Abstract
Hypertension‐mediated organ damage (HMOD) is frequently observed in hypertensive patients at different cardiovascular (CV) risk profile. This may have both diagnostic and therapeutic implications for the choice of the most appropriate therapies. Among different markers of HMOD, the most frequent functional and structural adaptations can be observed at cardiac level, including left ventricular hypertrophy (LVH), diastolic dysfunction, aortic root dilatation, and left atrial enlargement. In particular, LVH was shown to be a strong and independent risk factor for major CV events, namely myocardial infarction, stroke, congestive heart failure, CV death. Thus, early identification of LVH is a key element for preventing CV events in hypertension. Although echocardiographic assessment of LVH represents the gold standard technique, this is not cost‐effective and cannot be adopted in routine clinical practice of hypertension. On the other hand, electrocardiographic (ECG) assessment of HMOD relative to the heart is a simple, reproducible, widely available and cost‐effective method to assess the presence of LVH, and could be preferred in large scale screening tests. Several new indicators have been proposed and tested in observational studies and clinical trials of hypertension, in order to improve the relatively low sensitivity of the conventional ECG criteria for LVH, despite high specificity. This article reviews the differences in the use of the main conventional and the new 12 lead ECG criteria of LVH for early assessment of asymptomatic, subclinical cardiac HMOD in a setting of clinical practice of hypertension.
Keywords: cardiac organ damage, electrocardiogram, hypertension, hypertension‐mediated organ damage, left ventricular hypertrophy
1. INTRODUCTION
Essential hypertension (HTN) is one of the most common modifiable risk factors in the general population, being strongly and independently related to an increased risk of cardiovascular (CV) morbidity and mortality, independently by age and gender.1 Indeed, high blood pressure (BP) levels are associated with increased risk of major CV outcomes, including myocardial infarction, stroke, congestive heart failure (CHF), and CV death.1 In view of the progressive aging of the population, as well as of the increasing prevalence of CV and metabolic risk factors and comorbidities, early detection and prompt control of HTN represent key elements for reducing CV morbidity and mortality in both high‐ and low‐income countries. Recent observations, however, seem to suggest that lowering BP levels to targets may not be sufficient to reduce CV risk related to HTN.2
Clinical studies have consistently reported a high prevalence of markers of HTN‐mediated organ damage (HMOD) among hypertensive patients at different CV risk profile.3, 4, 5 These markers include structural and functional changes, mainly involving kidneys, arteries, brain, and heart.6, 7 At cardiac level, left ventricular hypertrophy (LVH) represents the main factor associated with a worse CV prognosis.8
From a pathophysiological point of view, persistently elevated BP levels are recognized to produce a hemodynamic overload, leading to functional adaptations and structural changes of LV geometry, which may, in turn, result into an increased LV mass and remodeling.9, 10 These adaptations, as well as the interactions with genetic, biochemical, neuro‐hormonal, and metabolic factors, are responsible for the development of LVH.11, 12, 13 Once established, LVH tends to promote the occurrence of unfavorable cardiac effects, such as atrial and ventricular arrhythmias, myocardial stiffness, diastolic dysfunction, reduced coronary blood flow, coronary artery disease, and congestive heart failure.14 Therefore, given these clinical consequences, current HTN guidelines recommend to perform systematic global CV risk assessment in each individual patient with high BP, including the detection of LVH.15
Worldwide, the most common first‐line method to evaluate LVH is the 12‐lead conventional electrocardiography (ECG), due to its widespread availability, favorable cost‐effective ratio and its ease of performance.16 One of the major limitations of ECG screening, however, is the well‐known relatively low sensitivity, despite a high specificity, with regard to the assessment of LVH,17 mostly in special populations, such as obesity group.18 In addition, the lack of concordance among the currently available ECG criteria for LVH, mostly related to the different thresholds and leads proposed by different criteria, may induce contrasting data on its prevalence and clinical implications.
Such improper diagnostic and therapeutic approach in daily clinical management of hypertensive outpatients.19, 20 On the basis of these considerations, the aim of this narrative review is to discuss the main conventional and novel 12‐lead ECG criteria for the assessment of LVH, in order to improve the diagnostic work‐up in hypertension.
