Abstract
Salt has been linked very closely to the occurrence and complications of arterial hypertension. A large percentage of patients with essential hypertension are salt‐sensitive; that is, their blood pressure increases with increased salt intake and decreases with its reduction. For this reason, emphasis is placed on reducing salt intake to better regulate blood pressure. In day‐to‐day clinical practice this is viewed as mandatory for hypertensive patients who are judged to be salt‐sensitive. Previous studies have highlighted the negative effect of high‐salt diets on macrovascular function, which also affects blood pressure levels by increasing peripheral resistances. More recent studies provide a better overview of the pathophysiology of microvascular disorders and show that they are largely due to the overconsumption of salt. Microvascular lesions, which have a major impact on the functioning of vital organs, are often not well recognized in clinical practice and are not paid sufficient attention. In general, the damage caused by hypertension to the microvascular network is likely to be overlooked, while reversion of the damage is only rarely considered as a therapeutic target by the treating physician. The purpose of this review is to summarize the impact and the harmful consequences of increased salt consumption in the microvascular network, their significance and pathophysiology, and at the same time to place some emphasis on their treatment and reversion, mainly through diet.
Keywords: hypertension, non‐pharmacological therapy, sodium
1. INTRODUCTION
Essential hypertension is a very important modifiable risk factor for the occurrence of severe cardiovascular complications. However, apart from its effects on the large vessels, hypertension may lead, even in its initial stages, to small‐vessel disease and dysfunction.1 This phenomenon occurs in two main ways. First, by increasing the media‐to‐lumen diameter ratio of the small vessels and second, by causing a decrease in their density (rarefaction).2 This reduction concerns the arterioles and capillaries and worsens with the stage and severity of the hypertension.3
On the other hand, salt has been linked very closely with the occurrence and complications of hypertension.4, 5 It has been estimated that up to 50% of all individuals with essential hypertension are salt‐sensitive: that is, their blood pressure (BP) increases with increased salt intake and decreases with its reduction.4, 6 For this reason, emphasis is placed on reducing salt intake to better regulate BP. In everyday clinical practice this is considered mandatory for hypertensive patients who are judged to be salt‐sensitive. Previous studies have shown the negative effect of a high‐salt diet on macrovascular dysfunction, which also affects BP levels via an increase in peripheral resistances.7 In previous years this was considered the main mechanism of participation of the peripheral vasculature in essential hypertension, as was illustrated by experimental salt‐induced exacerbation of hypertension in animal studies.8 Several other studies provide a better picture of the pathophysiology of microvascular disorders due to the overconsumption of salt, which are often not sufficiently recognized in clinical practice.9, 10, 11, 12 In addition, despite the significant role of salt intake in microvascular dysfunction, very little attention has been paid in the literature to how therapeutic interventions may be able to modify these changes. Generally, the damage caused by hypertension to the microvascular network tends to be overlooked, while the possibility of reversion is only rarely considered as a therapeutic target by the treating physician.
The purpose of this review is to summarize the impact and the harmful consequences of increased salt consumption in the microvascular network, their significance and pathophysiology, and at the same time to place some emphasis on their treatment and reversion, mainly through diet.
2. MICROVASCULAR DYSFUNCTION ASSOCIATED WITH HIGH‐SALT INTAKE IN EXPERIMENTAL SETTINGS
Extensive experimental and clinical data have demonstrated the adverse effect of increased salt intake on the microvascular circulation (Table 1).7, 8, 9, 10, 11, 13, 14, 15, 16, 17 Increased salt consumption may provoke water retention that leads to high renal flow conditions. This high‐flow condition—as studies with animal models show—may induce adverse microvascular remodelling through increased mechanical load, such as hear stress and hemodynamic forces.18 Beyond this, however, there is plentiful experimental evidence showing την resulting microvascular dysfunction directly associated with high‐salt diet. Microvascular rarefaction, that is, a reduction in the number of arterioles and capillaries, is a frequent complication of organs in hypertension19 that is rarely examined or considered in clinical practice. Administration of a high‐salt diet for four weeks has been shown to induce rarefaction in skeletal muscle, specifically a reduction in capillary density.9, 10 Other experimental animal studies have shown that a high‐salt diet, apart from microvascular rarefaction,9, 10 also results in inward remodelling.9, 11 The extracellular matrix is also a significant determinant of various microvascular cellular functions, although these have not been completely defined. In addition, it appears that an increased salt intake can also affect the microvascular network adversely via other mechanisms, such as the potentiation of action of vasoconstrictors11, 20 or disturbances of endothelial Ca2+ signalling.21 Previous experimental studies have shown that hypoxia‐induced dilation in the mesenteric resistance arteries of rats fed a high‐salt diet is significantly impaired as a result of abnormally high production of 20‐hydroxyeicosatetraenoic acid (HETE),22 a vasoconstrictor metabolite of cytochrome P 450 4A ω‐hydroxylase. This may provide a pathogenetic pathway linking increased salt intake to microvascular dysfunction. The CYP4A pathway contributes significantly to the induction of vascular oxidant stress and the concomitant microvascular dysfunction in salt‐sensitive rats on a high‐salt diet.23
Table 1.
