Abstract
Hypertension is strongly correlated with an increased risk of cardiovascular events. Recent studies have demonstrated that body fat percentage (BF%) is associated with cardiometabolic risk factors. The aim of this study was to investigate the association between a change in BF% and body mass index (BMI) and the incidence of new‐onset hypertension in a normotensive Korean cohort. At baseline (2001‐2002), 8848 participants aged 40‐70 years were recruited for the study; follow‐up surveys were completed in the year 2012. A total of 3902 adults (1866 men and 2036 women) were included in the final analysis. These subjects were divided into quartile groups according to changes in BF% and were followed for 8.4 years to monitor for the development of hypertension. A Poisson regression model was used to evaluate the relative risk (RR) for hypertension according to BF% change quartile. Additionally, we also stratified participants into four groups according to BMI change levels and body fat change levels. Finally, we compared two factors, BF% change, and BMI change, to determine which is more predictive of incident hypertension. In an adjusted model, compared with the lowest BF% quartile group, the risk of new‐onset hypertension significantly increased with BF% change: Changes in risk were 0%‐2.0% for quartile 3 subjects (RR: 1.32 [1.06‐1.63]) and 2.0%‐8.9% for quartile 4 participants (RR: 1.78 [1.43‐2.19]). We also revealed that the RR for new‐onset hypertension was 1.81 (95% CI: 1.47‐2.21) for quartile 4 group subjects, compared with subjects in quartile 1 (change in BMI −6.80 to −0.86% [kg/m2]). Body fat gain and BMI increase were predictors of hypertension in this community‐based Korean cohort.
Keywords: body mass index, fat percentage, hypertension, retrospective study
1. INTRODUCTION
Hypertension, an important risk factor for cardiovascular disease,1 is a major public health issue worldwide. The World Health Organization (WHO) estimated that, worldwide, approximately 40% of adults over the age of 25 had been diagnosed with hypertension in 2008. The number of people with hypertension increased from 600 million in 1980 to 1 billion in 2008.2 In Korea, the estimated prevalence of hypertension in 2017 was 27% in adults over the age of 30% and 60% in older adults.3 This increasing prevalence has been attributed to aging and behavioral risk factors, such as excess energy intake, physical inactivity, and obesity.
Body mass index (BMI) is the most commonly used indicator for evaluating degrees of obesity. Many previous studies have shown that higher BMI is associated with higher blood pressure4, 5 and that changes in BMI are related to incident hypertension in several prospective cohort studies.6, 7 However, BMI may not always be a reliable indicator of obesity, since increases in BMI may be the result of not only fat mass increase but also muscle mass increase.8 Recent studies have demonstrated that body fat percentage (BF%) is a risk factor for cardiovascular disease, including hypertension9; however, studies on changes in BF% or comparisons with BMI are limited.
The aim of our study was to retrospectively examine associations for changes in BF% with the incidence risk of hypertension in a large‐scale, community‐based Korean cohort observed over 10 years. We also compared the efficacy of changes in BF% and BMI for the prediction of hypertension risk.
2. METHODS
2.1. Study population
We used data from the Korean Genome and Epidemiology Study in the Korean General Population (KoGES). This population‐based retrospective cohort study was designed to assess environmental and lifestyle determinants for the prevalence and incidence of chronic degenerative disorders, such as hypertension, diabetes, osteoporosis, and cardiovascular disease. KoGES invited all adults in the rural and urban areas of Ansung and Ansan in South Korea, where demographic shifts are infrequent, and the population can be followed long‐term, to participate in the study. Detailed information on the study design and aims of the KoGES have been described in previous studies. In brief, the baseline survey, carried out from June 2001 to January 2003, included 8848 adults (3945 men and 4903 women) aged 40‐69 years. Study participants were invited to undergo a follow‐up visit biennially. Data from the baseline study to the sixth examination from March 2011 to February 2013 were used in the current study. All study participants were invited to participate in the follow‐up survey. Of the original 8848 participants, 2284 subjects had hypertension at baseline; 1454 refused to participate in the follow‐up examination. We excluded the subjects with hypertension at baseline and the 1454 subjects who had missing data. Those who had not undergone bioelectrical impedance analysis (BIA) were also excluded (n = 1206). The final sample size for the present analysis was 3902 participants (1866 men and 2036 women) without hypertension at baseline (Figure 1). The study protocol was approved by the Institutional Review Board of Yonsei University College of Medicine. All participants provided written informed consent to participate in the survey.
Figure 1.
