Abstract
The Science of Salt reviews identify, summarize, and critically appraise published studies on dietary salt and health outcomes according to pre‐specified methods. This review covers the period April 3 to October 30, 2018. Here, nineteen studies that fit pre‐specified criteria for review and summary are included. Three of these, one prospective cohort study, one randomized controlled trial, and a post hoc analysis of the Dietary Approaches to Stop Hypertension (DASH) sodium trial fulfilled the quality criteria for detailed critical appraisal, including risk of bias assessment, and commentary. Two trials demonstrated a positive association between salt intake and blood pressure. In a cohort of older Italians, increased risk of total mortality was observed with salt intake less than ~16 g/d (6300 mg sodium/d) at baseline; no association existed for incident cardiovascular disease (CVD) or CVD mortality. The paucity of published studies which met our criteria for methodological quality is of concern.
Keywords: hypertension, review, salt, sodium
1. INTRODUCTION
Non‐communicable diseases have been identified as one of the top ten threats to health in 2019 and are responsible for over 70% of global deaths.1 The World Health Organization (WHO) Global Action Plan for the reduction of non‐communicable diseases 2013‐2020 has identified reducing mean population sodium intake by 30% by 2025 as one of nine priority voluntary global targets for reduction of non‐communicable disease.2 This is supported by evidence showing that world population sodium intake remains substantially higher than levels recommended by the WHO.3, 4 In 2010, global mean sodium intake was estimated to be 3950 mg/d (95% uncertainty interval: 3890–4010 mg/d).4 By contrast, the WHO recommends a mean population sodium intake for adults of <2000 mg/d, with lower intakes for children proportional to energy intake.5
A consistent body of evidence shows a causal relationship between high sodium intakes and elevated blood pressure.6 Aside from the well‐documented association between high blood pressure and cardiovascular disease (CVD), substantial evidence also directly links high sodium intakes with cardiovascular outcomes including stroke and myocardial infarction.7, 8, 9 Despite this, there is inconsistent data regarding the association between sodium intake and mortality risk, particularly at lower levels of intake.10, 11 Debate also continues about the role of dietary sodium restriction in patient groups, such as in those with heart failure.12 Much of the debate about this conflicting evidence centers around methodological concerns, particularly the role of measurement error, confounding, reverse causality, and for heart failure interactions with potent diuretics.13 Despite this debate, dietary guidelines around the world consistently recommend reductions in population sodium intake.14, 15
The Science of Salt Weekly series of systematic reviews and critical appraisals is produced to inform scientific, clinical, and policy stakeholders of recent literature in the field of salt intake and health outcomes, with a focus to highlight studies that meet pre‐specified and rigorous methodological criteria. This paper includes articles published from April 3 to October 30, 2018.
2. METHODOLOGY
A detailed description of the methodological approach used to identify published articles for this review has been previously reported.16 Briefly, a weekly search of MEDLINE was undertaken using a search strategy adapted from a systematic review used to develop the WHO guideline on dietary sodium intake.5, 17, 18 The present review includes health outcome studies identified during the period April 3 to October 30, 2018 (Figure 1). Inclusion and exclusion criteria of studies included this review are outlined in Table S1. All eligible studies identified were screened independently by two authors (RM and KP) to determine which studies were eligible to undergo a detailed assessment and critical appraisal based on the outcomes examined and methodological quality, as described below. The criteria for assessing outcomes studies and methodological criteria are outlined in Tables S2 and S3. Other articles from the list of eligible studies, not chosen for critical appraisal, are discussed in this manuscript if judged by the authors to be impactful based on novelty of findings or potential for generating public discourse or scientific controversy, or for informing public health policy.
Figure 1.
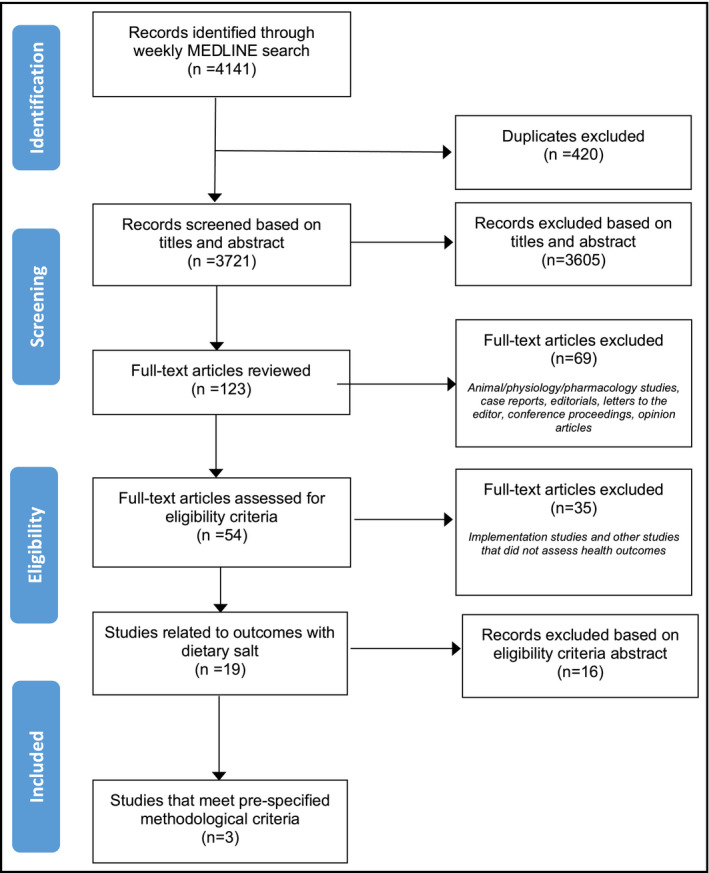
Study flow diagram for studies identified from April 2018 to October 2018
Firstly, articles have been assessed based on a hierarchy of health outcomes and classified based on relevance to patients (Table 1). These include mortality (Category I), morbidity (Category II) symptoms/quality of life/functional status (Category III), the clinical diagnosis, prevention or treatment of hypertension (Category IV), and other clinical surrogate outcomes (Category V). Studies on physiologic and biomarker surrogate outcomes (Category VI) were excluded from eligibility for detailed critical appraisal.
Table 1.