Overview of experimental data on the effects of salt on microcirculation
| Source | Animal model | Intervention | Summary of main findings |
|---|---|---|---|
| Boegehold et al7 | Dahl salt‐sensitive rats and Dahl salt‐resistant rats | High‐or normal‐salt diet for 4 wk | Increase in total peripheral resistance in the hypertensive Dahl rat, with the renal vasculature undergoing the largest resistance increase |
| Wu et al8 | Dahl salt‐sensitive rats and Dahl salt‐resistant rats | High‐salt–low‐potassium diet for 8 wk | Increased intra‐renal vessel remodeling in Dahl‐sensitive rats |
| Hansen‐Smith et al9 | Dahl salt‐sensitive rats and Dahl salt‐resistant rats | High‐salt–low‐potassium diet for 8 wks | Dahl salt rats present a greater degree of intra‐renal vessel remodeling |
| Hernandez et al10 | Rats | Angiotensin II infusion in rats on high‐salt diet | Angiotensin II blocked the rarefaction due to salt |
| Frisbee et al11 | Sprague Dawley rats with reduced renal mass hypertension | High‐salt or low‐salt diet and reduced for over 4 wk | Reduced arteriolar vasodilator reactivity developed with high‐salt diet and restored with low‐salt diet |
| Zhao et al14 | C57BL/6 mice with reduces renal mass | 3 mo of normal‐or high‐salt diet | High‐salt diet resulted in a lower arteriolar media/lumen area ratio |
| Bo et al15 | Young adults rats | Prenatal high‐salt diet | Vessel tension and intracellular Ca(2+) concentrations associated with phenylephrine‐induced pressor responses were increased in the mesenteric arteries of the high‐salt offspring. PKC α‐and δ‐isoforms were upregulated in mesenteric arteries of the high‐salt offspring |
| Rong et al16 | Rats | High‐salt vs normal‐salt diet for 4 wk | High‐salt intake upregulates the pro‐renin receptors expression in the glomeruli and proximal tubules and tubules of medullary rays in rat nephron |
| Weber et al20 | Sprague‐Dawley rats | High‐salt (or a low‐salt diet for 3 d (short‐term) or 4‐8 wk (chronic) | Chronic (but not short‐term) high‐salt diet selectively potentiated angiotensin II‐induced constriction of skeletal muscle resistance arteries |
| Zhu et al21 | Male Sprague‐Dawley rats | Low‐salt or high‐salt diet for 3 d with or without low‐dose angiotensin II infusion | Reduced circulating angiotensin II levels during elevated dietary salt lead to elevated superoxide levels, impaired endothelial Ca2+ signaling, and reduced NO production in the endothelium. |
| Wang et al22 | Rats | Short‐term (3‐d) high‐or low‐salt intake | High‐salt HS increased the expression of CYP450‐4A enzymes in the rat mesenteric vasculature, 20‐hydroxyeicosatetraenoic acid contributed to the vasoconstrictor response to norepinephrine in mesenteric resistance arteries which was greater in rats fed a high‐salt diet |
| Lukaszewicz et al23 | Dahl salt‐sensitive rats and salt sensitive Brown Norway rats | High‐salt or normal‐salt diet. Middle cerebral arteries were treated with N‐methyl‐sulfonyl‐12,12‐dibromododec‐11‐enamide, 15 (Z)‐20‐HEDE, N(G)‐nitro‐L‐arginine methyl ester, indomethacin or N‐(methylsulfonyl)‐2‐(2‐propynyloxy)‐benzenehexanamide | Pharmacological interventions that either inhibit the catalytic function of CYP4A enzymes or antagonize the actions of 20‐hydroxyeicosatetraenoic acid both restored vascular relaxation in cerebral resistance arteries of rats |
| Simon et al25 | Male Sprague‐Dawley rats | Treatment with angiotensin II subcutaneously for 4 or 12 wk on normal‐sodium diet or on high‐sodium diet | Angiotensin induced structural vascular changes and increased wall‐to‐lumen ratio that are dose‐and time‐dependent and synergistically enhanced by dietary sodium supplementation |