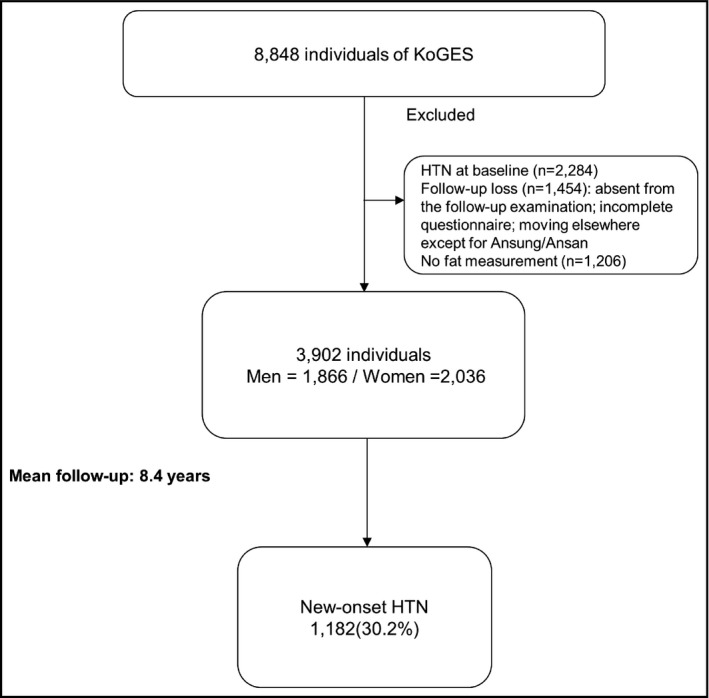
Study population
2.2. Data collection
At baseline and at the follow‐up examination, study participants completed a standardized medical history and lifestyle questionnaire and underwent a comprehensive health examination according to standard procedures. Body weight and height were measured, while participants were wearing light indoor clothing without shoes. Blood pressure was measured from the right arm using a standard mercury sphygmomanometer (Baumanometer) after the participant had rested for at least 5 minutes in a quiet room. With the participant seated, an appropriately sized cuff, chosen for each subject according to mid‐arm circumference, was applied snugly around the upper right arm at the heart level. Two measurements separated by at least 5 minutes were made, and the mean of the two measurements was used for analysis. Smoking status was determined based on self‐reports. Non‐smokers were defined as participants who had smoked <100 cigarettes (<5 packs of cigarettes) in their lifetime. Current smokers were defined as participants who had smoked ≥100 cigarettes in their lifetime and who reported “currently smoking” in the questionnaire. Former smokers were defined as participants who had smoked ≥100 cigarettes in their lifetime, but reported that they “abstain from smoking” on the questionnaire. A venous blood sample was drawn from study participants after fasting for ≥12 hours or overnight. Fasting glucose was determined by a glucose oxidase‐based assay. Serum concentrations of low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, and triglycerides (TG) were determined by enzymatic methods (Advia 1650, Bayer). We used the 2018 Korean guidelines for the management of hypertension: systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.10 We also used the WHO's diagnostic criteria for type 2 diabetes mellitus (T2DM): 8‐hour fasting blood glucose (FBG) ≥7.0 mmol/L (126 mg/dL) or HbA1c level ≥6.5%.11 In addition, subjects who reported undergoing hypoglycemic therapy during the follow‐up period were considered to have new‐onset T2DM. The details of the design and procedures have been described previously.
2.3. Statistical analysis
All analyses were performed using SAS version 9.2 (SAS Institute). P values < .05 were considered statistically significant. Baseline characteristics of the study population according to BMI were compared using an independent t test or the Mann‐Whitney U test for categorical variables. Continuous variables with normal distributions were expressed as a mean standard deviation. Since triglyceride levels were markedly skewed, they are expressed as a median instead of a mean. To investigate the relationship between baseline BMI and hypertension, we divided the study population into the following four groups: reference, overweight, obesity, and severe obesity. The basic characteristics of the study population according to BMI were compared using one‐way analysis of variance for continuous variables and the chi‐square test for categorical variables. Multivariable logistic regression was used to assess the association of baseline obesity with hypertension. We adjusted for age, diabetes, hypertension, smoking, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, HDL cholesterol, and eGFR. We also assessed the association of changes in BMI and BF% levels with new‐onset hypertension. BMI change was calculated as “BMI at 1st period survey—end point BMI” or “BMI at 1st period survey—BMI at the period when subjects were diagnosed with hypertension.” BF% change was calculated “BF% at 1st period survey—end point BF%” or “BF% at 1st period survey—BF% at the period when subjects were diagnosed with hypertension.” We divided the study population into quartiles according to BMI change and BF% change during follow‐up periods. The cutoff values were −0.86%, 0%, and 0.91% for BMI change and −2.1%, 0%, and 2.0% for BF% change. We developed two different models to estimate the risk of new‐onset hypertension: The basic model was based on parameters shown to be associated with hypertension: age, gender, systolic BP, diastolic BP, fasting blood sugar, total cholesterol, and smoking history. The body fat change model (Figure 2A) was created by adding BF% change in the basic model; the BMI change model (Figure 2B) entailed adding BMI change instead of BF% change. For each model, the area under a receiver operating curve (AROC) was calculated. We also calculated cutoff values, which were defined as that closest to the upper left corner. The probabilities of new‐onset hypertension for BF% change and BMI change are presented with smooth spline curves using R package version 3.4.2 (Institute for Statistics and Mathematics).
