Abstract
The present study was aimed at assessing the relationships between absolute and individual residual blood pressure (BP) variability and cognitive function in a general population. This cross‐sectional study evaluated cognitive function using minimental state evaluation (MMSE) in 471 subjects enrolled in the PAMELA study. MMSE was calculated 10 years after initial enrollment of the subjects in the PAMELA study. Measurements included office, home, and 24‐hour ambulatory BP monitoring. BP variability was obtained by calculating: (a) 24‐hour standard deviation (SD) for systolic and diastolic BP and (b) individual residual BP variability. Mean age (±SD) of the subjects enrolled was 63 ± 5.7 years at the initial evaluation, with a 10‐year increase when MMSE was performed. There was no significant difference in BP or heart rate values measured at office, home, or during 24‐h BP monitoring between subjects with MMSE < 24 and those with ≥24. BP variability measured by SBP and DBP SD was also similar between these two groups. However, individual residual BP variability was significantly greater in subjects with lower MMSE and this difference became more pronounced when the study population was divided in three groups according to MMSE score (10‐20, 21‐23, 24‐30). Individual residual SBP and DBP variability gradually decreased with the increase in MMSE score. Our data show that a sensitive parameter for the development of cognitive impairment is not BP or absolute BP variability but rather its short‐term erratic component, which has been previously shown to be an important prognostic marker for organ damage, cardiovascular, and all‐cause mortality.
Keywords: blood pressure variability, cognitive function, hypertension
1. INTRODUCTION
The relationships between blood pressure (BP) and hypertension with cognitive dysfunction have been recognized in elderly population.1 However, the association between midlife hypertension and cognitive dysfunction remains unclear, deserving further additional investigation. Furthermore, published studies did not show consistent results regarding the BP‐lowering interventions, cognitive decline, or dementia.2
In a previous study, we have shown that 24‐hour mean BP attenuation of nocturnal hypotension and erratic diastolic BP variability independently predict the mortality risk, the erratic BP variability component representing the most important factor.3 The influence of BP variability on cognitive function has been previously reported even in midlife.4, 5 However, data are not consistent and some studies reported no significant relationship between BP variability and cognitive function.6 Our study group detected two main reasons for short‐term BP variation responsible for >95% BP variability in global population: day/night and pre/postprandial BP difference.3 However, residual variance could account for about 50% of BP variability averaged for each subject.3 No data are available on the influence of residual BP variability on cognitive function in a general or a hypertensive population.
Cognitive function assessment still remains difficult to be performed and correctly interpreted. Studies using minimental state evaluation (MMSE) for evaluation of cognitive function showed that MMSE has a sensitivity and specificity of 94% and 83%, respectively, in the prediction of uncontrolled hypertension.7 The authors showed that the predictive accuracy of MMSE to detect uncontrolled hypertension was close to 24‐hour ambulatory BP monitoring accuracy.7 This was the reason why we used this test for cognitive function evaluation.
The effect of sex on cognitive function in hypertension has not been established yet. However, there are indications that female sex is associated with greater risk of cognitive dysfunction in older subjects.8 Other investigators showed that differences in sex related cognitive functions were the consequence of differences in education.9
The aim of this study was to evaluate BP and BP variability, including individual residual variability, in subjects from the general population of the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study10 with different levels of cognitive function assessed by MMSE. The second aim was to determinate the influence of age and sex on the relationships between BP variability and cognitive function.
2. METHODS
2.1. Study Population
The PAMELA study started in 1990‐1991 and included individuals aged between 25 and 74 years, randomly selected from the residents in Monza to be representative of its population,10 using the criteria of the World Health Organization Monitoring Diseases (WHO‐MONICA) project performed in the same geographic area.11
2.2. Entry data
Methods used in the PAMELA study have been previously explained in detail.12 All study subjects underwent a comprehensive clinical examination at the outpatient clinic of the Saint Gerardo University Hospital in Monza. All participants signed an informed consent. Data taken from study participants included full medical history, blood and urine samples, physical examination, and three sphygmomanometric BP measurements in the sitting position. Body weight and height were measured in all subjects.
