 | 1.03 | 1.0‐1.05 | 0.017 | 1.03 | 1.01‐1.05 | 0.006 |
| Stage of hypertension | |||||||||
| Stage 2 vs Stage 1 | 1.07 | 0.68‐1.72 | 0.762 | NI | NI | ||||
| Stage 3 vs Stage 1 | 1.42 | 0.94‐2.20 | 0.103 | NI | NI | ||||
| Stage 3 vs Stage 2 | 1.33 | 1.01‐1.75 | 0.045 | NI | NI | ||||
| Management of hypertension | |||||||||
| Irregular vs Regular | 2.44 | 1.89‐3.14 | <0.001 | 2.56 | 1.76‐3.74 | <0.001 | 2.38 | 1.69‐3.36 | <0.001 |
| Never treat vs Regular | 2.16 | 1.49‐3.13 | <0.001 | 2.40 | 1.39‐4.17 | 0.002 | 1.85 | 1.13‐3.05 | 0.015 |
| Never treat vs Irregular | 0.89 | 0.60‐1.31 | 0.542 | 0.94 | 0.52‐1.68 | 0.823 | 0.78 | 0.46‐1.31 | 0.348 |
| Uncontrolled hypertension | 2.63 | 2.09‐3.34 | <0.001 | 2.80 | 1.98‐3.97 | <0.001 | 2.53 | 1.84‐3.48 | <0.001 |
A bidirectional eliminated stepwise multivariate logistic regression model fitted with R function “glm,” model with the minimum Akaike information criterion (AIC) was used to calculate the adjusted OR (AOR) and 95% CI, and coefficient of variables was tested by Wald chi‐square test. The stage of hypertension was classified according to JNC 6. Uncontrolled hypertension means systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg; NI, not included in the model.
To analyze medication compliance, patients were subjected to three levels of medication compliance, which included patients on regular medication, irregular medication, and never treated. After adjusting for other factors, we found that patients who had never been treated and who were taking irregular medication had an increased risk of AAD compared to those who took regular medication (irregular vs regular: AOR 2.44, 95% CI: 1.89‐3.14, P < 0.001; never treated vs regular: AOR 2.16, 95% CI: 1.49‐3.13, P < 0.001), while the risk of AAD in the patients who had never been treated and who were taking irregular medication was comparable (AOR 0.89, 95% CI: 0.6‐1.31, P = 0.542). Uncontrolled hypertension (AOR 2.63, 95% CI: 2.09‐3.34) was also associated with an increased risk of AAD. These differences remained significant in both type A and type B dissection subgroup analyses. The results of the univariate analysis of logistic regression are shown in Table S2. The incidence of hypertension was re‐estimated after discharge (Table 5). In AAD patients without hypertension history, 25/115 (21.7%) and 53/118 (44.9%) could be diagnosed as having hypertension, contributing to an incidence of hypertension of 91.5% and 87.0% for type A and type B aortic dissection, respectively.
Table 5.
Awareness rate of hypertension in AAD patients
| Total | Type A | Type B | |
|---|---|---|---|
| Patients, n | 825 | 385 | 440 |
| Known hypertension, n (%) | 592 (71.8) | 270 (70.1) | 322 (73.2) |
| Unknown hypertension, n (%) | 233 (28.2) | 115 (29.9) | 118 (26.8) |
| Newly diagnosed hypertension, n (%) | 78 (33.5) | 25 (21.7) | 53 (44.9) |
| Total hypertensive patients, n (%) | 670 (81.2) | 295 (76.6) | 370 (85.2) |
| Awareness rate, % | 88.4 | 91.5 | 87.0 |
Awareness rate = (patients with known hypertension history/total hypertensive patients) × 100%
4. DISCUSSION
Clinical comorbidities, such as Marfan syndrome, BAV, Turner syndrome, and hypertension, have been considered risk factors for AAD for decades. Consistent with previous evidence, we also confirmed some of these risk factors in our study. Clinical comorbidities attributed to genetic disorders only occurred in a small number of AAD patients, for whom there was a lack of effective intervention. In contrast, the prevalence (approximately 65%‐75%) of hypertension was significantly higher in AAD patients.1 Furthermore, hypertension could be controlled by adequate anti‐hypertensive therapy in most cases. Therefore, hypertension appears to be the most important controllable factor in AAD development.