1.1. Pathophysiology of left ventricular hypertrophy in essential hypertension
1.1.1. Early stages of left ventricular hypertrophy
Left ventricular hypertrophy is a maladaptive response to hemodynamic overload and neuro‐hormonal imbalance that can be observed at cardiac level in hypertensive patients.21, 22, 23 In the early stages of HTN, LVH counterbalances the abnormal cardiac wall stress, whereas in the subsequent stages of the disease, long‐standing and elevated BP may lead to an increase of LV wall thickness without altering LV mass.21, 22, 23 This condition is referred as concentric LV remodeling, and may lead toward the development of subsequent stages of LVH, which have been related to markedly higher risk of major CV complications compared to LV remodeling or normal LV geometry.24, 25, 26
Although the mechanisms underlining this process have not been fully elucidated, several studies demonstrated that it is mainly characterized by both in parallel growth of new sarcomeres along the longitudinal axes, thus expanding the cross‐sectional area of myocytes, and by the deposition of new fibrous tissue in the interstitial compartment.24, 25, 26 Diwan et al (2007) showed that the workload of this process requires an elevated oxygen consumption; therefore, LVH is eventually vulnerable to decompensation.27
1.1.2. Non‐hemodynamic factors for left ventricular hypertrophy
It should be also noted, however, that other non‐hemodynamic factors may substantially contribute to modulating the hypertrophic response of the LV.28 Among these factors, abnormal neuroendocrine stimulation plays a major role in the development and progression from normal LV geometry toward LVH.29 Grassi G et al (2006) demonstrated that the sympathetic nervous system, activated by mineralocorticoids, leads to baroreceptor dysfunction, impaired arterial compliance, abnormal myocardial, vascular fibrosis, metabolic effects (eg, insulin resistance).30 All these factors may promote development and progression of LVH.30 On the other side, the activation of the renin‐angiotensin‐aldosterone system (RAAS)31, 32 and the imbalance of endothelin‐133, 34 and natriuretic peptides35 network is also responsible for the generation of reactive oxygen species, vascular inflammation, and cardiac remodeling, which further promote this abnormal response to increased BP load. In particular, current evidence suggests that the RAAS significantly contributes to the development of diastolic dysfunction in HTN and plays an important role in its progression toward CHF by promoting an increase in collagen production with a subsequent enhancement of myocardial fibrosis and stiffness.36
1.1.3. Subsequent stages of left ventricular hypertrophy
Although LVH is initially an adaptive process to the increased pressure overload, the presence of LVH is also the primary element responsible for the progression from HTN to hypertensive heart disease (HHD).37 Indeed, the prototypal outcome of HHD progression is the well‐known “burned‐out” effect of the LV.14, 38 This process is characterized by an evolution from LVH associated with diastolic dysfunction, abnormal LV relaxation, and impaired filling properties, toward LV enlargement and systolic dysfunction.36, 39 Thus, HHD includes a wide range of clinical manifestations from asymptomatic LVH to symptomatic CHF, which, in turn, may occur as preserved ejection fraction (HFpEF > 50%), mid‐range ejection fraction (HFmEF 40%‐50%), and reduced ejection fraction (HFrEF < 40%).40 It should be also noted, however, that echocardiographic assessment of LVH has distinct prognostic value with respect to that obtained by ECG‐detected LVH.41, 42
1.2. Hypertension‐induced LVH and related cardiovascular complications
Left ventricular hypertrophy is an independent risk factor for CV morbidity and mortality.43, 44, 45 It has been also related to a significantly higher and independent risk of non‐fatal CV events, including arrhythmias, coronary artery disease, myocardial infarction, peripheral atherosclerotic disease, and CHF.46
One of the most frequent HTN‐induced LVH complications is represented by the development of cardiac arrhythmias, which may be related to intrinsic modifications of the cardiac vasculature and structure, thus leading to an increased risk of sudden cardiac death and CHF.47, 48 The increased amount/accumulation of fibrous tissue represents the main cause of the genesis of arrhythmias.48 The large amount of extracellular collagen deposition can lead to side‐to‐side electric coupling alterations between myocardial fibers, thus resulting in irregular cardiac contraction, increased dispersion of repolarization, and a vast array of intraventricular electrical pathways. Each one facilitates micro‐re‐entry and promotes arrhythmogenesis.49
Among supraventricular arrhythmias, atrial fibrillation is the most common electrical alteration in LVH patients. Instead, among ventricular arrhythmias, sustained ventricular tachyarrhythmia is considered the major cause of death in patients with LVH.50 Once sustained and malignant ventricular arrhythmias have established, the risk of sudden cardiac death increases. In a recent meta‐analysis, the incidence of ventricular arrhythmias was 5.5% in patient with LVH compared to 1.2% in patients without LVH. In addition, a recent study reported LVH as a potential risk stratification factor due to its association with sudden cardiac death.51
In the setting of HTN, proliferation and hypertrophy of the vascular smooth muscle cells can be observed, thus resulting in vascular wall thickening and consequently leading to a reduced coronary flow reserve.52 These structural (vascular) changes along with low coronary blood flow can increase the risk of acute coronary syndrome and myocardial infarction.53, 54, 55 Changes of extracellular matrix which promoted vascular dysfunction, as well as impaired mechano‐elastic properties of cardiac myocytes, may culminate in the development of diastolic dysfunction.56
Furthermore, several studies underlined the importance to consider ECG‐related LVH criteria as markers of electrical anomalies, because their presence is associated with a marked increase in severity of the disease and its complications, whereas the reduction of the ECG‐detected LVH bears a more favorable prognosis.57
1.3. Conventional electrocardiographic criteria for left ventricular hypertrophy
Several ECG criteria have been proposed for the diagnosis of LVH in a setting of clinical practice, mostly based on QRS voltage criteria (Table 1).17 Sensitivity and specificity of different criteria have been discussed elsewhere.58 Schematic representation of main conventional ECG criteria used so far has been reported on Figure 1 (panel A). Although negativity of the ECG criteria does not mean that anatomic LVH is ruled out, due to the low sensitivity of this method, they are considered a valid and independent prognostic marker of cardiac organ damage and increased CV risk.15
Table 1.