Figure 2.
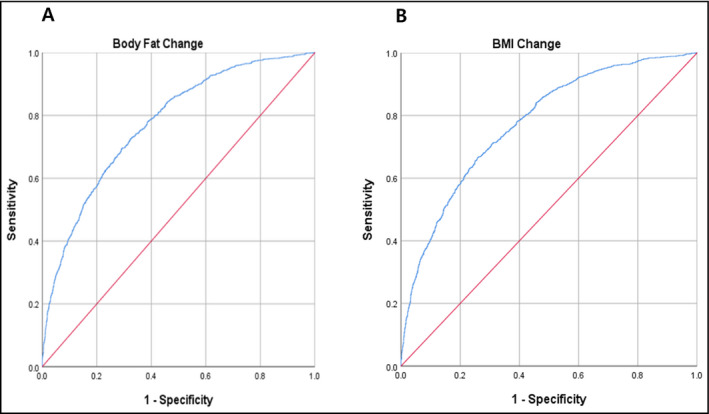
ROC curve defining cutoff values predicting incidence of hypertension, presenting the predictive power for incident hypertension according to body fat change (A) and for incident hypertension according to BMI change (B)
3. RESULTS
The baseline characteristics of the study population according to body mass index are shown in Table 1. eGFR level was lower in subjects with severe obesity than in those with normal weight. Total cholesterol and triglyceride levels were higher in subjects with severe obesity than in those with normal weight. HDL cholesterol was lower in subjects with severe obesity than in subjects with normal weight. The percentage of current smokers was lower in subjects with severe obesity than in those with normal weight. Also, systolic/diastolic blood pressure, participants who had hypertension and diabetes, and had taken dyslipidemia or hypertension medication, was higher in participants with severe obesity than in those of normal weight. Table 2 demonstrates odds ratios (ORs) and 95% CIs for risk of hypertension according to severity of baseline BMI. According to the journal,12 we set the normal range of BMI 18.5‐22.9 kg/m2. Compared with subjects in the normal weight group for baseline BMI group (18.5‐22.9 kg/m2), the ORs for subjects grouped in the severe obesity group (30.0‐kg/m2) were 4.91 (3.91‐6.17) after adjusting for age and gender; 2.13 (1.52‐2.97) after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, and smoking history; and 1.81 (1.29‐2.56) after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, smoking history, fasting glucose, total cholesterol, HDL cholesterol, and eGFR. Table 3 presents the ORs for risk of hypertension according to BF% in men and women. According to guidelines on BF%,13 we set the normal range of body fat of BMI 18.5‐22.9 kg/m2. In Table 3, compared with men in the normal range of BF% group (13%‐24%), men in the third tertile of BF% showed ORs of 2.50 (1.97‐3.16) after adjusting for age; 1.88 (1.33‐2.67) after adjusting for age, systolic blood pressure, and diastolic blood pressure; and 1.60 (1.11‐2.30) after adjusting for age, systolic blood pressure, diastolic blood pressure, smoking history, diabetes, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, and eGFR. Meanwhile, compared with women in the normal range of BF% group (25%‐36%), those in the third tertile of BF% showed ORs of 3.23 (2.29‐4.55) in model 1; 2.18 (1.36‐3.50) after adjustment in model 2; and 1.91 (1.18‐3.10) after adjustment in model 3. Table 4 shows the results of Poisson regression model analysis of the associations between change in BMI and BF% with new‐onset hypertension. Compared with participants in the first quartile of BMI change (−6.80% to −0.86%), the relative risks for subjects categorized in the highest quartile were 1.62 (1.33‐1.98) after adjusting for age and gender; 1.64 (1.34‐2.00) after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, and smoking history; and 1.81 (1.47‐2.21) after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, smoking history, glucose, diabetes, hypertension, total cholesterol, HDL cholesterol, triglycerides, and eGFR. Hypertension incidence percentage increased as change in BMI quartile increased. Compared with the lowest quartile, the relative risks (95% CI) for incidence of hypertension were 1.00 (0.86‐1.23) for Q2, 1.07 (0.88‐1.32) for Q3, and 1.64 (1.34‐2.00) for Q4 after adjusting for age, gender, systolic blood pressure, diastolic blood pressure, and smoking history. After additional adjustment for BMI, fasting glucose, diabetes, hypertension, total cholesterol, HDL cholesterol, triglycerides, and eGFR, the relative ratios (95% confidence intervals) for incidence of hypertension were 1.07 (0.87‐1.31) for Q2, 1.16 (0.95‐1.43) for Q3, and 1.81 (1.47‐2.21) for Q4. ROC curves for multivariate models are presented in Figure 2. According to the ROC curve analysis, the optimal cutoff point of Figure 2.A was 0.297, with a sensitivity of 0.699, a specificity of 0.697, and an AUC of 0.775 (0.759‐0.790). The optimal cutoff point of Figure 2.B was 0.298, with a sensitivity of 0.700, a specificity of 0.699, and an AUC of 0.775 (0.759‐0.791), which showed that both factors had high predictive power. We also investigated the continuous relationship between BF% and BMI change and development of hypertension. The hazard ratios of hypertension (with 95% CI) for between BF% and BMI change are presented with smooth spline curves (Figure 3). We found an increasing trend in the incidence of hypertension with higher body fat and BMI.