The primary hemodynamic mechanisms underlying AAD are increased wall stress and wall weakness. These two factors contribute differently in hypertensive patients compared to patients with structural morbidities, such as Marfan syndrome.13 In the setting of hypertension, elevated aortic pressure was the primary cause of increased wall stress. Although elevated blood pressure was not considered the only factor, hypertension was indeed the main facilitator of increased AAD risk. Incidence of aortic dissection and/or rupture significantly correlated with aortic diameter and age in patients with a moderately dilated ascending aorta.14 Hypertension was associated with an increase in aortic stiffness and aortic root size, and most notably with the size of the supra‐aortic ridge and proximal ascending aorta.15, 16 Furthermore, hypertension‐related atherosclerosis may play a role in AAD development. Agmon et al17 found that hypertension was independently associated with aortic atherosclerosis, especially complex aortic atherosclerosis with protruding plaques >4 mm in thickness, mobile debris, or ulceration. Advanced atherosclerosis that causes inflammation, destruction, and calcification of the aortic wall may result in decreased aortic elasticity and increased hemodynamic stress, thus leading to AD.18 In clinical practice, Marfan patients with elevated blood pressure are usually at higher risk of aortic dilatation and progression to AD. However, the rate of dilation can be decreased by anti‐hypertensive therapy.19, 20 These observations suggest that reasonable blood pressure control may delay the progression or reduce the incidence of AAD. Indeed, in the present study, we found that patients with uncontrolled hypertension had an increased risk of AAD.
Although population awareness and medical compliance of hypertension have improved with time, there are still challenges in improving hypertension management in underdeveloped countries.3, 4 Jilin province is located in northeast China, which is an underdeveloped area with climate variabilities. The climate in Jilin province is cold and dry in the winter, and hot and rainy in the summer. The fluctuations in temperature during seasonal changes might contribute to cardiovascular disease. In addition, patients in Jilin usually have limited health awareness and unhealthy living habits. Many patients eat high‐salt and fat‐containing foods and do not attend regular physical examinations. Thus, the role of hypertension management in AAD development may be affected by geography. Our study revealed several characteristics of hypertension management in AAD patients in Jilin province. First, the incidence of hypertension in AAD patients might be underestimated if only self‐reported hypertension history is considered. We found that the incidence of hypertension prevalence was 70.1% for type A and 73.2% for type B dissection. However, in patients admitted for AAD without a known hypertension history, 25 (21.7%) of type A and 53 (44.9%) of type B patients were diagnosed with hypertension after hospitalization, increasing hypertension prevalence to 76.6% for type A and 85% for type B patients. Therefore, the actual prevalence of hypertension in type A and type B dissection was 91.5% and 87.0%, respectively. The awareness rate of hypertension was 88.4% in AAD patients. Secondly, we found that hypertensive patients with AAD tended to have a higher classification, longer history, and poorer control rates of hypertension, which suggest that the impact of hypertension on AAD development may be persistent, progressive, and eventually increase without management. The control rates of hypertension in the control and AAD groups were 54% and 28%, respectively, according to our results. However, both rates were higher than those reported in the general Chinese population (18%‐30%).2, 4 This might be due to differences in inclusion criteria between studies. Thirdly, our results suggest that hypertensive patients with AAD had poor medication adherence and blood pressure control, both of which were associated with AAD risk. It is reasonable to conclude that good medication compliance may contribute to effective blood pressure control. However, the correlation between medication compliance and blood pressure control was 0.233 (P < 0.001), 0.209 (P < 0.001), and 0.253 (P < 0.001) in our total cohort, type A dissection, and type B dissection, respectively, which indicates that regular medication itself cannot guarantee optimal blood pressure control. Actually, blood pressure control with anti‐hypertensive therapy is a complicated process and may be impacted by multiple factors such as eating habits, emotional health, and individual differences in pharmacokinetics. This may explain why the correlation coefficient between medication compliance and blood pressure control was only approximately 0.2 in our study. It is also interesting that good medication compliance, regardless of blood pressure control, decreased the risk of AAD. No interaction was found between medication compliance and blood pressure control (data not shown). This might be because patients with good medication compliance live healthier lives and have more health awareness, or because irregular medication impacts the stability of the plasma concentrations of anti‐hypertensive drugs that cause blood pressure fluctuation. However, we should point out that blood pressure control in our study was self‐reported by patients and not prospectively recorded. Thus, further research is needed to investigate the relationship between blood pressure control and medication compliance.