Summary of 19 studies identified from April 3 to October 30 2018 and eligibility for detailed critical appraisal
| Study (Country) | Study Design | Study Duration | Method of sodium intake measurement | Outcomes | Eligible for detailed critical appraisal | Reason for exclusion from detailed critical appraisal |
|---|---|---|---|---|---|---|
| Category I—Mortality outcomes | ||||||
| Lelli et al, Italy28 | Cohort Study | 9 y | 24‐h urine | Mortality and incident cardiovascular events | Yes | |
| Mente et al, 21 countries (PURE Study)29 | Cohort Study | 9 y | Spot urine (Kawasaki formula) | Blood pressure, cardiovascular disease, and mortality | No | Spot urine used for sodium intake assessment |
| Category II—Morbidity outcomes | ||||||
| Abreu et al (Portugal)32 | Population data analysis (Retrospective cohort analysis) | Data included from 202 to 2015 (Acute Coronary Syndrome) and 202 to 2016 (Other CVD hospital admission data) | None | Acute Coronary Syndrome, Stroke (ischemic and hemorrhagic, Acute Myocardial Infarction) Unstable Angina | No | No measure of sodium intake |
| Li et al (China)33 | Cross‐sectional | N/A | 24‐h urine and spot urine | Coronary heart disease | No | Cross‐sectional study |
| Mirmiran (Iran)30 | Cohort | 6.3 y | FFQ | Chronic Kidney Disease | No | FFQ used to assess sodium intake |
| Yoon et al (Republic of Korea)31 | Cohort | Median follow‐up 123 mo | FFQ | Chronic Kidney Disease | No | FFQ used to assess sodium intake |
| Category III—Symptoms/Quality of Life/Functional Status Outcomes | ||||||
| Fatahi et al22 | Systematic Review and Meta‐Analysis of cross‐sectional and cohort studies | N/A | dietary or 24‐h urinary sodium measurement | Osteoporosis, bone mineral density, and bone mineral content | No | Only one cohort study included in Systematic Review (Chan et al 2011) which assessed multiple nutrients by FFQ at baseline |
| Category IV—Clinical surrogate outcomes (Blood pressure) | ||||||
| Awadalla et al (Sudan)34 | Cross‐sectional | N/A | Questionnaire | Hypertension (Systolic blood pressure (SBP) >140 mm Hg and/or diastolic >90 mm Hg) | No | Sodium intake assessed by questionnaire only |
| Carranza‐Leon et al (USA)35 | Cross‐sectional | N/A | Spot urine (Kawasaki formula) | Blood pressure | No | Spot urine used for sodium intake assessment |
| Garofalo et al23 | Systematic Review and Meta‐Analysis of RCTs | N/A | 10/11 studies reported 24‐h urinary sodium | Blood pressure, change in kidney function (proteinuria, albumuria or GFR) change in body weight or fluid, change in urinary sodium and potassium. | No | Trials range from 1 to 24 wk—not eligible since all studies need to be ≥4 wk |
| Janda et al24 | RCT | 12 wk | Spot urine | Blood pressure | No | Spot urine used for sodium intake assessment |
| Murao et al (Japan)36 | Cross‐sectional | N/A | Spot urine | Blood pressure | No | Spot urine used for sodium intake assessment |
| Murtaugh et al (USA)25 | RCT (reanalysis of DASH‐sodium trial results) | 12 wk | 24‐h urine | Blood pressure | Yes | |
| Musso et al (USA)27 | Controlled trial (not randomized) | 9 wk | 24‐h urine | Blood pressure | No | Not a RCT, and difference between sodium intake only 460 mg/d |
| Yang et al (China)26 | RCT | 6 mo | 24‐h urine | Blood pressure | Yes | |
| Category V—Physiologic surrogate outcomes | ||||||
| Padiha et al (Brazil)37 | Cross‐sectional | N/A | 24‐h urine | serum cholesterol | No | Cross‐sectional study |
| Other outcomes | ||||||
| Bolin et al (USA)38 | Cross‐sectional | N/A | Questionnaire | Adherence to Low salt diet | No | Sodium intake assessed by questionnaire only |
| Cogswell et al USA39 | Cross‐sectional | N/A | 24‐h urine (NHANES survey 2014) | Sodium intake | No | Sodium intake is outcome |
| dos Santos et al, Brazil40 | Cross‐sectional | N/A | Spot urine (Kawasaki formula) | Sodium intake | No | Sodium intake is outcome |
Secondly, selection of studies for detailed critical appraisal and commentary (Table 2) involved using methodological quality criteria outlined in Table S3. Included in the appraised articles were randomized controlled trials (RCTs) that allocated at least one group of participants to reduced sodium intake and one group to higher sodium intake (control group) with no concomitant interventions, achieved an intake difference of ≥920 mg sodium (2.3 g salt) between intervention and control conditions, and measured sodium intake with a 24‐hour urine collection. Cohort studies included in the detailed appraisals had a prospective design; included 400 or more participants (continuous outcomes) or events (dichotomous outcomes); measured sodium intake with a 24‐hour urine collection, food record, or 24‐hour food recall. Studies with a primary outcome of blood pressure and hypertension (Category IV) were only eligible for detailed critical appraisal if they were RCTs or systematic reviews of RCTs meeting the minimum methodological criteria listed above and were ≥4 weeks duration. RCTs and cohort studies assessing Category I, II, or V outcomes were eligible for appraisal if they were ≥1‐year duration. RCTs and cohort studies assessing Category III outcomes, eligible studies were ≥4 weeks duration.
Table 2.