Table 1.
Baseline characteristics of the study population according to body mass index
| Normal (BMI 18.5‐22.9) | Overweight (BMI 23.0‐24.9) | Obese (BMI 24.9‐29.9) | Severe_Obesity (BMI 30.0‐) | P‐value† | |
|---|---|---|---|---|---|
| No. subjects (%) | 2566 | 2322 | 3352 | 440 | |
| Age (years) | 52.4 ± 9.4 | 51.7 ± 8.8 | 52.2 ± 8.6 | 52.4 ± 8.6 | .63 |
| BMI (kg/m2) | 21.4 ± 1.2 | 24.0 ± 0.6 | 26.8 ± 1.3 | 31.7 ± 1.8 | <.001 |
| Estimated GFR | 87.7 ± 16.0 | 85.1 ± 15.6 | 83.7 ± 15.6 | 82.8 ± 14.5 | <.001 |
| Gender, male n, (%) | 1248 (50.9) | 1075 (48.1) | 1509 (47.3) | 113 (27.2) | <.001 |
| Fasting glucose (mg/dL) | 84.7 ± 20.9 | 87.4 ± 22.0 | 89.7 ± 22.1 | 86.2 ± 12.2 | <.001 |
| Total cholesterol (mg/dL) | 190.4 ± 34.7 | 199.0 ± 37.2 | 205.0 ± 36.5 | 208.4 ± 38.5 | <.001 |
| Triglyceride (mg/dL) | 104 (75‐145) | 124 (89‐184) | 146 (102‐210) | 153 (108‐226) | <.001 |
| HDL cholesterol (mg/dL) | 53.3 ± 12.6 | 49.2 ± 11.3 | 46.7 ± 10.6 | 46.8 ± 10.5 | <.001 |
| Smoking status, n (%) | |||||
| Never | 1357 (55.3) | 1334 (59.6) | 1904 (59.6) | 322 (77.6) | <.0001 |
| Current | 757 (30.8) | 550 (24.6) | 736 (23.1) | 54 (13.0) | |
| Ex | 340 (13.9) | 353 (15.8) | 552 (17.3) | 39 (9.4) | |
| Systolic BP (mm Hg) | 117.2 ± 18.2 | 119.6 ± 18.6 | 123.7 ± 18.5 | 130.5 ± 20.5 | <.001 |
| Diastolic BP (mm Hg) | 76.9 ± 11.8 | 78.9 ± 11.8 | 82.2 ± 11.8 | 86.2 ± 12.2 | <.001 |
| Hypertension, n (%)a | 537 (21.9) | 613 (27.4) | 1291 (40.4) | 218 (52.5) | <.001 |
| Diabetes, n (%)b | 216 (8.4) | 257 (11.1) | 531 (15.8) | 99 (22.4) | <.001 |
| Medications, n (%) | |||||
| Dyslipidemia | 5 (0.2) | 21 (0.9) | 27 (0.8) | 4 (0.9) | .010 |
| Hypertension | 159 (6.2) | 63 (2.7) | 597 (17.8) | 119 (27.1) | <.001 |
| Diabetes | 85 (3.3) | 93 (4.0) | 147 (4.4) | 24 (5.4) | .108 |
All data are presented as mean ± SD, proportions, or medians (interquartile ranges) for skewed variables.
Abbreviations: BMI, body mass index; BP, blood pressure.
† P value was calculated by one‐way ANOVA for continuous variables and chi‐square test for categorical variables.
Hypertension was defined as a systolic BP ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg, or previous use of antihypertensive medication.
Diabetes was defined as fasting plasma glucose level ≥ 126 mg/dL or use of hypoglycemic agent or insulin.