Main electrocardiographic criteria for detection of left ventricular hypertrophy
| Criterion | Definition | Cutoff values |
|---|---|---|
| Lewis voltage | R in I + S in III – R in III – S in I | ≥17 mm |
| Gubner‐Underleider voltage | R in I + S in III | ≥25 mm |
| Sokolow‐Lyon voltage | S in V1 + R in V5/V6 | ≥35 mm |
| R in aVL voltage | R in aVL | >11 mm |
| Cornell voltage | S in V3 + R in aVL | >20 mm (men); 28 mm (women) |
| Cornell product | (S in V3 + R in aVL) x QRS duration (msec) | ≥2440 mm*msec |
| Romhilt‐Estes score | 0‐7 items, including: (1) R or S wave in the limb leads ≥20 mm; or S wave in V1 or V2 ≥ 30 mm; (2) R wave in V5 or V6 ≥ 30 mm (3 points); (3) left atrial involvement—terminal deflection of P wave in V1 is 1 box wide, and 1 box deep or more (3 points); (4) left axis deviation; (5) QRS duration ≥ 0.09 second (1 point); (6) Intrinsicoid deflection in V5 and V6 ≥ 0.05 second (1 point); (7) ST‐T segment changes (LV strain) | ≥5 |
| Left ventricular strain | ST segment depression and T wave inversion | |
| Framingham criterion | Left ventricular strain + at least one voltage criterion (R in aVL or Gubner‐Underleider or Sokolow‐Lyon or S in V1/V2 ≥ 25 mm or R in V5/V6 ≥ 25 mm) | |
| Perugia criterion | Left ventricular strain and/or Cornell voltage and/or Romhilt‐Estes score ≥5 | |
| VAT | Time interval between the beginning of the QRS complex to the peak of the R wave | >0.05 |
| Tp‐Te Interval | Time interval between the peak and the end of the T wave in one precordial lead (mostly V5) | Not available |
Figure 1.
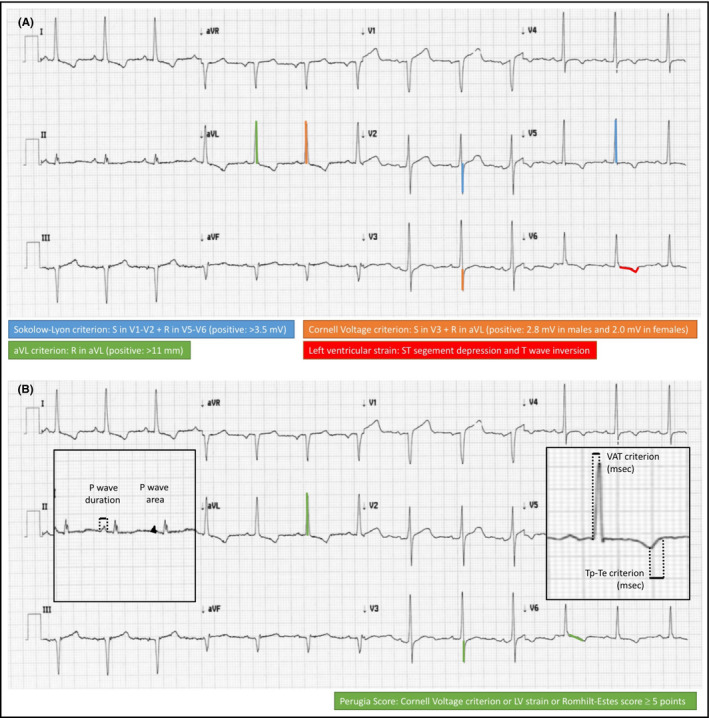
Illustrative representation of conventional (panel A) and novel (panel B) criteria for electrocardiographic detection of left ventricular hypertrophy
One of the most historically reliable ECG criterion is the Sokolow‐Lyon voltage, which can be obtained by the sum of S wave detected in V1 lead and R wave measured in V5/6 leads (positive: ≥35 mm).17 This is characterized by relatively low sensitivity and high specificity. Among other criteria, Gubner‐Ungerleider and Framingham59 criteria have a very low sensitivity, although high specificity.58 Similarly, Lewis voltage and the R wave in V6 lead/R wave in V5 lead ratio have high specificity and low sensitivity.60
The criterion with the highest specificity (98% in men and 95% in woman) is the Cornell Voltage, which can be derived by the sum of S wave in V3 and of R wave in aVL (positive: >28 mm in men, >20 mm in woman).61 Analysis of the Framingham cohort reported a relatively low sensitivity of this criterion in this population (about 10% in men and 22% in woman).24 Later, the Cornell product criterion (which is the Cornell Voltage criterion multiplied for the QRS duration; positive: >2.4 mV) has demonstrated to provide better sensitivity and specificity than those provided by Cornell Voltage in a large cohort of hypertensive patients with ECG evidence of LVH.62
Lately, the Romhilt‐Estes score includes the following points63: (1) the R or S wave in the limb leads greater than or equal to 20 mm; or S wave in V1 or V2 greater than or equal to 30 mm; (2) R wave in V5 or V6 greater than or equal to 30 mm (3 points); (3) left atrial involvement—terminal deflection of P wave in V1 is 1 box wide, and 1 box deep or more (3 points); (4) left axis deviation—QRS axis is −30 degrees or more negative (2 points); (5) QRS duration greater than or equal to 0.09 second (1 point); (6) Intrinsicoid deflection in V5 and V6 greater than or equal to 0.05 second (1 point); (7) ST‐T segment changes ("LV strain" = ST‐T vector shifted opposite to QRS vector): without digitalis (3 points) or with digitalis (1 point). Indeed, it incorporates abnormalities in QRS axis, duration, amplitude, QRS onset‐to‐peak time, P wave, ST‐T morphology.