Summary of three articles critically appraised
| Study (Country) | Study Design | Participants | Study Duration | Dietary salt “dose” (actual mean intake per day) | Method of sodium intake measurement | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Category I—Mortality outcomes | |||||||
| Lelli et al, Italy28 | Cohort Study |
N = 920 adults aged 65‐102; median age 74.5 y, 55.4% women; from two Italian cities (Greve in Chianti and Bagno in Ripoli), participating in the InCHIANTI cohort study. Analysis of incident cardiovascular events included only those with no history of CVD at baseline (N = 514) |
Median follow‐up time 9 y | Overall mean, median, and range not presented. Participants were divided into quartiles of salt intake (<9.25 g/d, 9.25‐13.60 g/d, 13.61‐17.6 and >17.6 g/d) for survival analysis | Single 24‐h urine at baseline. | All‐cause mortality and incident cardiovascular events (angina pectoris, myocardial infarction, heart failure, and stroke) | Increased risk of mortality in those in the lowest two quartiles of sodium intake: 46.2% in quartile 1 (<9.25 g salt/d) and 34.7% in quartile 2 (9.25‐13.60 g salt/d). A negative association between salt intake and mortality risk and was only observed below estimated intakes of 15.5 g salt/d. The overall association between decreasing 24‐h sodium excretion and total mortality was statistically significant in all models (adjusted HR 1.12, 95% CI 1.04, 1.22). In those without prevalent cardiovascular disease at baseline (N = 514), there was a weak inverse but not statistically significant association between sodium intake and risk of cardiovascular disease, (adjusted RR 0.96, 95% CI 0.90, 1.02) |
| Category II—Morbidity Outcomes | |||||||
| None | |||||||
| Category III—Symptoms/Quality of life/Functional status outcomes | |||||||
| None | |||||||
| Category IV—Clinical surrogate outcomes (Blood Pressure) | |||||||
| Murtaugh et al (USA)25 | Single‐blind randomized controlled feeding study (crossover). (post hoc analysis of DASH‐sodium trial results) | 379 non‐Hispanic black and white adult participants with complete dietary data from the original 412 participants in the DASH‐sodium trial. Participants had untreated pre or stage 1 hypertension | Two‐week run‐in high sodium control diet, followed by randomization to one of three sodium levels in random order for 30 each. Each diet period was separated by an, on average, 5‐d washout period |
Low salt: 3.7 g/d Mid salt: 6.2 g/d High salt: 8.2 g/d |
Single 24‐h urine collection during last week of each diet period |
Blood pressure measured at
|
The slope of the association between sodium intake and blood pressure in both diet groups (DASH and control) was greater at lower energy intakes than higher energy intakes. The relationship between sodium intake and blood pressure was attenuated on the DASH diet compared with the control diet at all levels of energy intake. These associations were shown when the analyses were stratified by race (except diastolic blood pressure (DBP) among those of white race), with slightly larger differences in those of black race compared to whites. There were smaller differences in blood pressure by energy intake among obese compared with non‐obese participants |
| Yang et al (China)26 | Single‐blind randomized controlled trial | 126 adult Han Chinese aged 50 to 80 y with mild to moderate hypertension (average SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg or being treated with antihypertensive drugs) | After a 4‐wk run‐in period, participants were categorized into Isolated systolic hypertension (ISH) or non‐isolated systolic hypertension (NISH) groups. They were then randomized into either intervention LSSalt (low sodium salt) or control NSalt (normal salt) groups and followed up for 6 mo | Intervention groups: 10 g/d salt and 8.8 g/d salt for ISH and NISH groups, respectively. Control groups: 14.2 g/d salt and 12.9 g/d salt for ISH and NISH groups, respectively | Single 24‐h urine collection at 6 mo | Blood pressure measured at baseline, randomization, and every 2 mo for 6 mo | Use of a LSSalt was associated with a statistically significant reduction in urinary sodium excretion at 6 mo compared with using NSalt in both ISH and NISH participants (P < 0.001). For ISH participants, there was a mean (SD) sodium reduction of 50 (59.3) mmol/d (1150 (1364)mg/d) in the low salt group compared with only 4.97(54.3) mmol/d (114 (1249) mg/d)in the control group. For NISH participants, there was a mean (SD) reduction of 91.7 (47.3) mmol/d (2109 (1088) mg/d) in the low sodium salt group, and a mean difference of 16.0 (85.0) mmol/d (368 (1995) mg/d) in the normal salt group. Use of a LSSalt was associated with a statistically significantly lower SBP at 6 mo for the ISH participants. Compared with the mean of the ISH control group, the LSSalt ISH group had a mean difference SBP of −10.18 mm Hg (95% CI −17.2, −3.13). There was no difference in SBP at 6 mo for NISH participants, and there was no difference in DBP at 6 mo for either group |
Three independent reviewers (RMM, KSP, and SR) assessed articles selected for a detailed critical appraisal for risk of bias. Randomized control trials were assessed using the Cochrane risk of bias tool.19 Observational, non‐randomized studies were assessed using a modified Cochrane risk of bias tool.20 For meta‐analyses, the AMSTAR tool was applied.21
3. RESULTS
The weekly search identified 4141 citations over the period from April 3 to October 30, 2018, of which 19 studies including dietary sodium and health outcomes met the criteria for full review (Table 1). These included systematic review and meta‐analyses (n = 2),22, 23 randomized controlled trials (n = 3, including one post hoc analysis of a randomized controlled trial),24, 25, 26 a non‐randomized controlled trial (n = 1),27 prospective cohort studies (n = 4),28, 29, 30, 31 a retrospective cohort study using population data (n = 1),32 and cross‐sectional studies (n = 8).33, 34, 35, 36, 37, 38, 39, 40 Two studies assessed mortality outcomes,28, 29 four studies assessed morbidity outcomes,30, 31, 32, 33 and one study assessed symptoms/quality of life/functional status outcomes.22 Eight studies assessed clinical surrogate outcomes,23, 24, 25, 26, 27, 34, 35, 36 one study assessed physiologic surrogate outcomes,37 and three studies assessed other outcomes38, 39, 40 according to our pre‐specified categories. Of these, three studies met the minimum methodological criteria for detailed critical appraisal.25, 26, 28
Sixteen studies were not eligible for detailed critical appraisal due to study design (eight were cross‐sectional studies, one meta‐analysis included trials that were <4 weeks in duration, one non‐randomized controlled trial) and methodological issues. Six studies used spot urine samples to estimate sodium intake, three studies used Food Frequency Questionnaires, and two used a dietary habits questionnaire. Other reasons for exclusion from detailed critical appraisal included no measure of sodium intake (n = 1) and sodium being the outcome measure (n = 2).
The three studies critically appraised comprise of one prospective cohort study,28 one RCT,26 and a post hoc analysis of the DASH‐sodium trial data.25 The prospective cohort study included participants from the InCHIANTI study (median age 74.5) conducted in two Italian cities and showed all‐cause mortality was higher in those who consumed <6250 mg/d of sodium (equivalent to 15.63 g salt) at baseline over a mean follow‐up period of 9 years. There was no association between sodium intake and incident CVD in adjusted models.28 An RCT investigating a 6‐month intervention of low sodium salt use in a free‐living population in China reported that low sodium salt use was associated with decreased sodium intake, as measured by 24‐hour urinary sodium excretion, and reduced systolic blood pressure (SBP) in those diagnosed with isolated systolic hypertension, but not in those with non‐isolated systolic hypertension.26 A post hoc analysis of data from the DASH sodium controlled feeding trial examined the relationship between sodium intake, energy intake, race (black or white race), and obesity. Authors reported a significant interaction between sodium intake, energy, and obesity status for SBP. The association between sodium intake and blood pressure was stronger at lower energy intake levels than high energy intakes and stronger in the control diet compared with the DASH diet arms of the trial.25
A summary of study characteristics and risk of bias assessments included in the detailed critical appraisal are highlighted below. A more comprehensive summary of the risk of bias assessments is included in Table S4.