The same day when physical examination was performed all subjects underwent 24‐hour ambulatory BP monitoring with adequate ambulatory BP monitoring device (Spacelabs 90207) set to obtain automated BP and heart rate oscillometric readings every 20 minutes over 24 hours. Subjects were asked to follow their usual activities during the monitoring period, to hold the arm still during BP measurement. All ambulatory BP recordings were analyzed to obtain 24‐hour daytime and nighttime average systolic and diastolic (SBP and DBP), after editing for artifacts. Each individual was given a validated semiautomatic BP measuring device (Philips, model HP 5331) for taking BP at home at 7.00 AM and 7.00 PM, using the arm contralateral to the one used for ambulatory BP monitoring.
In each individual, calculation was made of 24‐hour average systolic BP, diastolic BP, and HR average and standard deviation (SD) for 24‐hour SBP and DBP (index of BP variability). Additionally, each single systolic BP reading collected over the 24 hours (total: 72 recordings) was averaged for all individuals, the Fast Fourier transform spectral analysis was applied to the overall circadian BP profile so obtained to identify the cyclic components that accounted for most (>95%) of the SBP and DBP SD. All these components were afterward verified for their ability to fit the SBP and DBP profiles in each subject and the sum squared of the differences between the observed and the fitted profile was taken as reflecting in each individual the SBP and DBP variability unexplained by the cyclic components, accounting for nearly all the systolic BP variability in the population as a whole.13 This was termed “individual residual variability” and it was taken to reflect the erratic SBP and DBP variations. When averaged for all of the subjects, the residual variance accounted for 55.3 ± 17.9% and 53.88 ± 16.9% of the SBP and DBP residual variability, respectively.3 Traditional parameters of BP variability were also used: (a) SD of average 24‐hour BP and (b) coefficient of variation (CV) of 24‐hour BP that represents the average SD of BP divided by the corresponding mean BP (CV = SD/BP average values).
The residual variability is the average of the difference between the original value and the estimated value of the model. The model is created for each subject and is the sum of 2 cyclical components: 24‐ and 12‐hour.
All participants were followed from the time of the initial medical visit (from 1990 to 1993) to 2003, for a total time interval of 148 ± 27 months (median 156). Only 8 out of 2051 participants of the PAMELA Study were lost during follow‐up (0.39%). Cognitive function was assessed in 471 subjects via MMSE test only at the evaluation performed in 2001‐2002, taking as reference clinic data collected at the first PAMELA examination carried out 10 years before. The MMSE is a 17‐item scale of orientation, calculation, registration, attention, short‐term memory, visuospatial skills, and praxis, with a maximum possible score of 30.14 Higher MMSE scores indicate better cognitive function. The test requires for its performance 5‐10 minutes. It is extensively used for clinical and research purposes to assess cognitive impairment. It can be used to evaluate the severity and progression of cognitive impairment and to follow the cognitive changes in an individual over time. The same cutoff values of MMSE score were used for all participants and there were no individualized adjustment of these values.
All subjects were divided first into the two groups according to the results of MMSE (<24 and ≥24). Afterward all participants were classified according to their MMSE score into normal (score: 25‐30), mild cognitive impairment (score: 21‐24), and moderate cognitive impairment (score: 10‐20) groups. There was no subject with severe cognitive impairment (score: 0‐9). These cutoff values were previously validated by other authors.7
2.3. Data analysis
In each subject, three office and two home BP as well as HR values collected over 24 hours were separately averaged. All ambulatory BP recordings were analyzed to obtain 24‐hour, daytime and nighttime average systolic and diastolic BP (SBP and DBP), as well as mean BP, after editing for artifacts. All subjects were divided first into the two groups according to the results of MMSE (<24 and ≥24) and afterward in three groups (10‐20, 21‐24 and 25‐30).