However, a recent systematic review reports that conventional ECG criteria should not be used to rule out the presence of LVH in patients with HTN, because of their low sensitivity.64
1.4. New electrocardiographic criteria for left ventricular hypertrophy
Over the last few years, several new ECG criteria have been proposed and tested in relatively small studies including both normotensive individuals and hypertensive outpatients, in order to overcome the intrinsic limitations of the above mentioned conventional ECG criteria and improve diagnostic accuracy of ECG detection of LVH.65, 66 Illustrative representation of novel criteria for ECG detection of LVH are shown in Figure 1 (panel B).
Among these new criteria, the Perugia criterion has firstly been introduced to ameliorate the sensitivity of ECG findings for LVH diagnosis.66 It includes at least one of the following: S wave in V3 lead + R wave in aVL lead >24 mm (men) and >20 mm (women) or LV strain or Romhilt‐Estes score ≥5 points, yielded values of sensitivity, specificity, and accuracy of 34%, 93%, and 73%, respectively, in subjects with uncomplicated essential hypertension.60 This criterion was firstly tested in a clinical study aimed at comparing the accuracy and prognostic value of different ECG criteria by including 1717 Caucasian adult hypertensive patients, who were prospectively followed up for up to 10 years (mean 3.3) in Italy.67 At entry, the prevalence of LVH was 17.8% (Perugia score), 9.1% (Cornell), 3.9% (Framingham), 5.2% (Romhilt‐Estes), 6.4% (strain), and 13.1% (Sokolow‐Lyon).67 During follow‐up event rate of major CV events was higher in the subjects with than in those without LVH (all P < .001) according to any criteria, with the only exception of the Sokolow‐Lyon index.67 At multivariate analysis, an independent association between LVH and CV risk was observed for both the Perugia score (hazard ratio [HR] 2.04, 95% confidence interval [CI] 1.5‐2.8) and the Framingham (HR 1.91, 95% CI 1.1‐3.2), Romhilt‐Estes (HR 2.63, 95% CI 1.7‐4.1) and strain methods (HR 2.11, 95% CI 1.4‐3.2).67 However, Perugia score showed the highest population‐attributable risk for CV events, accounting for 15.6% of all cases, whereas the Framingham, Romhilt‐Estes and strain methods accounted for 3.0%, 7.4%, and 6.8% of all events, respectively. In particular, LVH diagnosed by the Perugia score was associated with an increased risk of CV mortality (HR 4.21, 95% CI 2.1‐8.7).67
Another important prognostic index is the value of R wave in aVL lead (positive: ≥11 mm),68 which has been also recommended by the latest set of European guidelines on HTN.15 Indeed, recent studies have demonstrated the importance of R wave in aVL due to its significant correlation with the LV mass.61, 68 Moreover, significant correlation between QT interval and LVH has been observed in several studies. In the CARdiovascular Living and Ageing in Halle (CARLA) study, prolonged QT interval adjusted for heart rate (QT corrected, QTc) >500 ms was associated with increasing LV mass.69
In the few last years, new parameters, closely related to an increased LV mass, LV diastolic dysfunction and risk of cardiac arrhythmias, have been proposed. These parameters include the time interval between the peak and the end of the T wave (Tp‐Te) 70 and ventricular activation time (VAT).71
In the Tp‐Te interval, the peak of the T wave represents the end of the epicardial action potential, whereas the end of the T wave represents the end of the mid‐myocardial action potential, thus reflecting the transmural dispersion of repolarization.70 Despite the fact that a clear cutoff value has not been established yet, longer Tp‐Te interval has been observed in untreated hypertensive outpatients than in normotensive individuals, being significantly related to increased LV mass and high 24‐hour ambulatory BP levels.72 Moreover, prolonged Tp‐Te (91 ± 12.24 vs 74 ± 9.96; P < .001), Tp‐Te/QT (0.24 ± 0.027 vs 0.20 ± 0.025; P < .001) and Tp‐Te/QTc (0.22 ± 0.023 vs 0.18 ± 0.023; P < .001) were significantly increased in non‐dipper hypertensive patients than dippers with metabolic syndrome.73 In addition, abnormal Tp‐Te interval has been reported in patients with coronary artery disease.74, 75, 76 Indeed, a more recent analysis from the PAMELA study has shown that Tp‐Te interval is independently correlated with an increased risk of CV events.77 It should be noted, however, that in this latter study, Tp–Te measurements were adjusted for heart rate using the modified Bazett's formula, that is interval.