4. DETAILED CRITICAL APPRAISALS OF SELECTED STUDIES
Is sodium intake associated with increased risk of cardiovascular disease and mortality? Prospective cohort study
Lelli D, Antonelli‐Incalzi R, Bandinelli S, et al. Association between sodium excretion and cardiovascular disease and mortality in the elderly: a cohort study. Journal of the American Medical Directors Association. 2018;19(3):229‐234.
Design: Prospective observational study.
Setting: Two Italian cities (Greve in Chianti and Bagno in Ripoli), participating in the InCHIANTI cohort study.
Participants: N = 920 adults aged 65‐102 years; median age 74.5 years, 55.4% women; median follow‐up 9 years.
Exposure: Sodium intake, estimated by a single 24‐hour urine sample at baseline.
Outcomes: Mortality (all cause) collected from mortality registers (n = 920). For participants without a history of CVD at baseline (n = 514), incident CVD events (angina pectoris, myocardial infarction, heart failure, and stroke) were assessed using follow‐up questionnaires and reviewing clinical documentation. Mortality risk by sodium intake was examined using Kaplan‐Meier curves and cox regression analysis. For incident CVD, regression models were used to calculate relative risks (RR).
Risk of bias:
Sampling: Low risk.
Representativeness: Low risk.
Reliability/validity of exposure: High risk.
Reliability/validity of outcome: Unclear risk.
Blinding of outcome assessment: Unclear risk.
Risk of selective outcome reporting: Low risk.
Confounding: High risk.
Sources of funding: Italian Ministry of Health and US National Institute on Ageing.
Summary of results. When the total sample participants were stratified into quartiles of sodium intake (cutoffs for sodium intake quartiles were <3.70 g/d, 3.70‐5.43 g/d, 5.44‐7.04 g/d, and >7.04 g/d; equivalent to <9.25 g/d, 9.25‐13.60 g/d, 13.61‐17.6 g/d, and >17.6 g/d of salt), there was increased cumulative risk of mortality in the lowest two quartiles of sodium intake: 46.2% in quartile 1 and 34.7% in quartile 2. There was no difference in mortality risk between the third and fourth quartile. The negative association between sodium intake and risk of mortality was not linear and was only observed below estimated sodium excretion of 6.25 g/d (15.63 g salt). The overall association between decreasing 24‐hour sodium excretion and total mortality was statistically significant in the fully adjusted model (adjusted Hazard ratio [HR] 1.12, 95% CI 1.04, 1.22). In frail and non‐frail individuals without CVD at baseline, similar associations with all‐cause mortality were observed (frail: HR 1.23, 95% CI 1.02, 1.50; non‐frail: HR 1.11, 95% CI 1.01, 1.22). In those without prevalent cardiovascular disease at baseline (N = 514), there was a weak inverse association between sodium intake and risk of CVD (RR 0.95; 95% CI 0.90‐1). However, when adjusted for confounders no association was reported (adjusted RR 0.96, 95% CI 0.90, 1.02).
Comment. This study found that sodium intakes below 6.25 g/d (15.63 g/d salt) were associated with increased risk of all‐cause mortality in Italian community‐dwelling adults older than 65 years of age. Sodium intake was not associated with incident CVD. The strengths of this study include its prospective design, long‐term follow‐up, and adjustment for multiple potential confounding factors. There are several limitations related to dietary sodium exposure and assessment, which could lead to substantial misclassification. Although 24‐hour urine collection is considered the gold standard measure of dietary sodium intake, there appears to have been no attempts to validate whether collected samples were complete. Incomplete urines are particularly of concern as non‐adherence even to placebo is associated with increased mortality as demonstrated in randomized controlled trials.41, 42 Furthermore, due to large day‐to‐day variability of intake and excretion, a single 24‐hour urine sample is unlikely to give a valid estimate of usual intake, with at least 3 non‐consecutive days required.43, 44, 45 Additionally, sodium excretion was only measured at baseline; thus, estimates do not reflect any changes in intake over the 9‐year follow‐up period. Sodium intakes are high for the cohort,46 with the lowest quartile cutoff (<3.7 g/d, or <9.25 g/d salt) substantially above the mean level recommended by WHO of 2 g/d (5 g/d salt). Although analyses are adjusted for multiple confounders (including age, sex, education, renal function, smoking, body mass index, energy intake, medication use, presence of hypertension or diabetes, and frailty), the possibility of residual confounding and reverse causality exists. Of note, those in the lower sodium group were older, more likely to be women, more sedentary, had lower glomerular filtration rate, and had higher SBP and prevalence of dementia. Physical activity is a driver of sodium (and energy) intake and is strongly associated with decreased mortality and longevity in older adults.
What is the relationship between dietary sodium density with blood pressure in the Dietary Approaches to Stop Hypertension (DASH) sodium trial?
Murtaugh MA, Beasley JM, Appel LJ, et al. Relationship of sodium intake and blood pressure varies with energy intake: secondary analysis of the DASH (Dietary Approaches to Stop Hypertension)‐sodium trial. Hypertension. 2018;71:858‐865.
Design: Single‐blind randomized controlled crossover feeding study. This is a post hoc analysis of the DASH‐sodium trial conducted between 1997 and 1999.47
Setting: Four clinical centers in the United States.
Study duration: Fourteen‐day run‐in period with three 30‐day intervention periods.
Participants: Three hundred and seventy‐nine of 412 participants from the DASH‐sodium trial. Participants were healthy, community‐dwelling adult men and women, aged 22 years or older who had blood pressure measurements of 120‐159 mm Hg systolic and 80‐96 mm Hg diastolic. Only those who identified their race as black or white were included in this secondary analysis.
Intervention: Participants were randomized to either a DASH diet or a control diet that was designed to represent a “typical American” diet. Within each diet allocation, participants received three different levels of dietary sodium density (mg/kcal) daily in random order: 0.5 mg sodium/kcal (equivalent to 1150 mg/d sodium, 2.9 g salt per 2100 kcal), 1.1 mg sodium/kcal (2300 mg sodium; 5.8 g salt per 2100 kcal), and 1.6 mg sodium/kcal (3450 mg sodium; 8.6 g salt per 2100 kcal). During the run‐in period (2 weeks), all participants consumed the high sodium control diet. This was a controlled feeding study, and all caloric foods and beverages were provided for the duration of the study; caloric provision was adjusted so participants' weight remained constant.