Table 2.
Odds ratios and 95% confidence intervals for risk of baseline hypertension according to severity of baseline body mass index (BMI)
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|
| Body mass index | |||
| Normal (18.5‐22.9 kg/m2) | 1.00 | 1.00 | 1.00 |
| Overweight (23.0‐24.9 kg/m2) | 1.50 (1.18‐2.04) | 1.35 (1.10‐1.67) | 1.26 (1.53‐2.96) |
| Obesity (25.0‐29.9 kg/m2) | 2.79 (1.18‐2.04) | 2.16 (1.79‐2.60) | 1.92 (1.59‐2.33) |
| Severe_Obesity (30.0‐kg/m2) | 4.91 (3.91‐6.17) | 2.13 (1.52‐2.97) | 1.81 (1.29‐2.56) |
(Adjusted R‐squared = 0.693)
Model 1: Adjusted for age and gender.
Model 2: Adjusted for age, gender, systolic blood pressure, diastolic blood pressure, and smoking.
Model 3: Adjusted for age, gender, diabetes, hypertension, smoking, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, eGFR, and HDL cholesterol.
Table 3.
Odds ratios and 95% confidence intervals for risk of baseline hypertension according to baseline body fat percentage
| Men (N = 3292) | Model 1 RR (95% CI) | Model 2 RR (95% CI) | Model 3 RR (95% CI) |
|---|---|---|---|
| Body fat percentage | |||
| 40‐69 y | |||
| Q1 (13.0%‐23.9%) | 1.00 | 1.00 | 1.00 |
| Q2 (24.0%‐28.9%) | 1.81 (1.53‐2.14) | 1.56 (1.22‐2.00) | 1.43 (1.10‐1.85) |
| Q3 (29.0%‐40.0%) | 2.50 (1.97‐3.16) | 1.88 (1.33‐2.67) | 1.60 (1.11‐2.30) |
| Women (N = 3492) | Model 1 RR (95% CI) | Model 2 RR (95% CI) | Model 3 RR (95% CI) |
|---|---|---|---|
| Body fat percentage | |||
| 40‐69 y | |||
| Q1 (25.0%‐35.9%) | 1.00 | 1.00 | 1.00 |
| Q2 (36.0%‐40.9%) | 1.52 (1.26‐1.84) | 1.09 (0.83‐1.42) | 1.01 (0.77‐1.32) |
| Q3 (41.0%‐49.9%) | 3.23 (2.29‐4.55) | 2.18 (1.36‐3.50) | 1.91 (1.18‐3.10) |
(Adjusted R‐squared = 0.675 in men and 0.697 in women)
Model 1: Adjusted for age
Model 2: Adjusted for age, systolic blood pressure, diastolic blood pressure, and smoking.
Model 3: Adjusted for age, systolic blood pressure, diastolic blood pressure, smoking, diabetes, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, and eGFR.
Table 4.
Relative risk and 95% confidence intervals for risk of new‐onset hypertension according to change of body mass index (BMI) and body fat percentage
| Model 1 RR (95% CI) | Model 2 RR (95% CI) | Model 3 RR (95% CI) | |
|---|---|---|---|
| BMI change | |||
| Q1 Loss (−6.80 to −0.86) | 1.00 | 1.00 | 1.00 |
| Q2 Loss (−0.86 to 0) | 0.98 (0.807‐1.21) | 1.00 (0.86‐1.23) | 1.07 (0.87‐1.31) |
| Q3 Gain (0 to 0.91) | 1.07 (0.88‐1.31) | 1.07 (0.88‐1.32) | 1.16 (0.95‐1.43) |
| Q4 Gain (0.91 to 13.2) | 1.62 (1.33‐1.98) | 1.64 (1.34‐2.00) | 1.81 (1.47‐2.21) |
| Body fat change | |||
| Q1, Loss (−7.0% to −2.1%) | 1.00 | 1.00 | 1.00 |
| Q2 Loss (−2.1% to 0%) | 1.11 (0.90‐1.37) | 1.10 (0.89‐1.36) | 1.12 (0.91‐1.34) |
| Q3 Gain (0% to 2.0%) | 1.23 (1.01‐1.52) | 1.25 (1.02‐1.55) | 1.32 (1.06‐1.63) |
| Q4 Gain (2.0 ‐ 8.9%) | 1.63 (1.32‐2.00) | 1.62 (1.32‐2.00) | 1.78 (1.43‐2.19) |
Model 1: Adjusted for age and gender.
Model 2: Adjusted for age and gender, systolic blood pressure, diastolic blood pressure, and smoking.
Model 3: Adjusted for age, gender, diabetes, hypertension, smoking, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, eGFR, and HDL cholesterol.