Values were expressed as median (interquartile range) or percentages. Continuous variables were compared by Mann‐Whitney test or Kruskal‐Wallis test. Chi‐square test or Fisher's exact test were used for categorical data. A P value less than 0.05 was statistically significant. Statistical analysis was performed by SAS System (version 9.4; SAS Institute Inc, Cary, NC, USA).
3. RESULTS
There was no significant difference in age, hypertension prevalence, and BP or heart rate values measured at office, home, or during the 24‐hour BP monitoring between subjects with MMSE < 24 and those with ≥24 (Table 1). BP variability measured with SBP and DBP SD was also similar between these two groups. However, residual variability for both SBP and DBP which could not be explained with cyclic BP changes (day/night and pre/postprandial BP changes) was significantly higher in subjects with MMSE < 24 than in those with MMSA ≥ 24 (Table 1 and Figure 1, upper panels). There was no difference in residual SBP and DBP variability between subjects with MMSE < 24 and those with MMSE ≥ 24 in both sex (Table 1).
Table 1.
Demographic, clinical and blood pressure data in the whole study population
| Minimental state evaluation score | ||
|---|---|---|
| 0‐23 | 24‐30 | |
| N | 26 | 445 |
| Age (years) | 64 (58‐69) | 63 (59‐69) |
| Body mass index (kg/m2) | 26.5 (23.7‐30.9) | 26.3 (23.8‐29.0) |
| Smoking (%) | 15.4 | 18.2 |
| Male (%) | 50.0 | 53.0 |
| Hypertensive drug (%) | 42.3 | 30.8 |
| Office SBP (mm Hg) | 145 (130‐162) | 140 (130‐156) |
| Office DBP (mm Hg) | 86 (84‐90) | 88 (80‐94) |
| Office HR (beat/min) | 68 (64‐76) | 68 (64‐76) |
| 24 h SBP (mm Hg) | 124 (118‐133) | 123 (115‐132) |
| 24 h DBP (mm Hg) | 77 (72‐80) | 75 (71‐81) |
| 24 h HR (beat/min) | 77 (70‐79) | 73 (68‐79) |
| Home SBP (mm Hg) | 140 (134‐150) | 132 (121‐146) |
| Home DBP (mm Hg) | 78 (71‐92) | 79 (73‐86) |
| Home HR (beat/min) | 69 (64‐75) | 71 (65‐77) |
| Residual variability SBP* | 11.5 (9.4‐12.9) | 10.1 (8.8‐11.8) |
| Residual variability DBP* | 9.5 (7.8‐10.9) | 8.3 (7‐9.9) |
| SD 24 h SBP | 15.1 (12.6‐17.3) | 14.4 (12.0‐17.2) |
| SD 24 h DBP | 13.1 (10.8‐15.4) | 12.1 (9.9‐14.3) |
| Male (n) | 13 | 236 |
| Residual variability SBP | 11.1 (10.2‐13.1) | 10.1 (8.7‐12.0) |
| Residual variability DBP | 9.1 (8.0‐10.3) | 8.4 (7.0‐9.7) |
| Female (n) | 13 | 209 |
| Residual variability SBP | 11.7 (9.4‐12.4) | 9.9 (8.9‐11.6) |
| Residual variability DBP | 9.9 (7.8‐11.2) | 8.3 (7.0‐10.3) |
Data are shown as median (IQR) or %.
DBP: diastolic blood pressure, HR: heart rate, SBP: systolic blood pressure, SD: standard deviation.
P < 0.05.
Figure 1.