Achieved sodium intake: Sodium intake was controlled by investigators and measured by a single 24‐hour urine sample during the last week of each 30‐day diet period. Energy intake was calculated for each participant from the nutrition composition of menus (including beverages) plus supplemental nutrition bars provided. The DASH‐sodium trial achieved sodium intakes of 1495 mg sodium, (3.7 g salt); 2461 mg sodium, (6.2 g salt); and 3266 mg sodium, (8.2 g salt) in the low, mid, and high sodium groups, respectively, for both diet allocations. For the purpose of this analysis, participants were divided into tertiles of energy intake (<2200 kcal/d, 2200‐3000 kcal/d, and ≥3000 kcal/d) and sodium density (with mean of 0.6 mg/kcal, 1.2 mg/kcal, and 1.7 mg/kcal in the low, mid, and high sodium density groups, respectively).
Outcomes: Clinic blood pressure was measured in the sitting position with random‐zero sphygmomanometers. Blood pressure was measured twice during the run‐in period, weekly during the first 3 weeks of each of the three 30‐day intervention periods, and at 5 clinic visits during the last 9 days (at least 2 during the final 4 days) of each intervention period. Mixed effects models were used to assess the relationship between sodium intake, energy intake, and blood pressure overall and by race and body size.
Risk of bias:
Random sequence generation: High risk.
Allocation concealment: Unclear risk.
Blinding of participants and personnel: High risk.
Blinding of outcome assessors: Low risk.
Incomplete outcome reporting: Low risk
Selective reporting: Low risk.
Other sources of bias: Low risk.
Source of funding: National Heart Lung and Blood Institute; National Center for Advancing Translational Sciences of the National Institutes of Health.
Summary of results. A positive association between sodium intake and both systolic and diastolic blood pressure in both the DASH and control diets was observed. However, the strength of the relationships varied with energy intake, with stronger associations at lower energy intakes than higher energy intakes (interaction of sodium and energy intake, P < 0.001). However, the relationship between sodium intake and blood pressure was attenuated on the DASH diet compared with the control diet at all levels of energy intake, suggesting that other DASH diet components also influenced changes in blood pressure. There was no significant interaction between race and energy, or among race, Na intake, and energy for SBP or DBP. For example, on the control diet at 2300 mg sodium, SBP was 3.8 mm Hg higher at 2100 kcal compared with 3200 kcal in blacks, whereas there was no significant difference in white participants. There were smaller differences in blood pressure by energy intake among obese compared with non‐obese participants. For example, on the control diet at 2300 mg sodium intake, SBP was 4.7 mm Hg (95% CI, 1.0, 8.5) higher at 2100 kcal compared with 3200 kcal in the non‐obese, whereas there was no difference in SBP in obese participants. On the DASH diet at 2300 mg sodium intake, there were no significant differences in SBP at 2100 kcal vs 3200 kcal in either group; however, diastolic blood pressure was higher in the non‐obese (2.1 mm Hg; 95% CI, 0.1‐4.4) but not in the non‐obese.
Comment. This secondary analysis of data from the DASH‐sodium trial examined the relationship between sodium intake, energy intake, and blood pressure, and shows that sodium density, not just sodium intake, is an important predictor of blood pressure response for black, white, obese, and non‐obese participants. The DASH‐sodium trial has several strengths: It is a feeding study with tightly controlled energy intakes monitored by food diaries, and levels of sodium intake verified by 24‐hour urine collection. The crossover design at different sodium levels controls for individual factors associated with blood pressure. The study achieved adequate differentiation in sodium intakes, which is relevant to current intakes and those recommended by dietary guidelines. A potential weakness of the current post hoc analysis, as discussed by the authors, is that energy intake is included as an exposure of interest but was not included in the randomized design of the trial, and there is potential for confounding by sex and physical activity. The study was limited to two groups: whites and blacks, with those who identified as Hispanic or “other” race/ethnicity excluded from the analyses. The authors suggest that sodium density may be more important than sodium intake in predicting blood pressure in whites and blacks.
Does using a low sodium salt result in changes in blood pressure in those with isolated systolic and non‐isolated systolic hypertension?
Yang G‐H, Zhou X, et al. Effects of a low salt diet on isolated systolic hypertension: a community‐based population study. Medicine. 2018;97(14).
Design: Single‐blind RCT.
Setting: Ten communities in the rural Hedong District, Tianjin China.
Study duration: Six months, preceded by a 4‐week run‐in period.
Participants: A total of 126 Han Chinese adults (57% female) aged 50‐80 years with mild to moderate hypertension (meeting one of SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, or being treated with antihypertensive drugs).
Intervention: Participants were divided into two groups: isolated systolic hypertensive (ISH; n = 51) and non‐isolated systolic hypertension (NISH; n = 75) based on baseline blood pressure or previous diagnosis. Within each group, participants were randomized to receive either low sodium salt (65% sodium chloride, 30% potassium chloride, 5% calcium salts, and 12 mg/kg folic acid) or normal salt (sodium chloride).
Achieved sodium intake: Urinary sodium excretion was measured at baseline and again by a single 24‐hour urine sample at 6 months. Mean sodium excretion for those in the low sodium salt groups was 4048 mg/d sodium (10 g salt) and 3519 mg/d sodium (8.8 g salt) for ISH and NISH groups, respectively. Mean sodium excretion in the normal salt groups was 5658 mg sodium/d (14.2 g salt) and 5175 mg sodium/d (12.9 g salt) for ISH and NISH groups, respectively.
Outcomes: Clinic blood pressure (primary outcome) was measured every 2 months by two experienced physicians using detection of Korotkoff sounds. Blood pressure was measured three times, and the mean of the second and third measurements was used. A fourth measurement was taken if the difference in the second and third measurements was ≥10 mm Hg. At 6 months, 24‐hour urinary potassium and calcium levels were measured, as well as serum lipids, glucose, insulin, renin activity, angiotensin II, and atrial naturetic peptide.
Risk of bias:
Random sequence generation: High risk.
Allocation concealment: High risk.
Blinding of participants and personnel: Unclear risk
Blinding of outcome assessors: Unclear risk.