| Simon et al26 | Rats | 12 wk of treatment: angiotensin II; 2% NaCl diet; cold exposure; angiotensin II plus 2% NaCl diet; angiotensin II plus cold exposure; cold exposure plus 2% NaCl diet; angiotensin II plus 2% NaCl diet plus cold exposure; and control | Increase of blood pressure in angiotensin II‐treated plus salt‐fed rats. The blood pressure rise of the former group was accompanied by an increased wall‐to‐lumen ratio of cortical resistance arteries and decreased glomerular volume |
| Boegehold28 | Dahl salt‐sensitive rats | High‐or low‐salt diets for 4 wk | High‐salt intake reduced the passive diameter of large arterioles by 20% and was associated with increased tone of proximal, but not distal, arterioles |
3. MICROVASCULAR DYSFUNCTION ASSOCIATED WITH HIGH‐SALT INTAKE IN HUMAN STUDIES
Aside from experimental studies, various human studies have identified mechanisms of microvascular dysfunction in individuals with high‐salt intake. (Table 2) The proven harmful effect of increased dietary sodium intake on endothelial function has been demonstrated during the evaluation of cutaneous microvasculature in otherwise healthy adults, independently of changes in BP.29 Greaney et al has also reported that ingestion of a high Na+ diet for 7 days had no significant effect on BP, but reduced the dilator response of cutaneous arterioles to localized heating through a selective loss of the NO‐dependent component of this dilation.13 High dietary salt intake in healthy young persons is associated with reduced vascular NO bioactivity.13, 30 Intravenous sodium loading may cause an acute increase in microvascular permeability in healthy male subjects, attributable to adverse effects on the endothelial surface layer.31 Even short‐term salt intake reduces brachial artery endothelial function and switches the mediator of vasodilation in the microcirculation to a non–nitric‐oxide dependent mechanism, even in healthy adults.32
Table 2.
Parameters studied as markers of microvascular dysfunction in relation to salt intake
| Authors | Sample | Number of participants | Intervention | Drug treatment | Parameter studied | Findings |
|---|---|---|---|---|---|---|
| Greaney et al17 | Normotensive adults | 12 | Randomised to a 7 d low‐sodium or a 7 d high‐sodium diet | None | Nitric oxide (NO)‐dependent vasodilatation, maximal cutaneous vascular conductance | Dietary sodium loading impairs cutaneous microvascular function independently of blood pressure |
| Rorije et al31 | Normotensive males | 12 | A low‐sodium diet (less than 50 mmol/d) and a high‐sodium diet (more than 200 mmol/d) for 8 d in randomized order | None | Microvascular permeability | Acute intravenous sodium loading resulted in increased transcapillary escape rate of 125I‐labeled albumin whereas chronic dietary sodium loading did not affect it |
| Cavka et al32 | Young females | 54 | Randomised to either high‐salt diet (∼14 g/d NaCl) or low‐salt diet (<2.3 g/d) NaCl) for 7 d. | None | Post‐occlusive reactive hyperemia in skin microcirculation assessed by laser Doppler flowmetry | High‐salt diet significantly impaired post‐occlusive reactive hyperemia |
| Engelen et al36 | Patients with type 1 diabetes | 1,212 | Analyses of dietary salt intake | Insulin | Microalbuminuria | Higher dietary salt intake may be positively associated with microalbuminuria |