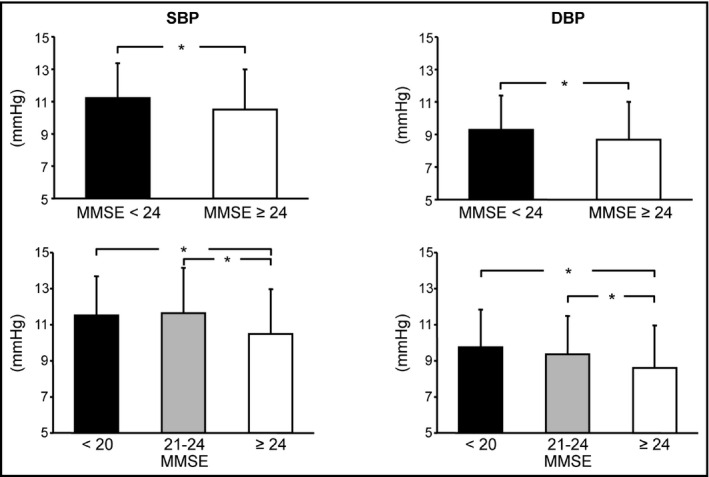
Residual systolic and diastolic blood pressure (SBP and DBP) variability in subjects of the PAMELA study classified in two or three groups (upper and lower panel, respectively) according to Minimental state evaluation score (MMSE). Data are shown as means ± standard deviations. Asterisks (P < 0.05) refer to the statistical significance between groups.
In patients aged less than 75 years, there was no difference age, hypertension prevalence, and BP or heart rates measured at office, home, or during 24‐hour BP monitoring between subjects with MMSE < 24 and those with ≥24 (Table 2). There was also no difference in traditional parameters of BP variability (SD and CV) between these two groups (Table 2). However, there was significantly higher residual BP variability among subjects with MMSE < 24. In the individuals older than 75 years, there was no difference in any demographic, clinical and BP parameter, including BP variability.
Table 2.
Demographic, clinical, and blood pressure data in the subjects younger than 75 y
| Minimental state evaluation score | ||
|---|---|---|
| 0‐23 | 24‐30 | |
| N | 12 | 251 |
| Hypertension (%) | 66.7 | 60.0 |
| Office SBP (mm Hg) | 139 (129‐151) | 138 (126‐152) |
| Office DBP (mm Hg) | 87 (84‐93) | 87 (80‐94) |
| Office HR (beat/min) | 68 (64‐72) | 68 (64‐76) |
| 24 h SBP (mm Hg) | 123 (119‐129) | 122 (115‐131) |
| 24 h DBP (mm Hg) | 78 (75‐80) | 76 (71‐82) |
| 24 h HR (beat/min) | 78 (72‐80) | 74 (69‐79) |
| Home SBP (mm Hg) | 139 (121‐149) | 128 (118‐142) |
| Home DBP (mm Hg) | 87 (75‐98) | 78 (73‐85) |
| Home HR (beat/min) | 70 (57‐78) | 71 (66‐78) |
| Dipper SBP (%) | 66.7 | 63.2 |
| Dipper DBP (%) | 75.0 | 85.6 |
| SD 24 h SBP | 15.13 (12.62‐17.25) | 14.43 (12.02‐17.15) |
| SD 24 h DBP | 13.12 (10.79‐15.41) | 12.13 (9.93‐14.28) |
| CV 24 h SBP | 0.12 (0.11‐0.14) | 0.12 (0.1‐0.14) |
| CV 24 h DBP | 0.18 (0.14‐0.2) | 0.16 (0.13‐0.19) |
| Residual variability SBP* | 12.4 (10.6‐13.1) | 9.5 (8.5‐11.1) |
| Residual variability DBP* | 10.4 (9.0‐11.5) | 7.8 (6.6‐9.5) |
DBP: diastolic blood pressure, HR: heart rate, SBP: systolic blood pressure.
P < 0.05.