Incomplete outcome reporting: High risk.
Selective reporting: Low risk.
Other sources of bias: Low risk.
Source of funding: National Science Foundation of China, Tianjin Municipal Science and Technology Committee, and intramural research program from Logistics University of Chinese People's Armed Police Forces.
Summary of results. Use of a low sodium salt was associated with a statistically significant reduction in urinary sodium excretion at 6 months compared with using normal salt (sodium chloride) in both ISH and NISH participants (P < 0.001). For ISH participants, there was a mean (SD) decrease in sodium excretion of 1150 (1364) mg/d in the low salt group compared with only a slight decrease in sodium excretion 114 (1249) mg/d in the control group. For NISH participants, there was a mean (SD) reduction in sodium excretion of 2109 (1088) mg/d in the low sodium salt group and a mean decrease of 368 (1995) mg/d in the normal salt group. There were also increases in urinary potassium and calcium among users of low sodium salt. Use of a low sodium salt compared with normal salt was associated with a statistically significantly lower SBP at 6 months for the ISH participants. The authors report that compared with the mean of the ISH control group, the low salt ISH group had a mean difference SBP of −10.18 mm Hg (95% CI −17.2, −3.13). There was no difference in SBP at 6 months for NISH participants in the low sodium salt group (mean difference −5.1 mm Hg (95% CI −2.02, 12.2), and there was no difference in diastolic blood pressure at 6 months for either group. For both urinary sodium excretion and blood pressure, the intervention effect (between group difference) was not reported.
Comment. The reporting of this trial of low sodium salt does not conform to the CONSORT statement,48 which limits interpretation. The failure to report intervention effects for either urinary sodium excretion or blood pressure is also a limitation. Other potential limitations include failure to test for completeness of 24‐hour urine collections (not reported) and lack of detail regarding how blood pressure was measured—including whether participants were seated or supine.49 As this was a relatively small trial (n = 51 ISH and n = 75 NISH), low power may have limited the ability to show effects, as demonstrated with relatively wide confidence intervals in blood pressure results. Strengths of the study include the range of biochemical results and apparent high retention of participants. Results suggest that use of low sodium salt can reduce sodium intake and reduce SBP in those with ISH among Chinese adults over a 6‐month period.
5. DISCUSSION
This systematic review identified only three out of nineteen studies that met the pre‐specified criteria for quality in a 6‐month period. Of these, a cohort study identified an association between lower sodium intake (<6.25 g/d) and increased mortality in Italian adults, most of whom consumed well above recommended sodium intakes assessed by single 24‐hour urine collections at baseline.28 Two randomized controlled trials confirmed the previously identified positive association between sodium intake and elevated blood pressure. A post hoc analysis of the DASH sodium controlled feeding trial showed that among a subset of participants of black and white race, sodium density was related to blood pressure, and this association was stronger among those with lower energy intake than higher energy intakes.25 A randomized controlled trial in a community‐dwelling Chinese population showed that use of low sodium salt was associated with lower sodium intakes (mean difference of 1150 and 2093 mg/d in isolated and non‐isolated systolic hypertension participants, respectively, from baseline to follow‐up) over a 6‐month period and was associated with lower SBP in those with isolated systolic hypertension, but not in those with non‐isolated systolic hypertension,26 although the relatively low numbers in the study suggest that this may have been under‐powered to show an effect.
Two important themes are highlighted by this review. Firstly, there remains a relatively small proportion of studies that meet our criteria for high‐quality studies, which was highlighted in our previous review.50 In particular, the use of inadequate sodium intake assessment in observational studies is problematic. Six studies used spot urine collections to estimate usual sodium intake,24, 29, 33, 35, 36, 40 despite the large body of literature showing that spot urine collections lack precision and accuracy for assessment of usual sodium intake in individuals.51, 52 The risk of misclassification is high in these studies as demonstrated by Bland‐Altman analyses consistently showing poor agreement between measures of spot and 24‐hour urinary excretion for individuals.51 Furthermore, at a population level, estimates of sodium intake from spot urine samples are also biased. Of greater concern is the proportional bias that exists whereby the level of over‐ or under‐estimation is proportional to mean sodium excretion. Two cohort studies included in this review used FFQ to assess the association between sodium intake at baseline and incidence of chronic kidney disease among people with normal kidney function in Korea31 and Iran.30 A recent review found that FFQs are not adequate for quantifying sodium intake in individuals.53 Although dietary assessment methods are necessary in people with established CKD (in whom urinary excretion is impaired), both these studies included only people with normal renal function at baseline and so multiple 24‐hour urine collections would have been a more accurate measure of usual intake. A meta‐analysis identified by this review, but not included in the detailed critical appraisal and commentary, included cohort and cross‐sectional studies that examined the association of sodium intake with bone mineral density and risk of osteoporosis.22 It included studies that used a range of methods to assess sodium intake, such as FFQ, dietary recalls, and urinary excretion (including one study which used spot urinary sodium) to assess usual sodium intake. The findings were that there was a significant association between sodium intake assessed by dietary assessment methods and risk of osteoporosis, but not when dietary sodium was assessed by urinary excretion.22 This study highlights the importance of accurate measurement in observational studies.