Figure 3.
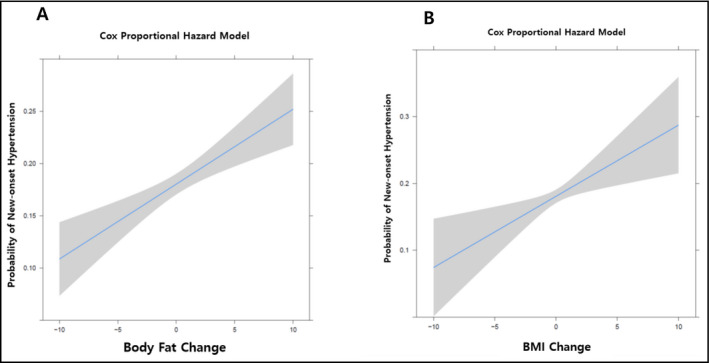
Relative hypertension incidence according to body fat change and BMI change. A, Probability of hypertension for body fat change. B, Probability of hypertension for BMI change
4. DISCUSSION
In this large retrospective, community‐based KoGES cohort study, we found that body fat and BMI increases were associated with new‐onset hypertension. These associations remained after adjusting for potential surrogate markers for hypertension. Our results are consistent with emerging evidence demonstrating that BMI increase leads to elevation of blood pressure.14 Moreover, our study showed that body fat changes are also positively associated with blood pressure. Some explanatory biological mechanisms may be offered.
Nitric oxide (NO) availability, an important factor regulating vascular tone,15 is reduced in obese subjects and obesity‐related hypertensive patients. Reduced NO availability is also responsible for increased vascular oxidative stress. Obesity induces increased exposure to fatty acids and generation of superoxides and results in uncoupling activation, reflecting a significant impairment in mitochondrial oxidative capacity.16 Weight gain results in the activation of this metabolic effect. To compensate for weight gain, presynaptic secretion of norepinephrine occurs. Since norepinephrine is directly recorded at sympathetic nerves, these nerves are activated.17 Indeed, the presence of obesity and hypertension has been found to exhibit associations with sympathetic nervous system activation in pathophysiological conditions.18 Meanwhile, microneurographic studies have shown that sympathetic activation is associated with visceral body fat distribution,19 as well as waist circumference of waist‐to‐hip ratio.20 The activation of the kidney's sympathetic nervous system has been observed in obesity because of the renal tubular re‐absorption of sodium.21 This sympathetic nervous system activation results in vascular or tubular compression and glomerulosclerosis. As nephrons are damaged, hyperfiltration is increased.21 Increased hyperfiltration may induce an adipokine‐induced incremental increase in mineralocorticoids, specifically aldosterone.22 Sympathetic outflow to the kidneys can also result in activation of the renin‐angiotensin system. In obese subjects with hypertension, angiotensin 2 receptor blockers can reduce sympathetic nerve activation and blood pressure.23 Leptin and adiponectin are the most important adipocyte factors controlling blood pressure by regulating arterial tone. Leptin increases energy expenditure and decreases appetite,24 and some studies have shown that leptin levels can be predictive of the onset of hypertension.25 Also, recent studies report that the renin‐angiotensin system mediates the effects of leptin on renal sympathetic activity and combustion of brown adipose tissue. Therefore, the renin‐angiotensin system could mediate the action of leptin on energy expenditure and blood pressure.26 Adiponectin, a potential antihypertension protective agent, is reduced in obese subjects.24, 27
Despite our recruitment of a large number of subjects extracted from the 8848 participants, adjustment for confounding factors, and 10‐year follow‐up, this study has some limitations. First, BMI is not a perfect index of obesity; variations in lean body mass, including bone mass and muscle mass, affect BMI. The measurement of fat mass using the principle of bioelectrical impedance analysis, which suggests that electrical current flows faster in muscle than fat mass, can be biased if participants have residual urine or consume a meal before measuring BMI. To minimize this error, subjects were measured after fasting and voiding. Second, the study does not account for other metabolic origins of weight change such as new‐onset cancer, hypothyroidism, and Cushing's syndrome. Third, the ethnic and regional homogeneity of the study population is a limitation. The subjects are Koreans, particularly from Ansung and Ansan, Korea. Generalization of findings to the entire Asian population is difficult. These findings need to be confirmed for other regions and other ethnic groups. Fourth, our analyses partially rely on past history questionnaires (eg, cancer history, baseline hypertension history, and smoking history). Underreporting by those with a history of smoking or cancer is possible. Fifth, the reasons for weight loss are variable. For