When patients were divided in three different groups according to MMSE (score 10‐20, 21‐23, and 24‐30) there was no difference in clinical and demographic characteristics (Table 3). Office and 24‐hour BP and heart rate were similar between these groups. Interestingly, home SBP was higher and home DBP was lower in the subjects with the lowest MMSE than in the other two groups (Table 3). Conventional parameters of BP variability (SD and BP) did not differ between these 3 groups (Table 3). Residual SBP and DBP variability gradually decreased with increasing MMSE (Table 3 and Figure 1, lower panels).
Table 3.
Demographic, clinical, and blood pressure data in the subjects divided according to the minimental state evaluation in three groups
| minimental state evaluation | |||
|---|---|---|---|
| 10‐20 | 21‐24 | 25‐30 | |
| N | 10 | 29 | 432 |
| Age | 64 (60‐73) | 63 (57‐68) | 63 (59‐69) |
| Body mass index (kg/m2) | 24.3 (23.5‐27.0) | 27.6 (23.9‐30.5) | 26.3 (23.8‐29.0) |
| Smoking (%) | 20.0 | 13.8 | 18.3 |
| Male (%) | 40.0 | 55.2 | 53.0 |
| Antihypertensive therapy (%) | 30.0 | 41.4 | 30.8 |
| Office SBP (mm Hg) | 142 (126‐162) | 146 (130‐160) | 140 (130‐156) |
| Office DBP (mm Hg) | 85 (82‐88) | 84 (82‐94) | 88 (80‐94) |
| Office HR (beat/min) | 72 (68‐78) | 68 (60‐72) | 68 (64‐76) |
| 24 h SBP (mm Hg) | 124 (114‐133) | 124 (118‐134) | 123 (115‐131.6) |
| 24 h DBP (mm Hg) | 73 (71‐77) | 78 (72‐80) | 75 (71‐81) |
| 24 h HR (beat/min) | 77 (74‐79) | 72 (69‐78) | 73 (68‐79) |
| Home SBP (mm Hg)* | 142 (129‐154) | 135 (126‐150) | 132 (121‐146) |
| Home DBP (mm Hg)* | 72 (69‐83) | 79 (74‐87) | 79 (73‐86) |
| Home HR (beat/min) | 69 (65‐71) | 69 (63‐75) | 71 (65‐78) |
| SD 24 h SBP | 16.5 (12.9‐17.1) | 14.5 (12.6‐18.4) | 14.4 (12.0‐17.1) |
| SD 24 h DBP | 13 (12.2‐14.4) | 13.1 (10.7‐15.6) | 12.1 (9.9‐14.2) |
| CV 24 h SBP | 0.13 (0.11‐0.13) | 0.12 (0.1‐0.14) | 0.12 (0.1‐0.14) |
| CV 24 h DBP | 0.18 (0.14‐0.2) | 0.18 (0.15‐0.21) | 0.16 (0.13‐0.19) |
| Residual variability SBP* | 11.8 (10.8‐13.1) | 11.4 (9.5‐13.2) | 10.0 (8.8‐11.8) |
| Residual variability DBP* | 10.1 (8.8‐10.9) | 9.4 (7.8‐11.2) | 8.3 (7.0‐9.9) |
| Male (n) | 4 | 16 | 229 |
| Residual variability SBP | 12.1 (11.0‐13.9) | 11.3 (9.6‐13.9) | 10.1 (8.7‐11.9) |
| Residual variability DBP | 9.6 (8.9‐10.5) | 9.5 (7.7‐11.6) | 8.4 (7.0‐9.6) |
| Female (n) | 6 | 13 | 203 |
| Residual variability SBP | 11.8 (9.3‐12.4) | 11.7 (9.4‐12.6) | 9.9 (8.9‐11.6) |
| Residual variability DBP | 10.2 (7.4‐12.0) | 9.2 (7.8‐11.2) | 8.2 (7.0‐10.2) |
| Age < 75 (n) | 4 | 15 | 244 |
| Residual variability SBP* | 11.7 (8.9‐12.9) | 12.3 (10.2‐13.9)** | 9.5 (8.5‐11.0) |
| Residual variability DBP* | 9.6 (7.4‐11.7) | 10.3 (8.2‐11.6)** | 7.8 (6.5‐9.3) |
| Age ≥ 75 (n) | 6 | 14 | 188 |
| Residual variability SBP | 11.8 (10.8‐13.1) | 9.7 (8.4‐11.7) | 11.1 (9.3‐12.8) |
| Residual variability DBP | 10.1 (9.1‐10.9) | 8.5 (7.4‐9.9) | 8.9 (7.7‐10.5) |
DBP: diastolic blood pressure, HR: heart rate, SBP: systolic blood pressure, SD: standard deviation.