The second theme highlighted in this review is the apparent discrepancy in relation to sodium intake and health outcomes shown by observational (cohort) studies and randomized trials, the cohort study suggesting evidence of harm at lower intakes (increased mortality) and the RCTs showing evidence of benefit (reduced blood pressure). The apparent discrepancy between findings of observational studies and RCTs is pivotal to much of recent controversy regarding recommendations to reduce population sodium intake.13 Several other cohort studies have reported increased risk of adverse health‐related outcomes with lower levels of sodium intake,29 mostly in studies that use poor methodology to quantify sodium intake (spot urine) at baseline10, 11, 29 or in diseased cohorts rather than general healthy population groups.54 Use of spot urine collections to estimate intake at baseline is particularly problematic due to its large variability and systemic bias when compared with 24‐hour excretion.51, 55 Validation studies that use Bland‐Altman plots56 to examine agreement between estimates based on spot urine and 24‐hour measurements show large mean differences and wide limits of agreement. For example, a validation in a multi‐ethnic sample in South Africa compared three formulae for converting spot urine to 24‐hour urinary excretion estimates and demonstrated large differences for the Kawasaki and Tanaka formulae (−2242 mg and −837 mg, respectively). Estimates based on the INTERSALT formula over‐estimated sodium excretion below 5000 mg/d and underestimated excretion above 5000 mg/d. Estimates using all three formulae showed wide limits of agreement, indicating very poor ability to predict 24‐hour excretion on an individual basis.52
A recent examination of the difference between estimates from spot urine and 24‐hour urinary assessment in relation to mortality in a cohort of patients who participated in the Trials of Hypertension Prevention (TOHP) trial demonstrates the errors inherent in the use of spot urine in cohort studies.55 This reanalysis of data including a median follow‐up period of 24 years showed that when baseline sodium intake was measured using 24‐hour urine collections a linear positive association with mortality was demonstrated, whereas when Kawasaki formula estimates were used the association changed and there was the suggestion of a J‐shaped curve. This analysis suggests that the apparent J‐shaped relationship between sodium intake estimates from spot urine and clinical outcomes in several published studies10, 11, 29 is likely due to measurement error in sodium intake rather than a true association. In other cohort studies with 24‐hour urinary assessment at baseline, confounding and reverse causality must also be considered. Participants with low sodium intake are more likely to have other adverse clinical risk factors compared to those with higher sodium intake as demonstrated by several studies.28, 54 While there are few randomized controlled trials of dietary sodium interventions and clinical outcomes, a meta‐analysis of trials in 2011 found that sodium reduction was associated with a 20% reduction in cardiovascular events compared with the respective control condition (RR 0.8, 95% CI 0.64, 0.99).7.
Adequately powered clinical trials of nutritional interventions with hard clinical outcomes rather than surrogate endpoints and sufficient follow‐up time lack feasibility. This is due to the difficulty in randomizing free‐living individuals to a dietary intervention and long‐term maintenance of dietary changes. Randomized controlled trials are unlikely, therefore, to replace cohort studies completely in this context. However, observational studies must be undertaken with rigorous methodology, including multiple complete 24‐hour urine assessments at baseline and follow‐up, subject inclusion and exclusion criteria that minimize the likelihood of reverse causality, measurement of potential confounders and appropriate statistical methods to reduce the likelihood of confounding, and adequate follow‐up periods. Then, examination of the totality of the evidence including both surrogate and clinical outcomes, accounting for strengths and weaknesses of different study designs, should be used to guide policy.
6. CONCLUSIONS
The Science of Salt reviews aim to inform scientists, clinicians, and policy makers about recent evidence on dietary salt (sodium) and disease in humans. This seventh Science of Salt review provides further evidence of the positive association between sodium intake and blood pressure, and highlights the association between sodium density (sodium by energy intake) and blood pressure, particularly in some population groups. A cohort study demonstrated increased mortality at lower sodium intakes in Italian adults with high sodium intakes. Only three of 19 studies met the criteria for detailed critical appraisal due to methodological aspects and outcomes assessed. The number of studies that used spot urine to estimate usual sodium intake in individuals is concerning, especially given the growing body of evidence of the inaccuracy of this method. It is vital that public health policymakers use only rigorous studies to inform practice in this area.
DISCLOSURES
Norm Campbell was a paid consultant to the Novartis Foundation (2016‐2017) to support their program to improve hypertension control in low to middle income countries which includes travel support for site visits and a contract to develop a survey.
Supporting information
McLean RM, Petersen KS, Arcand J, et al. Science of Salt: A regularly updated systematic review of salt and health outcomes studies (April to October 2018). J Clin Hypertens. 2019;21:1030–1042. 10.1111/jch.13611
REFERENCES
- 1. World Health Organization . Ten threats to global health in 2019. Geneva: World Health Organization; 2019. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Accessed March 10, 2019.
- 2. World Health Organization . Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 3. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38(3):791‐813. [DOI] [PubMed] [Google Scholar]
- 4. Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3(12):e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Guideline: Sodium Intake for Adults and Children. Geneva, Switzerland: World Health Organization (WHO); 2012. [PubMed] [Google Scholar]
- 6. He FJ, Li J, MacGregor GA. Effect of longer‐term modest salt reduction on blood pressure. Cochrane Database of Syst Rev. 2013;(4):CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta‐analysis of outcome trials. Lancet. 2011;378(9789):380. [DOI] [PubMed] [Google Scholar]
- 8. Strazzullo P, D'Elia L, Kandala N, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. J Am Med Assoc. 2011;305(17):1777‐1785. [DOI] [PubMed] [Google Scholar]
- 11. Mente A, O'Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388(10043):465‐475. [DOI] [PubMed] [Google Scholar]
- 12. Mahtani KR, Heneghan C, Onakpoya I, et al. Reduced salt intake for heart failure: a systematic review. JAMA Intern Med. 2018;178(12):1693‐1700. [DOI] [PubMed] [Google Scholar]
- 13. Webster J, Waqanivalu T, Arcand J, et al. Understanding the science that supports population‐wide salt reduction programs. J Clin Hypertens. 2017;19(6):569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Academies of Sciences Engineering and Medicine . Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 15. Expert Working Group for Sodium . Australian and New Zealand nutrient reference values for sodium. Australian Government Department of Health and the New Zealand Ministry of Health: Australian Government Department of Health and the New Zealand Ministry of Health; 2017.