example, weight loss associated with physical training is beneficial to health. However, weight loss due to cancer or malnutrition is harmful. Also, weight loss does not reflect individualized socioeconomic and health state. Sixth, the reasons for the changes in BMI and body fat of the participants are not clear. Seventh, there were differences (eg, age, BMI, BPs) between subjects with follow‐up loss or missing data and those included in this analysis. Therefore, there might be selection bias in the current study. Finally, newly diagnosed hypertension may be underestimated as this may have occurred after follow‐up loss or completion of the 10‐year follow‐up period. Nevertheless, this study has many advantages compared with previous studies. Several studies have investigated the association of BMI or fat percentage and hypertension.28, 29 Channanath et al28 reported that BMI is associated with hypertension: Specifically, risk of hypertension increases with obesity levels and is higher in patients with diabetes than in patients without diabetes. Ukawa et al29 showed an association between BMI at 65 years of age and the development of hypertension during the subsequent 5 years. However, most of these studies were cross‐sectional and conducted in small populations with only short‐term follow‐up data. Tests with predictive values of elevated risk of development of hypertension prior to development of clinical symptoms are of increasing clinical importance because regular health checkups are becoming widely common.30 Additionally, hypertension is a significant prognostic factor of many diseases, including chronic kidney disease,31 arterial aneurysmal rupture, cerebral vascular accidents,32 and cardiovascular disease.33 Many studies have shown associations for hypertension with these diseases, and the importance of controlling blood pressure has been emphasized for preventing such diseases. There are many causes of hypertension including age, fasting glucose, excessive sodium diet, cholesterol, smoking history, and diabetes mellitus.34, 35, 36 Furthermore, fat mass is also known as a risk factor for hypertension.37 Finally, we found a predictor of hypertension using BMI and BF%. A unique aspect of our study is that we suggest that a change in BMI and fat percentage is an appropriate predictor hypertension incidence. We have verified that these two factors affect the incidence of hypertension in the long‐term retrospective KoGES cohort study.
In conclusion, BMI and BF% were proportional to the incidence of hypertension in KoGES study subjects. This suggests that increased BMI and BF% can play a role in early prediction of hypertension. These findings justify genetic studies of risk factors related to BMI and BF% and their associations with hypertension in Koreans. The findings may spur detailed studies of hypertension prevention methods that include the use of dietary agents.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
DHJ and SBL: contributed to the hypothesis and conception of the article. DHJ, YJK, and SBL: contributed to the design of the work. ARJ: contributed to the introduction. DHJ and YJK: contributed to the statistical analysis or methods and interpretation of data for the work. SBL: contributed to the results and the discussion. DHJ and SBL: drafted the manuscript. SBL, YJK, and DHJ: revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of article ensuring integrity and accuracy.
SOURCES OF SUPPORT FOR THIS RESEARCH
This study was conducted with bioresources from the National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea.
Supporting information
ACKNOWLEDGMENTS
Data in this study were collected from the Korean Genome and Epidemiology Study (KoGES; 4851‐302) conducted by the National Research Institute of Health, Centers for Disease Control and Prevention, and Ministry for Health and Welfare, Republic of Korea.
Lee S‐B, Cho A‐R, Kwon Y‐J, Jung D‐H. Body fat change and 8‐year incidence of hypertension: Korean Genome and Epidemiology Study. J Clin Hypertens. 2019;21:1849–1857. 10.1111/jch.13723
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 3. Korean Statistical Information Service . Hypertension: ≥30 years, by sex. Ministry of Health and Welfare, Korea National Health and Nutrition Examination Survey: Annual 1998–2017. http://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_11702_N105&conn_path=I2&language=en. Accessed June 25, 2019.