P < 0.05 general test.
P < 0.05 vs 25‐30.
There was no difference in residual BP variability among men with different MMSE score (Table 3). The same results were found in women (Table 3). Interestingly, the subjects younger than 75 years had the highest SBP and DBP variability in the group with MMSE score between 21 and 24 and the lowest BP variability among the subjects with MMSE score between 25 and 30 (Table 3). In the participants older than 75 years, there was no difference in BP variability among groups with various MMSE score (Table 3).
4. DISCUSSION
Our study revealed several important findings that deserve to be discussed. First, no difference in BP values assessed at office, home, and during 24‐hour BP monitoring between subjects with different MMSE scores was found. Second, BP variability measured with traditional parameters was not different among individuals with various levels of cognitive function, in global population, and in both sex. Third, residual individual variability was significantly higher in participants with lower MMSE score in the whole population and in subjects younger than 75 years, but not in the individuals older than 75 years.
Our findings did not reveal significant difference in BP between subjects with different MMSE score. On the other hand, Sayed et al7 showed that BP was gradually increased with increment of MMSE score. However, our study included subjects selected from the general population and Seyed and coworkers study included only hypertensive patients older than 65 years.7 Cho et al15 demonstrated that higher BP variability, but not average ambulatory BP level, was related to cognitive impairment in elderly patients with well‐controlled BP. Nevertheless, this investigation included significantly older patients (77.7 ± 8.3 years) than our study (approximately 63 years) and researchers used different test for cognitive assessment (Japanese version of the Montreal Cognitive Assessment) and not MMSE.15
More recently, a 5‐year longitudinal study showed no significant association between MMSE score and daytime and nighttime BP.16 Interestingly, cognitive function assessed with MMSE was significantly associated only with SBP, but not DBP variability.16 This relationship between SBP variability and cognitive deterioration was also revealed in the other study that involved subjects with mild cognitive dysfunction, Alzheimer disease, and healthy old individuals.17 Nevertheless, there are also authors who did not find the relationship between BP variability and global cognitive function measured by MMSE.6 Our study also did not show significant difference in SBP or DBP variability between participants with different MMSE score. However, our study population substantially differs from the mentioned studies because it involved global population and not hypertensive patients or subjects who already have cognitive impairment.
ASCOT‐BPLA study showed that residual visit‐to‐visit variability in SBP in treated patients was a strong predictor of stroke and coronary events, independent of mean SBP in clinic or during ambulatory BP monitoring.18 Our previous study from the PAMELA data showed that DBP residual variability was independently associated with cardiovascular and total mortality in global population.3 Additionally, our data revealed that individual residual SBP and DBP variability was independently of sex, age, and SBP associated with left ventricular mass index.13 Interestingly, this study did not show significant relationship between standard parameters of BP variability (SD and cyclic components) with LV mass index.13 In the current study, we confirmed the importance of residual individual BP variability, which was the only associated with the reduction of cognitive function assessed by MMSE.