Available evidence has also demonstrated that Tp‐Te interval may be considered a reliable measure of transmural dispersion of the LV repolarization, which is increased in LVH and responsible for the increased risk of arrhythmogenesis.78 In recent clinical studies, the Tp‐Te interval has been analyzed in the precordial leads, particularly V5,72 and this might be responsible for the higher able to detect the diffusion of the electrical field through the ventricle walls. The choice to use the precordial leads as the main referral gives a high sensitivity to the Tp‐Te interval with respect to the peripheral leads, where this interval may represent a dispersion index of the global repolarization, by including the apical‐basal and the interventricular dispersion.79 An increased LV transmural dispersion is linked to a high probability of developing cardiac arrhythmias, because the repolarization dispersion and the refractory time may be at a short distance to each other, thus generating a very rapid repolarization gradient.80, 81 Thus, it is the rapidity of the repolarization gradient that determines the arrhythmogenesis potential rather than the total width of the dispersion. The apical‐basal or the interventricular dispersion, in this context, may be less indicative, since it may be or not be associated to a rapid repolarization gradient with or without the associated risk of arrhythmogenesis. Further, the Tp‐Te interval is considered as a predictive index of ventricular tachyarrhytmias 82 and of an high risk of mortality in patients with LQTS, Brugada syndrome 83 and in patients treated with primary coronary intervention after 1 year from the ischemic event.84
Other finding of a recent study showed that smoking is associated with hyperactivity of the sympathetic system and LV repolarization abnormalities, including abnormal Tp‐Te interval, thus contributing to the increasing prevalence of ventricular arrhythmias among smokers.85 The clinical significance of Tp‐Te interval justifies the need of additional studies to better clarify its prognostic value in terms of CV risk.
Another not conventional parameter recently found is the intrinsicoid deflection or the ventricular activation time (VAT).71 It is the time required by the ventricle to depolarize and it can be estimated by measuring the interval from the beginning of the QRS complex to the peak of the R wave. In a recent study, VAT was considered as a potential marker for diastolic dysfunction, one of the major cardiac functional alteration in the course of hypertension.71 A value of VAT in V5 and V6 >0.05 seconds is a criterion used in the Romhilt‐Estes score regarding the LVH. It has been shown that an increase of VAT is associated with an increase in atrial and septum diameters, an increase in left ventricle mass index and a low e’ velocity at TDI, besides a minor e’/a’ ratio at the echocardiography.71
Finally, it has been evaluated also the P wave, since in literature it has been correlated at the echocardiographic level to left atrial alteration, which is associated with the presence of left ventricular diastolic dysfunction. In particular, four criteria (average duration, maximum duration, dispersion, and area of the P wave) are increased in patients with diastolic dysfunction and in patients with left atrial diameter >40 mL/m2 respect to those without diastolic dysfunction and a left atrial volume <40 mL/m2, by attributing to P wave parameters a predictive role of increased risk of left atrial enlargement, LV diastolic dysfunction and atrial arrhythmias.86
2. PERSPECTIVES AND CONCLUSIONS
Electrocardiographic assessment of LVH represents an easy to perform, widely available, repeatable, and cost‐effective method to assess the presence of LVH in the setting of clinical practice of HTN. In view of the mounting prevalence of HTN at global level, we suggest that this method is used in large screening evaluations of hypertensive patients, as also recommended by current guidelines. Up to date, several studies have been performed to assess the most reliable ECG criteria to diagnose LVH and to prevent its related complications. On the basis of the currently available evidence, several new ECG criteria can be proposed for being applied in routine practice, among which the assessment of Tp‐Te interval can be considered one of the most promising tool for early identification of electrophysiology risk in hypertensive patients with LVH. However, further studies are needed to improve and ameliorate the prognostic relevance of these new ECG criteria, as well as to test the potential effects of different antihypertensive therapies on ECG‐detected LVH regression and CV prognosis.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Francesca Miceli: involved in conception and design, drafting, and reviewing the manuscript. Vivianne Presta: involved in data searching and reviewing the manuscript. Barbara Citoni: involved in data searching and reviewing the manuscript. Flaminia Canichella: involved in data searching and reviewing the manuscript. Ilaria Figliuzzi: involved in data searching and reviewing the manuscript. Andrea Ferrucci: reviewed the manuscript. Massimo Volpe: reviewed the manuscript. Giuliano Tocci: involved in conception and design, drafting, reviewing and approving the final proofs.
Miceli F, Presta V, Citoni B, et al. Conventional and new electrocardiographic criteria for hypertension‐mediated cardiac organ damage: A narrative review. J Clin Hypertens. 2019;21:1863–1871. 10.1111/jch.13726
REFERENCES