- 16. Arcand JoAnne, Webster J, Johnson C, et al. Announcing "up to date in the science of sodium". J Clin Hypertens (Greenwich). 2016;18(2):85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. IOM (Institute of Medicine) . DRI, Dietary Refeence Intakes for Water, Potassium, Sodium Chloride and Sulfate. Washington, DC: National Academy Press. 2005. [Google Scholar]
- 18. Arcand J, Wong M, Santos JA, et al. More evidence that salt increases blood pressure and risk of kidney disease from the Science of Salt: a regularly updated systematic review of salt and health outcomes. J Clin Hypertens. 2017;19:813‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins J, Deeks JJ, Altman DG. (Eds). Chapter 16: Special topics in statistics. In: Higgins J, Green S (Eds.), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). London, UK: The Cochrane Collaboration, 2011. www.cochrane-handbook.org. [Google Scholar]
- 20. McLaren L, Sumar N, Barberio AM, et al. Population‐level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst Rev. 2016;9:CD010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fatahi S, Namazi N, Larijani B, Azadbakht L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta‐analysis. J Am Coll Nutr. 2018;37(6):522‐532. [DOI] [PubMed] [Google Scholar]
- 23. Garofalo C, Borrelli S, Provenzano M, et al. Dietary salt restriction in chronic kidney disease: a meta‐analysis of randomized clinical trials. Nutrients. 2018;10(6):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janda J, Veleminsky M, Sulakova T, et al. Effect of the DASH‐diet and salt Kardisal® on blood pressure in adolescents with prehypertension. Neuro Endocrinol Lett. 2018;38(8):544‐548. [PubMed] [Google Scholar]
- 25. Murtaugh MA, Beasley JM, Appel LJ, et al. Relationship of sodium intake and blood pressure varies with energy intake: secondary analysis of the DASH (Dietary approaches to stop hypertension)‐sodium trial. Hypertension. 2018;71(5):858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang G‐H, Zhou X, Ji W‐J, et al. Effects of a low salt diet on isolated systolic hypertension: a community‐based population study. Medicine. 2018;97(14):e0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musso N, Carloni B, Chiusano MC, Giusti M. Simple dietary advice reduces 24‐hour urinary sodium excretion, blood pressure, and drug consumption in hypertensive patients. J Am Soc Hypertens. 2018;12(9):652‐659. [DOI] [PubMed] [Google Scholar]
- 28. Lelli D, Antonelli‐Incalzi R, Bandinelli S, Ferrucci L, Pedone C. Association between sodium excretion and cardiovascular disease and mortality in the elderly: a cohort study. J Am Med Dir Assoc. 2018;19(3):229‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mente A, O'Donnell M, Rangarajan S, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community‐level prospective epidemiological cohort study. Lancet. 2018;392(10146):496‐506. [DOI] [PubMed] [Google Scholar]
- 30. Mirmiran P, Nazeri P, Bahadoran Z, Khalili‐Moghadam S, Azizi F. Dietary sodium to potassium ratio and the incidence of chronic kidney disease in adults: a longitudinal follow‐up study. Prevent Nutr Food Sci. 2018;23(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoon C‐Y, Noh J, Lee J, et al. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int. 2018;93(4):921‐931. [DOI] [PubMed] [Google Scholar]
- 32. Abreu D, Sousa P, Matias‐Dias C, Pinto FJ. Cardiovascular disease and high blood pressure trend analyses from 2002 to 2016: after the implementation of a salt reduction strategy. BMC Public Health. 2018;18(1):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C‐L, Wang H‐J, Si Q‐J, Zhou J, Li K‐L, Ding YU. Association between urinary sodium excretion and coronary heart disease in hospitalized elderly patients in China. J Int Med Res. 2018;46(8):3078‐3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Awadalla H, Elmak NE, El‐Sayed EF, et al. Hypertension in Sudanese individuals and associated risk factors: the critical intersection between salt and sugar intake. Cardiovas Diagn Ther. 2018;8(4):432‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carranza‐Leon D, Octaria R, Ormseth MJ, et al. Association between urinary sodium and potassium excretion and blood pressure and inflammation in patients with rheumatoid arthritis. Clin Rheumatol. 2018;37(4):895‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murao S, Takata Y, Yasuda M, Osawa H, Kohi F. The Influence of sodium and potassium intake and insulin resistance on blood pressure in normotensive individuals is more evident in women. Am J Hypertens. 2018;31(8):876‐885. [DOI] [PubMed] [Google Scholar]
- 37. Padilha BM, Ferreira RC, Bueno NB, et al. Association between blood cholesterol and sodium intake in hypertensive women with excess weight. Medicine. 2018;97(15):e0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolin LP, Horne CE, Crane PB, Powell JR. Low‐salt diet adherence in African Americans with hypertension. J Clin Nursing. 2018;27(19‐20):3750‐3757. [DOI] [PubMed] [Google Scholar]
- 39. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24‐hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. dos Santos EM, Brito DJdA, Calado IL, et al. Sodium excretion and associated factors in urine samples of African descendants in Alcântara, Brazil: a population based study. Ren Fail. 2018;40(1):22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Avins AL, Pressman A, Ackerson L, Rudd P, Neuhaus J, Vittinghoff E. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. J Gen Intern Med. 2010;25(12):1275‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Curtis J, Larson JC, Delzell E, et al. Placebo adherence, clinical outcomes and mortality in the Women’s Health Initiative randomized hormone therapy trials. Med Care. 2011;49(5):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Birukov A, Rakova N, Lerchl K, et al. Ultra‐long–term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am J Clin Nutr. 2016;104(1):49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olde Engberink R, van den Hoek TC, van Noordenne ND, van den Born B‐J, Peters‐Sengers H, Vogt L. Use of a single baseline versus multiyear 24‐hour urine collection for estimation of long‐term sodium intake and associated cardiovascular and renal risk. Circulation. 2017;136(10):917‐926. [DOI] [PubMed] [Google Scholar]
- 45. Sakaki M, Tsuchihashi T, Arakawa K, Fukui H, Kameda W, Tominaga M. Long‐term variability of urinary salt excretion and blood pressure in hypertensive patients. Hypertens Res. 2014;37(10):939. [DOI] [PubMed] [Google Scholar]
- 46. Cappuccio FP, Ji C, Donfrancesco C, et al. Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL‐GIRCSI programme. BMJ Open. 2015;5(9):e007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 48. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;1(2):100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. TRUE Consortium . Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. J Hypertens Caridiol. 2016;2(2):39‐49. [DOI] [PubMed] [Google Scholar]
- 50. Petersen KS, Rae S, Venos E, et al. Paucity of high‐quality studies reporting on salt and health outcomes from the science of salt: a regularly updated systematic review of salt and health outcomes (April 2017 to March 2018). J Clin Hypertens. 2019;21(2):307‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang L, Crino M, Wu J, et al. Mean population salt intake estimated from 24‐h urine samples and spot urine samples: a systematic review and meta‐analysis. Int J Epidemiol. 2016;45(1):239‐250. [DOI] [PubMed] [Google Scholar]
- 52. Swanepoel B, Schutte AE, Cockeran M, Steyn K, Wentzel‐Viljoen E. Monitoring the South African population’s salt intake: spot urine v. 24 h urine. Public Health Nutr. 2018;21(3):480‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR, Campbell NR. Assessment of dietary sodium intake using a food frequency questionnaire and 24‐hour urinary sodium excretion: a systematic literature review. J Clin Hypertens. 2017;19(12):1214‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. He FJ, Campbell N, Ma Y, MacGregor GA, Cogswell ME, Cook NR. Errors in estimating usual sodium intake by the Kawasaki formula alter its relationship with mortality: implications for public health. Int J Epidemiol. 2018;47(6):1784‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307‐310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