Our present data showed no difference in residual BP variability among men and women. This suggests that BP variability impacts both sexes and that sex hormones do not have protective or adverse effect on cognitive function. This is in agreement with some previous studies that included limited number of hypertensive patients did not show difference in MMSE score between women and men.6
Interestingly, the current data showed that difference in individual residual SBP and DBP variability was present only in younger (<75‐year old), but not in older patients (>75‐year old). This in interesting finding considering the fact that the most of studies was conducted in the elderly and showed the association between BP variability and cognitive dysfunction.4, 5, 7, 15 However, the agreement is lacking because the Honolulu‐Asia Aging Study revealed that SBP ≥ 160 mm Hg was related with 51% lower odds of poor performance on a test of global cognition compared with SBP < 100 mm Hg.19 Furthermore, the Framingham Heart Study did not find association between BP in late life and cognitive function.20 Our findings indicate that BP variability could be an important risk factor for cognitive decline even in younger subjects and not in elderly, as it is usually considered.
The association between BP variability and cognitive function could be explained by different pathophysiological mechanisms. It has been previously suggested that small vessel cerebrovascular disease could be one of the reasons for the relationship between BP variability and cognitive decline.21 The authors explained that increased BP variability was related to silent cerebral damage—cerebral white matter lesions diagnosed with MRI and computed topography.22 Goldstein and coworkers reported that 24‐hour SBP and its variability were associated with cognitive impairment and progression of white matter “hyperintensities,” a marker of small vessel cerebrovascular disease.23 The authors demonstrated that increased daytime SBP variability was associated with increased risk of severe white matter disease at follow‐up 5 years later and greater SBP nighttime variability was related with increased brain atrophy.21
Our data may indicate that BP variability could be a possible modifiable risk factor for cognitive decline not only in hypertensive, but also in global population. This refers not only in the elderly, but also in the subjects younger than 75 years. However, there is no much data regarding antihypertensive therapy treatment and its influence on BP variability in patients. Our findings suggest that residual BP variability is not only “noise,” as usually thought. Actually, it might represent the tendency for BP to vary in a rather “erratic” fashion that could also influence cognitive function. The reasons why the erratic BP variability negatively affected cognitive function more than absolute BP variability can only be only a matter of debate. A reasonable hypothesis would be that the relatively fast BP changes that are computed by individual residual BP variability might have more impact on the cardiovascular system by accelerating the atherosclerosis in small cerebral vessels, which ends with deteriorating of cerebral white matter and ultimately with cognitive impairment. However, one should underline the other sources of residual BP variability that range from measurement noise to acute physiological changes.
4.1. Limitations
Our study has a number of limitations. They include the limited number of subjects with reduced MMSE score. However, we studied normal population and not preselected hypertensive elderly subjects like the majority of other studies. Therefore, this could be also an unique feature of the present study. They also include the fact that MMSE was not performed at baseline of the PAMELA study and that no data were available on the etiology of cognitive dysfunction (Alzheimer, vascular, etc). MMSE could be influenced by age, education, culture, ethnicity, hearing or visual impairment, as well as any other relevant physical disabilities. However, our study included middle‐age population from one small town in Italy, which represents very homogenous population. Therefore, difference in culture and ethnicity were excluded. Both groups were of the similar age. MMSE is mostly performed orally, so visual impairment did not have significant role and hearing impairment in this age is still not prevalent. The education level in the PAMELA study was not investigated and this could be a limitation in the current study. The same cutoff values for MMSE score were used for all subjects, which could be considered as additional limitation. Finally in our PAMELA population, no neuroimaging data were available.
5. CONCLUSIONS
Our study suggests a possible relationship between BP and cognitive decline in the general population and provides evidence that the most sensitive prognostic variable for cognitive deterioration might not be the absolute BP or absolute BP variability but rather its short‐term individual residual component, which has been previously shown to represent the part of BP variability with major impact on left ventricular hypertrophy and cardiovascular mortality. However, further longitudinal studies are needed to investigate the potential predictive value of individual residual BP variability on cognitive dysfunction development in the general and hypertensive population, as well as the possible impact of antihypertensive therapy on the reduction of BP variability and improvement of cognitive function in hypertensive patients.
CONFLICT OF INTEREST
None.