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 2. Katholi RE, Couri DM. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int J Hypertens. 2011;2011:495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devereux RB, Bella J, Boman K, et al. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: the life study. Blood Press. 2001;10:74‐82. [DOI] [PubMed] [Google Scholar]
- 4. Wachtell K, Olsen MH, Dahlof B, et al. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: the life study. J Hypertens. 2002;20:405‐412. [DOI] [PubMed] [Google Scholar]
- 5. Hawkins NM, Wang D, McMurray JJ, et al. Investigators C, Committees. Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the charm programme. Heart. 2007;93:59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volpe M, Battistoni A, Tocci G, et al. Cardiovascular risk assessment beyond systemic coronary risk estimation: a role for organ damage markers. J Hypertens. 2012;30:1056‐1064. [DOI] [PubMed] [Google Scholar]
- 7. Tocci G, Figliuzzi I, Presta V, et al. Adding markers of organ damage to risk score models improves cardiovascular risk assessment: Prospective analysis of a large cohort of adult outpatients. Int J Cardiol. 2017;248:342‐348. [DOI] [PubMed] [Google Scholar]
- 8. Gosse P, Cremer A, Vircoulon M, et al. Prognostic value of the extent of left ventricular hypertrophy and its evolution in the hypertensive patient. J Hypertens. 2012;30:2403‐2409. [DOI] [PubMed] [Google Scholar]
- 9. Sciarretta S, Sadoshima J. New insights into the molecular phenotype of eccentric hypertrophy. J Mol Cell Cardiol. 2010;49:153‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corea L, Bentivoglio M, Verdecchia P, Motolese M. Factors influencing left ventricular hypertrophy in systemic hypertension. Am J Cardiol. 1983;51:1044. [DOI] [PubMed] [Google Scholar]
- 11. Cuspidi C, Sala C, Casati A, Bombelli M, Grassi G, Mancia G. Clinical and prognostic value of hypertensive cardiac damage in the pamela study. Hypertens Res. 2017;40(4):329‐335. [DOI] [PubMed] [Google Scholar]
- 12. Cicero AF, Rosticci M, Tocci G, et al. Serum uric acid and other short‐term predictors of electrocardiographic alterations in the brisighella heart study cohort. Eur J Intern Med. 2015;26(4):255‐258. [DOI] [PubMed] [Google Scholar]
- 13. Cicero AF, Rosticci M, Reggi A, et al. Relationship between serum uric acid and electrocardiographic alterations in a large sample of general population: Data from the brisighella heart study. High Blood Press Cardiovasc Prev. 2015;22:129‐134. [DOI] [PubMed] [Google Scholar]
- 14. Volpe M, Pagannone E, Tocci G, Rubattu S. Hypertension and heart failure: role of neurohormonal mechanisms. Clin Exp Hypertens. 2004;26:603‐610. [DOI] [PubMed] [Google Scholar]
- 15. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 16. Casiglia E, Schiavon L, Tikhonoff V, et al. Electrocardiographic criteria of left ventricular hypertrophy in general population. Eur J Epidemiol. 2008;23:261‐271. [DOI] [PubMed] [Google Scholar]
- 17. Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part v: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society: Endorsed by the international society for computerized electrocardiology. Circulation. 2009;119:e251‐261. [DOI] [PubMed] [Google Scholar]
- 18. Muiesan ML, Salvetti M, Di Castelnuovo A, et al. Obesity and ECG left ventricular hypertrophy. J Hypertens. 2017;35:162‐169. [DOI] [PubMed] [Google Scholar]
- 19. Felix‐Redondo FJ, Fernandez‐Berges D, Calderon A, Consuegra‐Sanchez L, Lozano L, Barrios V. Prevalence of left‐ventricular hypertrophy by multiple electrocardiographic criteria in general population: Hermex study. J Hypertens. 2012;30:1460‐1467. [DOI] [PubMed] [Google Scholar]
- 20. Tsiachris D, Chrysohoou C, Oikonomou E, et al. Distinct role of electrocardiographic criteria in echocardiographic diagnosis of left ventricular hypertrophy according to age, in the general population: the ikaria study. J Hypertens. 2011;29:1624‐1632. [DOI] [PubMed] [Google Scholar]
- 21. Otterstad JE, Smiseth O, Kjeldsen SE. Hypertensive left ventricular hypertrophy: pathophysiology, assessment and treatment. Blood Press. 1996;5:5‐15. [DOI] [PubMed] [Google Scholar]
- 22. Phillips RA. Etiology, pathophysiology, and treatment of left ventricular hypertrophy: focus on severe hypertension. J Cardiovasc Pharmacol. 1993;21(suppl 2):S55‐62. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez A, Ravassa S, Lopez B, et al. Myocardial remodeling in hypertension. Hypertension. 2018;72:549‐558. [DOI] [PubMed] [Google Scholar]
- 24. Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572‐580. [DOI] [PubMed] [Google Scholar]
- 25. Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550‐1558. [DOI] [PubMed] [Google Scholar]
- 26. Devereux RB, de Simone G, Ganau A, Koren MJ, Mensah GA, Roman MJ. Left ventricular hypertrophy and hypertension. Clin Exp Hypertens. 1993;15:1025‐1032. [DOI] [PubMed] [Google Scholar]
- 27. Diwan A, Dorn GW 2nd. Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology (Bethesda). 2007;22:56‐64. [DOI] [PubMed] [Google Scholar]
- 28. Evaristi MF, Caubere C, Harmancey R, et al. Increased mean aliphatic lipid chain length in left ventricular hypertrophy secondary to arterial hypertension: a cross‐sectional study. Medicine. 2016;95:e4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dang A, Zheng D, Wang B, et al. The role of the renin‐angiotensin and cardiac sympathetic nervous systems in the development of hypertension and left ventricular hypertrophy in spontaneously hypertensive rats. Hypertens Res. 1999;22:217‐221. [DOI] [PubMed] [Google Scholar]