- 4. Roka R, Michimi A, Macy G. Associations between hypertension and body mass index and waist circumference in U.S. adults: a comparative analysis by gender. High Blood Press Cardiovasc Prev. 2015;22(3):265‐273. [DOI] [PubMed] [Google Scholar]
- 5. Bays HE, Chapman RH, Grandy S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61(5):737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang M, Zhao Y, Sun H, et al. Effect of dynamic change in body mass index on the risk of hypertension: results from the rural Chinese cohort study. Int J Cardiol. 2017;238:117‐122. [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Chu C, Mu J. Relationship between body mass index changes and blood pressure changes from childhood to adulthood in a general Chinese population: a 26 year cohort follow‐up study. Blood Press. 2016;25(5):319‐326. [DOI] [PubMed] [Google Scholar]
- 8. De Schutter A, Lavie CJ, Gonzalez J, Milani RV. Body composition in coronary heart disease: how does body mass index correlate with body fatness? Ochsner J. 2011;11(3):220‐225. [PMC free article] [PubMed] [Google Scholar]
- 9. Yamashita K, Kondo T, Osugi S, et al. The significance of measuring body fat percentage determined by bioelectrical impedance analysis for detecting subjects with cardiovascular disease risk factors. Circ J. 2012;76(10):2435‐2442. [DOI] [PubMed] [Google Scholar]
- 10. Lee HY, Shin J, Kim GH, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II‐diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerner W, Bruckel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(7):384‐386. [DOI] [PubMed] [Google Scholar]
- 12. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157‐163. [DOI] [PubMed] [Google Scholar]
- 13. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694‐701. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Wang C, Liu Y, et al. Incident hypertension and its prediction model in a prospective northern urban Han Chinese cohort study. J Hum Hypertens. 2016;30(12):794‐800. [DOI] [PubMed] [Google Scholar]
- 15. Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(2):233‐246. [DOI] [PubMed] [Google Scholar]
- 16. Murray AJ, Panagia M, Hauton D, Gibbons GF, Clarke K. Plasma free fatty acids and peroxisome proliferator–activated receptor α in the control of myocardial uncoupling protein levels. Diabetes. 2005;54(12):3496‐3502. [DOI] [PubMed] [Google Scholar]
- 17. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96(10):3423‐3429. [DOI] [PubMed] [Google Scholar]
- 18. Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity‐related hypertension. Hypertension. 2000;36(4):538‐542. [DOI] [PubMed] [Google Scholar]
- 19. Grassi G, Dell'Oro R, Facchini A, Trevano FQ, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22(12):2363‐2369. [DOI] [PubMed] [Google Scholar]
- 20. Canoy D, Luben R, Welch A, et al. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC‐Norfolk) study. J Hypertens. 2004;22(11):2067‐2074. [DOI] [PubMed] [Google Scholar]
- 21. Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999;892:91‐107. [DOI] [PubMed] [Google Scholar]
- 22. Vogt B, Bochud M, Burnier M. The association of aldosterone with obesity‐related hypertension and the metabolic syndrome. Semin Nephrol. 2007;27(5):529‐537. [DOI] [PubMed] [Google Scholar]
- 23. Grassi G, Seravalle G, Dell'Oro R, et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21(9):1761‐1769. [DOI] [PubMed] [Google Scholar]
- 24. Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galletti F, D'Elia L, Barba G, et al. High‐circulating leptin levels are associated with greater risk of hypertension in men independently of body mass and insulin resistance: results of an eight‐year follow‐up study. J Clin Endocrinol Metab. 2008;93(10):3922‐3926. [DOI] [PubMed] [Google Scholar]
- 26. Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R566‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yiannikouris F, Gupte M, Putnam K, Cassis L. Adipokines and blood pressure control. Curr Opin Nephrol Hypertens. 2010;19(2):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Channanath AM, Farran B, Behbehani K, Thanaraj TA. Association between body mass index and onset of hypertension in men and women with and without diabetes: a cross‐sectional study using national health data from the State of Kuwait in the Arabian Peninsula. BMJ Open. 2015;5(6):e007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ukawa S, Tamakoshi A, Wakai K, Ando M, Kawamura T. Body mass index is associated with hypertension in Japanese young elderly individuals: findings of the new integrated suburban seniority investigation. Intern Med. 2015;54(24):3121‐3125. [DOI] [PubMed] [Google Scholar]
- 30. Jung DH, Byun YS, Kwon YJ, Kim GS. Microalbuminuria as a simple predictor of incident diabetes over 8 years in the Korean Genome and Epidemiology Study (KoGES). Sci Rep. 2017;7(1):15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28(2):74‐79. [DOI] [PubMed] [Google Scholar]
- 32. Pistoia F, Sacco S, Degan D, Tiseo C, Ornello R, Carolei A. Hypertension and stroke: epidemiological aspects and clinical evaluation. High Blood Press Cardiovasc Prev. 2016;23(1):9‐18. [DOI] [PubMed] [Google Scholar]
- 33. Patel P, Ordunez P, DiPette D, et al. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: the standardized hypertension treatment and prevention project. Rev Panam Salud Publica. 2017;41:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fakhruddin S, Alanazi WA, Alhamami HN, Briski KP, Jackson KE. Hyperglycaemia induced by chronic i.p. and oral glucose loading leads to hypertension through increased Na(+) retention in proximal tubule. Exp Physiol. 2018;103(2):236‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518‐2525. [DOI] [PubMed] [Google Scholar]
- 36. Libianto R, Batu D, MacIsaac RJ, Cooper ME, Ekinci EI. Pathophysiological links between diabetes and blood pressure. Can J Cardiol. 2018;34(5):585‐594. [DOI] [PubMed] [Google Scholar]
- 37. Takeoka A, Tayama J, Yamasaki H, et al. Intra‐abdominal fat accumulation is a hypertension risk factor in young adulthood: a cross‐sectional study. Medicine. 2016;95(45):e5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