| He et al33 | White, black and asian patients with untreated mildly raised blood pressure | 71 whites, 69 blacks, and 29 Asians | 12‐wk randomised double‐blind crossover trial to determine the effect of a modest reduction in salt intake | None | Skin capillary density at the dorsum and the side of the fingers | Improvement in both functional and structural capillary rarefactions |
| Govoni et al34 | Healthy participants | 137 | 12‐wk randomised controlled trial to compliance or not to UK dietary guidelines | None | Skin microcirculation, measured by skin video‐capillaroscopy on the dorsum of the finger, number of capillaries perfused under basal conditions, number of anatomic capillaries perfused during finger cuff inflation | Adherence to dietary guidelines may help maintain a healthy microcirculation |
| Jablonski et al35 | Healthy participants | 17 | Randomised to a 4 wk of both low and normal‐sodium intake | None | NO/ tetrahydrobiopterin (BH(4) bioavailability, and oxidative stress | Low salt enhances NO and BH(4) bioavailability and reduces oxidative stress |
| Ekinci et al37 | Hypertensive patients with type 2 diabetes | 32 | Randomised to 100 mmol/d NaCl or placebo | Telmisartan | Albuminuria | Albumin excretion rate response to telmisartan under habitual low‐salt intake is blunted by NaCl supplementation |
| Hwang et al43 | Non‐diabetic hypertensive patients | 245 | Randomised to low‐salt diet education or intensive low‐salt diet education | Olmesartan | Albuminuria | 24‐h urinary albumin excretion was decreased more in patients in the intensive low‐salt diet education group |
| Han et al38 | Participants from the Korea National Health and Nutrition Examination Survey (KNHANES) | 5187 | No intervention‐24‐h urinary sodium excretion | Unknown | Albuminuria | Salt intake is associated with the presence of albuminuria |
| Fox et al44 | Participants from the Framingham Offspring Study | 2700 | No intervention‐24‐h urinary sodium excretion | Unknown | Albuminuria | Urinary albumin excretion was strongly and positively associated with 24‐h urinary sodium excretion |
| Konta et al45 | Japanese individuals from the general population | 2321 | No intervention‐24‐h urinary sodium excretion | Unknown | Albuminuria | Urinary albumin excretion was associated with 24‐h urinary sodium excretion |
| Aaron et al46 | African‐American and white adults | 21,636 | No intervention‐questionnaire for dietary habits | Unknown | Albuminuria | Higher dietary Na/K and sodium intakes were associated with albuminuria |
| Sakabe et al47 | Patients with type 2 diabetes | 270 | No intervention‐questionnaire for dietary habits | Unknown | Albuminuria | Low daily salt intake was correlated with albuminuria |
Also the activity of the renin angiotensin system can be modified, since high‐salt intake upregulates prorenin‐receptor expression in the nephron, which seems to be involved in the regulation of renal dysfunction and proteinuria. 17 A high‐salt intake has a negative influence on the homeostatic regulation of vascular bed in hypertensive patients, to a large degree via mechanisms that activate the local renin–angiotensin–aldosterone system in the vessels,19 or via pathways that inhibit NO production.27 Finally, increased generation of cyclo‐oxygenase (COX‐1 and COX‐2)‐derived vasoconstrictor factors and endothelial activation may contribute to impaired vascular relaxation during high‐salt loading and COX‐1 derived vasoconstrictor metabolites play an important role in the regulation of microvascular blood flow during a high‐salt diet.30
Figure 1 sketches the pathophysiological links between salt in essential hypertension and microvascular dysfunction as described above.
Figure 1.