- 30. Grassi G. Sympathetic overdrive as an independent predictor of left ventricular hypertrophy: prospective evidence. J Hypertens. 2006;24:815‐817. [DOI] [PubMed] [Google Scholar]
- 31. Cowan BR, Young AA. Left ventricular hypertrophy and renin‐angiotensin system blockade. Curr Hypertens Rep. 2009;11:167‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pitt B. Regression of left ventricular hypertrophy in patients with hypertension: blockade of the renin‐angiotensin‐aldosterone system. Circulation. 1998;98:1987‐1989. [DOI] [PubMed] [Google Scholar]
- 33. Hua L, Li C, Xia D, et al. Relationship between hypertensive left ventricular hypertrophy and levels of endothelin and nitric oxide. Hypertens Res. 2000;23:377‐380. [DOI] [PubMed] [Google Scholar]
- 34. Schunkert H, Orzechowski HD, Bocker W, Meier R, Riegger GA, Paul M. The cardiac endothelin system in established pressure overload left ventricular hypertrophy. J Mol Med. 1999;77:623‐630. [DOI] [PubMed] [Google Scholar]
- 35. Dries DL. Natriuretic peptides and the genomics of left‐ventricular hypertrophy. Heart Fail Clin. 2010;6:55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sciarretta S, Paneni F, Palano F, et al. Role of the renin‐angiotensin‐aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond). 2009;116:467‐477. [DOI] [PubMed] [Google Scholar]
- 37. Tocci G, Sciarretta S, Volpe M. Development of heart failure in recent hypertension trials. J Hypertens. 2008;26:1477‐1486. [DOI] [PubMed] [Google Scholar]
- 38. Magri P, Rao MA, Cangianiello S, et al. Early impairment of renal hemodynamic reserve in patients with asymptomatic heart failure is restored by angiotensin ii antagonism. Circulation. 1998;98:2849‐2854. [DOI] [PubMed] [Google Scholar]
- 39. Palano F, Paneni F, Sciarretta S, Tocci G, Volpe M. [The progression from hypertension to congestive heart failure]. Recenti Prog Med. 2011;102:461‐467. [DOI] [PubMed] [Google Scholar]
- 40. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;2016(37):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 41. Narayanan K, Reinier K, Teodorescu C, et al. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11:1040‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346‐2351. [DOI] [PubMed] [Google Scholar]
- 43. Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334‐341. [DOI] [PubMed] [Google Scholar]
- 44. Kahn S, Frishman WH, Weissman S, Ooi WL, Aronson M. Left ventricular hypertrophy on electrocardiogram: prognostic implications from a 10‐year cohort study of older subjects: a report from the bronx longitudinal aging study. J Am Geriatr Soc. 1996;44:524‐529. [DOI] [PubMed] [Google Scholar]
- 45. Devereux RB, de Simone G, Ganau A, Roman MJ. Left ventricular hypertrophy and geometric remodeling in hypertension: stimuli, functional consequences and prognostic implications. J Hypertens Suppl. 1994;12:S117‐127. [PubMed] [Google Scholar]
- 46. Havranek EP, Emsermann CD, Froshaug DN, et al. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol. 2017;237:60‐63. [DOI] [PubMed] [Google Scholar]
- 48. Shenasa M, Shenasa H, El‐Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin. 2015;7:207‐220. [DOI] [PubMed] [Google Scholar]
- 49. Stevens SM, Reinier K, Chugh SS. Increased left ventricular mass as a predictor of sudden cardiac death: is it time to put it to the test? Circ Arrhythm Electrophysiol. 2013;6:212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aro AL, Reinier K, Rusinaru C, et al. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the oregon sudden unexpected death study and the atherosclerosis risk in communities study. Eur Heart J. 2017;38:3017‐3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aro AL, Reinier K, Phan D, et al. Left‐ventricular geometry and risk of sudden cardiac arrest in patients with preserved or moderately reduced left‐ventricular ejection fraction. Europace. 2017;19:1146‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015;131:1435‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wicker P, Tarazi RC. Coronary blood flow in left ventricular hypertrophy: a review of experimental data. Eur Heart J. 1982;3(suppl A):111‐118. [DOI] [PubMed] [Google Scholar]
- 54. Kobayashi K, Tarazi RC, Lovenberg W, Rakusan K. Coronary blood flow in genetic cardiac hypertrophy. Am J Cardiol. 1984;53:1360‐1364. [DOI] [PubMed] [Google Scholar]
- 55. Wicker P, Tarazi RC, Kobayashi K. Coronary blood flow during the development and regression of left ventricular hypertrophy in renovascular hypertensive rats. Am J Cardiol. 1983;51:1744‐1749. [DOI] [PubMed] [Google Scholar]
- 56. Heinzel FR, Hohendanner F, Jin G, Sedej S, Edelmann F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. J Appl Physiol. 1985;2015(119):1233‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bacharova L. Electrocardiography‐left ventricular mass discrepancies in left ventricular hypertrophy: electrocardiography imperfection or beyond perfection? J Electrocardiol. 2009;42:593‐596. [DOI] [PubMed] [Google Scholar]
- 58. Schillaci G, Battista F, Pucci G. A review of the role of electrocardiography in the diagnosis of left ventricular hypertrophy in hypertension. J Electrocardiol. 2012;45:617‐623. [DOI] [PubMed] [Google Scholar]
- 59. Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813‐822. [DOI] [PubMed] [Google Scholar]
- 60. Schillaci G, Verdecchia P, Borgioni C, et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol. 1994;74:714‐719. [DOI] [PubMed] [Google Scholar]