In our study, we also found that CKD was associated with increased AAD risk. There are several case reports stating that CKD patients developed AAD. Kidney failure was reported to complicate type B dissection,21 and the occurrence of kidney failure reciprocally correlated with the presence of AAD and AA.22, 23 Previous studies also found that patients with CKD, such as autosomal dominant polycystic kidney disease, had a significantly higher risk of developing AAD compared to the general population.24 Our study is the first to reveal a statistical positive relationship between CKD and AAD risk. However, for those severe CKD patients who required hemodialysis, we observed an opposite effect. Some individual cases had an increased incidence of AAD following dialysis.25, 26 This might be due to the “survivor bias.” Severe CKD patients on hemodialysis might not survive and therefore never be admitted to the hospital, or they might forego treatment due to economic issues or premature death, thus reducing the risk of AAD associated with hemodialysis observed in our study. It will be of great interest to clarify this issue through a large cohort study in the future.
4.1. Study limitations
This was a retrospective case‐control study. Information on hypertension management in the control group was collected via telephone interviews during patient follow‐up, and some information was provided by individuals other than the patients themselves, such as relatives. Furthermore, we were not able to contact some patients due to lack of correct contact information. Thus, our data might be incomplete or inaccurate. For anti‐hypertensive therapy, a detailed classification of medication was initially designed; however, this part of our data was incomplete and this design was not implemented. In addition, information regarding medication compliance was collected retrospectively through patient self‐reporting. Therefore, it is possible that these data contain inaccurate information. Finally, although an AOR for disease risk was calculated in our case‐control study, we were unable to examine the cause and effect relationship due to the retrospective nature of this study.
5. CONCLUSIONS
As a key risk factor, hypertension is associated with a significant risk of AAD, especially in patients with longer history and higher stage of hypertension. Hypertensive patients who receive irregular medication, never receive treatment, or have uncontrolled blood pressure are at a higher risk of AAD development. It is important to improve the management of hypertension to reduce AAD incidence in China's Jilin province.
CONFLICT OF INTEREST
We declare that we do not have any conflict of interest regarding this work.
AUTHOR CONTRIBUTIONS
Ning Dong designed the study and analyzed the results. Bo Li, Jian Xu, and Shibo Wei acquired the data. Bo Li and Hulin Piao validated the results. Ning Dong, Bo Li, and Hulin Piao interpreted the data. Kexiang Liu critically verified the statistics, validated the interpreted data, and revised the manuscript. Ning Dong and Hulin Piao drafted the manuscript. All authors approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Technology Development Project of Jilin Province (20130206025SF) and Development and Reform Commission of Jilin Province (2013C0234).