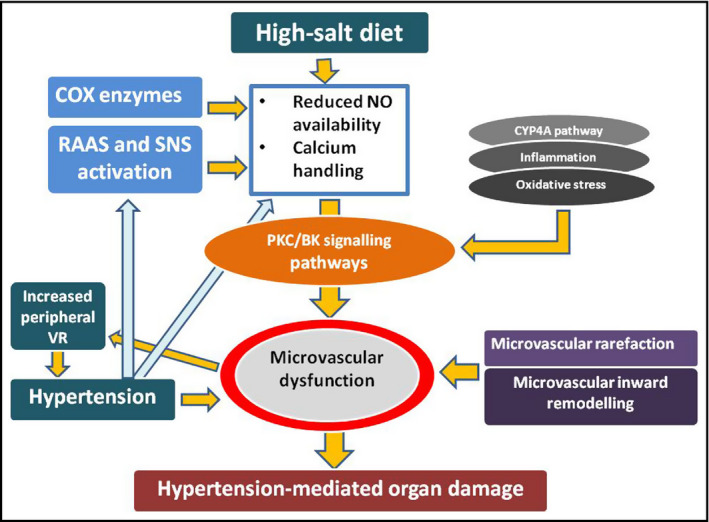
Pathophysiological links between salt consumption in essential hypertension and microvascular dysfunction. COX, cyclo‐oxygenase; CYP4A, cytochrome P450 4A; NO, nitric oxide; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system; VR, vascular resistance
4. DIETARY SALT INTAKE, MICROVASCULAR DISEASE, AND BLOOD PRESSURE CONTROL
Regardless of whether the patient is salt sensitive or not, the presence of microvascular dysfunction accompanies the pathogenetic processes of essential hypertension. Resistance arteries are key elements in blood pressure control and their remodelling plays a major role in the pathophysiology of hypertension. Microcirculatory dysfunction in patients with hypertension leads to the impairment of organ flow reserve, and thereby forms the basis of hypertension‐mediated organ damage. It is also a predictor of future cardiovascular events, and it has been demonstrated that hypertensive patients with an increased media‐to‐lumen diameter ratio of the small vessels in the subcutaneous tissue show greater mortality and morbidity over a 5‐6 year period.5
A diet containing large amounts of salt contributes to the increased incidence of the above pathophysiological processes. Our initial approach to regulating BP involves health and dietary interventions that include reducing sodium intake. Diuretics, which make up one of the main categories of antihypertensive drug, are aimed precisely at this mechanism: namely, the elimination of excess salt by the body. This plays an important role in clinical practice, primarily for hypertensive patients who are considered salt‐sensitive. However, little attention has been paid to the relationship between salt and microvascular dysfunction, which does not appear to be limited to salt‐sensitive hypertensives.
Decreasing salt consumption in hypertensive patients, even if it is not too high, has beneficial effects on the microcirculation by improving both functional and structural capillary rarefactions.33 In a 12‐week randomized controlled trial, skin microcirculation, measured by skin video‐capillaroscopy on the dorsum of the finger, and capillary recruitment (percentage difference between structural and functional capillary density) were beneficially affected by a low‐sodium diet (<6 g/d).34 Adherence to a healthy diet, including <5‐6 mg/d salt consumption, may help maintain a healthy microcirculation. Dietary sodium restriction confers a high degree of vascular protection, not only through its BP‐lowering effects, but also because it largely reverses microvascular endothelial dysfunction by enhancing NO, restoring tetrahydrobiopterin bioavailability and reducing oxidative stress.35 Other data from large clinical studies indicate that higher dietary salt intake in individuals with type 1 diabetes, as determined by 24‐hour urinary sodium excretion, may be positively associated with microalbuminuria, which is an index of microvascular dysfunction in the kidney, particularly in overweight individuals.36In contrast, the beneficial effect of angiostatin receptor inhibitors on microalbuminuria by may be blunted by salt supplementation,37 improving when intake is reduced.38 Table 1 shows the parameters studied as microvascular dysfunction markers in relation to salt intake. Clinical studies in this field are few and mainly aim at the study of microalbuminuria as a sign of microvascular damage to the kidney.