- 61. Gosse P, Jan E, Coulon P, Cremer A, Papaioannou G, Yeim S. ECG detection of left ventricular hypertrophy: the simpler, the better? J Hypertens. 2012;30:990‐996. [DOI] [PubMed] [Google Scholar]
- 62. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlof B. Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: the losartan intervention for endpoint reduction (life) in hypertension study. The life study investigators. Hypertension. 2000;36:766‐773. [DOI] [PubMed] [Google Scholar]
- 63. Romhilt DW, Estes EH Jr. A point‐score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752‐758. [DOI] [PubMed] [Google Scholar]
- 64. Bacharova L, Schocken D, Estes EH, Strauss D. The role of ECG in the diagnosis of left ventricular hypertrophy. Curr Cardiol Rev. 2014;10:257‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;69:1694‐1703. [DOI] [PubMed] [Google Scholar]
- 66. Verdecchia P, Angeli F, Reboldi G, et al. Improved cardiovascular risk stratification by a simple ECG index in hypertension. Am J Hypertens. 2003;16:646‐652. [DOI] [PubMed] [Google Scholar]
- 67. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383‐390. [DOI] [PubMed] [Google Scholar]
- 68. Verdecchia P, Angeli F, Cavallini C, et al. The voltage of R wave in lead aVL improves risk stratification in hypertensive patients without ECG left ventricular hypertrophy. J Hypertens. 2009;27:1697‐1704. [DOI] [PubMed] [Google Scholar]
- 69. Medenwald D, Kluttig A, Kors JA, et al. QT interval, general mortality and the role of echocardiographic parameters of left ventricular hypertrophy: results from the prospective, population‐based CARLA study. Eur J Prev Cardiol. 2016;23:428‐436. [DOI] [PubMed] [Google Scholar]
- 70. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol. 2008;41(6):575‐580. [DOI] [PubMed] [Google Scholar]
- 71. Boles U, Almuntaser I, Brown A, Murphy RR, Mahmud A, Feely J. Ventricular activation time as a marker for diastolic dysfunction in early hypertension. Am J Hypertens. 2010;23:781‐785. [DOI] [PubMed] [Google Scholar]
- 72. Ferrucci A, Canichella F, Battistoni A, et al. A novel electrocardiographic T‐wave measurement (Tp‐Te Interval) as a predictor of heart abnormalities in hypertension: a new opportunity for first‐line electrocardiographic evaluation. J Clin Hypertens (Greenwich). 2015;17:441‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karaagac K, Tenekecioglu E, Yontar OC, et al. Effect of non‐dipper and dipper blood pressure patterns on Tp‐Te interval and Tp‐Te/Qt ratio in patients with metabolic syndrome. Int J Clin Exp Med. 2014;7:1397‐1403. [PMC free article] [PubMed] [Google Scholar]
- 74. Conlon R, Tanner R, David S, et al. Evaluation of the Tp‐Te interval, Qtc and P‐wave dispersion in patients with coronary artery ectasia. Cardiol Res. 2017;8:280‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karaagac K, Yontar OC, Tenekecioglu E, et al. Evaluation of Tp‐Te interval and Tp‐Te/Qtc ratio in patients with coronary artery ectasia. Int J Clin Exp Med. 2014;7:2865‐2870. [PMC free article] [PubMed] [Google Scholar]
- 76. Tenekecioglu E, Karaagac K, Yontar OC, et al. Evaluation of Tp‐Te interval and tp‐te/qt ratio in patients with coronary slow flow Tp‐Te/Qt ratio and coronary slow flow. Eurasian J Med. 2015;47:104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bombelli M, Maloberti A, Raina L, et al. Prognostic relevance of electrocardiographic tpeak‐tend interval in the general and in the hypertensive population: data from the pressioni arteriose monitorate e loro associazioni study. J Hypertens. 2016;34:1823‐1830. [DOI] [PubMed] [Google Scholar]
- 78. Zhao Z, Yuan Z, Ji Y, Wu Y, Qi Y. Left ventricular hypertrophy amplifies the Qt, and Tp‐e intervals and the Tp‐e/ Qt ratio of left chest ECG. J Biomed Res. 2010;24:69‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Opthof T, Coronel R, Wilms‐Schopman FJ, et al. Dispersion of repolarization in canine ventricle and the electrocardiographic t wave: Tp‐e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341‐348. [DOI] [PubMed] [Google Scholar]
- 80. Akar FG, Yan GX, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of m cells underlies reentrant mechanism of torsade de pointes in the long‐Qt syndrome. Circulation. 2002;105:1247‐1253. [DOI] [PubMed] [Google Scholar]
- 81. Aiba T, Shimizu W, Hidaka I, et al. Cellular basis for trigger and maintenance of ventricular fibrillation in the brugada syndrome model: high‐resolution optical mapping study. J Am Coll Cardiol. 2006;47:2074‐2085. [DOI] [PubMed] [Google Scholar]
- 82. Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191‐200. [DOI] [PubMed] [Google Scholar]
- 83. Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐tend and tpeak‐tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the brugada syndrome. J Am Coll Cardiol. 2006;47:1828‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Erikssen G, Liestol K, Gullestad L, Haugaa KH, Bendz B, Amlie JP. The terminal part of the QT Interval (T peak to T end): a predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17(2):85‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ilgenli TF, Tokatli A, Akpinar O, Kilicaslan F. The effects of cigarette smoking on the Tp‐e interval, Tp‐e/Qt ratio and Tp‐e/Qtc ratio. Adv Clin Exp Med. 2015;24:973‐978. [DOI] [PubMed] [Google Scholar]
- 86. Tsai WC, Lee KT, Wu MT, et al. Significant correlation of P‐wave parameters with left atrial volume index and left ventricular diastolic function. Am J Med Sci. 2013;346:45‐51. [DOI] [PubMed] [Google Scholar]