Dong N, Piao H, Li B, Xu J, Wei S, Liu K. Poor management of hypertension is an important precipitating factor for the development of acute aortic dissection. J Clin Hypertens. 2019;21:804–812. 10.1111/jch.13556
REFERENCES
- 1. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35:2873‐U2893. [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524‐532. [DOI] [PubMed] [Google Scholar]
- 3. Marques‐Vidal P, Tuomilehto J. Hypertension awareness, treatment and control in the community: is the 'rule of halves' still valid? J Hum Hypertens. 1997;11:213‐220. [DOI] [PubMed] [Google Scholar]
- 4. Huang G, Xu J‐B, Zhang T‐J, et al. Prevalence, awareness, treatment, and control of hypertension among very elderly Chinese: results of a community‐based study. J Am Soc Hypertens. 2017;11(503‐512):e502. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Peng X, Nie X, et al. Burden of hypertension in China over the past decades: systematic analysis of prevalence, treatment and control of hypertension. Eur J Prev Cardiol. 2016;23:792‐800. [DOI] [PubMed] [Google Scholar]
- 6. Kang Q, Kang G, Li R, Zhu X, Yu Y, Yu Q. Relationship of gallbladder diseases with sociodemographic characteristics, lifestyle, and chronic diseases in Northeastern China. Int J Environ Res Public Health. 2018;15:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873‐2926. [DOI] [PubMed] [Google Scholar]
- 8. Zullig LL, Mendys P, Bosworth HB. Medication adherence: a practical measurement selection guide using case studies. Patient Educ Couns. 2017;100:1410‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen T, La Caze A, Cottrell N. What are validated self‐report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77:427‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413‐2446. [DOI] [PubMed] [Google Scholar]
- 11. Ho DE, Imai K, King G, Stuart E. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:804‐28. [Google Scholar]
- 12. Thase ME. Using biomarkers to predict treatment response in major depressive disorder: evidence from past and present studies. Dialogues Clin Neurosci. 2014;16:539‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robicsek F, Thubrikar MJ. Hemodynamic considerations regarding the mechanism and prevention of aortic dissection. Ann Thorac Surg. 1994;58:1247‐1253. [DOI] [PubMed] [Google Scholar]
- 14. Erbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart. 2006;92:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652‐658. [DOI] [PubMed] [Google Scholar]
- 16. Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension. 1996;28:47‐52. [DOI] [PubMed] [Google Scholar]
- 17. Agmon Y, Khandheria BK, Meissner I, et al. Independent association of high blood pressure and aortic atherosclerosis: a population‐based study. Circulation. 2000;102:2087‐2093. [DOI] [PubMed] [Google Scholar]
- 18. Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazarevic AM, Nakatani S, Okita Y, et al. Determinants of rapid progression of aortic root dilatation and complications in Marfan syndrome. Int J Cardiol. 2006;106:177‐182. [DOI] [PubMed] [Google Scholar]
- 20. Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994;330:1335‐1341. [DOI] [PubMed] [Google Scholar]
- 21. Li LI, Zhuang ShunJiu, Qi ShaoHong, et al. Acute thoracic aortic dissection (stanford type B) complicated with acute renal failure. Case Rep Vasc Med. 2013;2013:693435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dujardin A, Le Fur A, Cantarovich D. Aortic dissection and severe renal failure 6 years after kidney transplantation. Transplant Direct. 2017;3:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagiwara S, Saima S, Negishi K, et al. High incidence of renal failure in patients with aortic aneurysms. Nephrol Dial Transplant. 2007;22:1361‐1368. [DOI] [PubMed] [Google Scholar]
- 24. Sung P‐H, Yang Y‐H, Chiang H‐J, et al. Risk of aortic aneurysm and dissection in patients with autosomal‐dominant polycystic kidney disease: a nationwide population‐based cohort study. Oncotarget. 2017;8:57594‐57604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vieira MB, Ferreira T, Cotovio P, Ministro A. Type B aortic dissection in a chronic haemodialysis patient. BMJ Case Rep. 2015;2015:bcr2015213376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang G, Huang W, Shen C. Debakey III aortic dissection in a hemodialysis patient. Clin Kidney J. 2014;7:491‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