5. BP‐LOWERING EFFECTS FOLLOWING SALT CONSUMPTION MODIFICATION
Sodium intake is an important component of BP regulation in many hypertensive patients; reduced sodium intake could make a substantial contribution to the management of hypertension. The deleterious effects of a high‐salt diet are independent of elevated BP, regardless of salt‐sensitivity. They may occur even in normotensive individuals and are associated with impaired endothelial function.5
There is abundant evidence that a reduction in dietary sodium not only decreases the BP and the incidence of hypertension but also morbidity and mortality from CVD.39 However, the existing health policies have not been effective in achieving population goals for dietary sodium.39
INTERSALT, an international study of the relations of electrolyte excretion and other factors to blood pressure, involving more than 10,000 persons from 32 countries, has shown that the estimated mean change in systolic blood pressure associated with a 100‐mmol decrease in sodium intake was −3.1 mm Hg, with adjustment for age, sex, body mass index, and alcohol intake.6 The average BP change in a large metaanalysis of 40 sodium trials was −4.1/−2.5 mm Hg for a mean sodium reduction of 91 mmol/24 h, and this seems to be higher in older than in younger individuals.40 In addition, data from another metaanalysis show that increased potassium intake reduces BP in people with hypertension, in particular, the greatest decrease was seen in the group of studies with the highest sodium consumption (>4 g sodium/d).41
6. EFFECTS OF ANTIHYPERTENSIVE TREATMENT ON MICROVASCULAR DYSFUNCTION
Although pharmaceutical treatment in hypertension aims at improvement of hypertension‐mediated organ damage, insufficient attention has been given to its action on the microvascular net. Nonetheless, it appears that certain hypertension medication have a beneficial effect. To begin with, angiotensin converting enzyme inhibitors angiotensin receptor blockers have both proven their beneficial effect on improvement of microvascular dysfunction in essential hypertension.42 More specifically, medications that modify renin angiotensin aldosterone system activity but also calcium blockers φαίνεται seem to be much more effective in reducing media slash lumen ratio of small vessels compared to diuretics and beta blockers.42 In particular lercanidipine either as monotherapy for hypertension or in combination with enalapril, led to a significant improvement of retinopathy as well as media slash lumen ratio of microvessels.2 On the contrary, its beneficial effect disappeared with the co administration of diuretic. Also in combination with enalapril, it caused substantial improvement of the density of the microvascular net.2 Although not all agree,33, 43, 44 it appears that at the clinical level the importance of sodium intake is high, not only for hypertension‐mediated organ damage but also for the response to antihypertensive therapy.
It should be mentioned that in addition to hypertension treatment, hypolipidemic medications and statins in particular seem to have a beneficial effect on microcirculation even during acute administration48; this is recognized as an additional pleiotropic effect of statins.
7. CONCLUSIONS
The increase in BP with salt consumption shows great heterogeneity among hypertensive individuals. However, as a general rule it has a deleterious effect on the microcirculatory system that both creates future hypertensives, via an increase in peripheral resistances, and exacerbates the manifestations of hypertension. Salt consumption induces a systemic proinflammatory state while causing microvascular endothelial inflammation, anatomic remodelling and functional abnormalities, even in normotensive subjects.
Although some antihypertensive drugs appear to have a beneficial effect on the microvascular network, this is still largely ignored as a primary therapeutic goal and the main focus is on the macrovascular network. However, the microcirculation could be an important target and a possible increase in capillary density could reduce or improve hypertension‐mediated organ damage.
In clinical practice, instructions for a salt‐free diet are given when it is necessary to better control BP, while the proper importance is not assigned to the adverse effects of salt intake on the microcirculation. For this reason, it should be noted that a reduction in salt consumption is of great importance to everyone, regardless of whether they are salt‐sensitive or not.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Maria E. Marketou: conceived of the presented idea, wrote the paper, Spyros Maragkoudakis: Critical revision of the article, Ioannis Anastasiou: Wrote the paper, Helen Nakou: Critical revision of the article, Marina Plataki: Critical revision of the article, Panos E. Vardas: Critical revision of the article, Fragiskos I. Parthenakis: Critical revision of the article, final approval of the version to be published.