Tadic M, Cuspidi C, Bombelli M, Facchetti R, Mancia G, Grassi G. Relationships between residual blood pressure variability and cognitive function in the general population of the PAMELA study. J Clin Hypertens. 2019;21:39–45. 10.1111/jch.13428
REFERENCES
- 1. Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord. 2016;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kherada N, Heimowitz T, Rosendorff C. Antihypertensive therapies and cognitive function: a review. Curr Hypertens Rep. 2015;17(10):79. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Bombelli M, Facchetti R, et al. Long‐term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49(6):1265‐1270. [DOI] [PubMed] [Google Scholar]
- 4. Yano Y, Ning H, Allen N, et al. Long‐term blood pressure variability throughout adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin B, Viera AJ, Muntner P, et al. Visit‐to‐visit variability in blood pressure is related to late‐life cognitive decline. Hypertension. 2016;68(1):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsang S, Sperling SA, Park MH, Helenius IM, Williams IC, Manning C. Blood pressure variability and cognitive function among older African Americans: introducing a new blood pressure variability measure. Cogn Behav Neurol. 2017;30(3):90‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sayed K, Taha TT, Saad M, et al. Is mini‐mental score examination scoring a new predictor of uncontrolled hypertension? J Clin Hypertens. 2014;16(5):348‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldstein S, Katzel L. Gender differences in the relation of hypertension to cognitive function in older adults. Neurol Res. 2004;26(5):502‐506. [DOI] [PubMed] [Google Scholar]
- 9. Kim M, Park JM. Factors affecting cognitive function according to gender in community‐dwelling elderly individuals. Epidemiol Health. 2017;39:e2017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grassi G, Bombelli M, Sega R, et al. The PAMELA (Pressioni Arteriose Monitorate E Loro Associazioni) Study. High Blood Press Cardiovasc Prev. 2007;14(2):83‐88. [Google Scholar]
- 11. WHO MONICA Project Principal Investigators . The World Health Organization MONICA project: a major international collaboration. J Clin Epidemiol. 1988;41:105e14. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens. 1995;13:1377‐1390. [PubMed] [Google Scholar]
- 13. Sega R, Corrao G, Bombelli M, et al. Blood pressure variability and organ damage in a general population: results from the PAMELA Study. Hypertension. 2002;39:710‐714. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. ‘Mini‐mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 15. Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K. Relationship between blood pressure variability and cognitive function in elderly patients with good blood pressure control. Am J Hypertens. 31(3), 293‐298. [DOI] [PubMed] [Google Scholar]
- 16. McDonald C, Pearce MS, Kerr SR, Newton JL. Blood pressure variability and cognitive decline in older people: a 5‐year longitudinal study. J Hypertens. 2017;35(1):140‐147. [DOI] [PubMed] [Google Scholar]
- 17. Epstein NU, Lane KA, Farlow MR, et al. Cognitive dysfunction and greater visit‐to‐visit systolic blood pressure variability. J Am Geriatr Soc. 2013;61(12):2168‐2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. [DOI] [PubMed] [Google Scholar]
- 19. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late‐life cognitive function: the Honolulu‐Asia Aging Study. JAMA. 1995;274:1846‐1851. [PubMed] [Google Scholar]
- 20. Farmer ME, White LR, Abbott RD, et al. Blood pressure and cognitive performance: the Framingham Study. Am J Epidemiol. 1987;126:1103‐1114. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi Y, Wada M, Sato H, et al. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community‐based elderly Japanese. Am J Hypertens. 2014;27:1257‐1267. [DOI] [PubMed] [Google Scholar]
- 22. Gomez‐Angelats E, De La Sierra A, Sierra C, Parati G, Mancia G, Coca A. Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypertens. 2004;17:696‐700. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain a 5‐year follow‐up. Neurology. 2005;64(11):1846‐1852. [DOI] [PubMed] [Google Scholar]