Marketou ME, Maragkoudakis S, Anastasiou I, et al. Salt‐induced effects on microvascular function: The fifth column of hypertension‐mediated organ damage. J Clin Hypertens. 2019;21:749–757. 10.1111/jch.13535
REFERENCES
- 1. Eftekhari AΕ, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KJ. Disproportionally impaired microvascular structure in essential hypertension. J Hypertens. 2011;29:896‐905. [DOI] [PubMed] [Google Scholar]
- 2. De Ciuceis C, Salvetti M, Rossini C, et al. Effect of antihypertensive treatment on microvascular structure, central blood pressure and oxidative stress in patients with mild essential hypertension. J Hypertens. 2014;32:565‐574. [DOI] [PubMed] [Google Scholar]
- 3. Prasad A, Dunnill GS, Mortimer PS, MacGregor GA. Capillary rarefaction in the forearm skin in essential hypertension. J Hypertens. 1995;13:265‐268. [PubMed] [Google Scholar]
- 4. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481‐490. [DOI] [PubMed] [Google Scholar]
- 5. Rizzoni D, Porteri E, Boari G, et al. Prognostic significance of small‐artery structure in hypertension. Circulation. 2003;108:2230‐2235. [DOI] [PubMed] [Google Scholar]
- 6. Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT study. II. Estimates of electrolyte‐blood pressure associations corrected for regression dilution bias. The INTERSALT cooperative research group. Am J Epidemiol. 1994;139:940‐951. [DOI] [PubMed] [Google Scholar]
- 7. Boegehold MA, Huffman LJ, Hedge GA. Peripheral vascular resistance and regional blood flows in hypertensive Dahl rats. Am J Physiol. 1991;261(4 Pt 2):R934‐R938. [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Scholey JW, Sonnenberg H, Melo LG. Renal vascular morphology and haemodynamics in Dahl salt‐sensitive rats on high salt‐low potassium diet: neural and genetic influences. J Hypertens. 2000;18:783‐793. [DOI] [PubMed] [Google Scholar]
- 9. Hansen‐Smith FM, Morris LW, Greene AS, Lombard JH. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res. 1996;79:324‐330. [DOI] [PubMed] [Google Scholar]
- 10. Hernandez I, Cowley Jr AW, Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol. 1992;263:H664‐H667. [DOI] [PubMed] [Google Scholar]
- 11. Frisbee JC, Lombard JH. Development and reversibility of altered skeletal muscle arteriolar structure and reactivity with high salt diet and reduced renal mass hypertension. Microcirculation. 1999;6:215‐225. [PubMed] [Google Scholar]
- 12. Jacobsen JC, Hornbech MS, Holstein‐Rathlou NH. Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus. 2011;1:117‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greaney JL, DuPont JJ, Lennon‐Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590:5519‐5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Gao Y, Cao X, et al. High‐salt diet induces outward remodelling of efferent arterioles in mice with reduced renal mass. Acta Physiol. 2017;219:652‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bo Le, Jiang L, Zhou A, et al. Maternal high‐salt diets affected pressor responses and microvasoconstriction via PKC/BK channel signaling pathways in rat offspring. Mol Nutr Food Res. 2015;59:1190‐1199. [DOI] [PubMed] [Google Scholar]
- 16. Rong R, Ito O, Mori N, et al. Expression of (pro)renin receptor and its upregulation by high salt intake in the rat nephron. Peptides. 2015;63:15662. [DOI] [PubMed] [Google Scholar]
- 17. Cavka A, Cosic A, Jukic I, et al. The role of cyclo‐oxygenase‐1 in high‐salt diet induced microvascular dysfunction in humans. J Physiol. 2015;593:5313‐5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dumont O, Pinaud F, Guihot AL, Baufreton C, Loufrani L, Henrion D. Alteration in flow (shear stress)‐induced remodelling in rat resistance arteries with aging: improvement by a treatment with hydralazine. Cardiovasc Res. 2008;77:600‐608. [DOI] [PubMed] [Google Scholar]
- 19. Boddi M, Poggesi L, Coppo M, et al. Human vascular renin–angiotensin system and its functional changes in relation to different sodium intakes. Hypertension. 1998;31:836‐842. [DOI] [PubMed] [Google Scholar]
- 20. Weber DS, Frisbee JC, Lombard JH. Selective potentiation of angiotensin II‐induced constriction of skeletal muscle resistance arteries by chronic elevations in dietary salt intake. Microvasc Res. 1999;57:310‐319. [DOI] [PubMed] [Google Scholar]
- 21. Zhu J, Drenjancevic‐Peric I, McEwen S, et al. Role of superoxide and angiotensin II suppression in salt‐induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol. 2006;291:H929‐H938. [DOI] [PubMed] [Google Scholar]
- 22. Wang J, Roman RJ, Falck JR, de la Cruz L, Lombard JH. Effects of high‐salt diet on CYP450‐4A omega‐hydroxylase expression and active tone in mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1557‐H1565. [DOI] [PubMed] [Google Scholar]
- 23. Lukaszewicz KM, Falck JR, Manthati VL, Lombard JH. Introgression of Brown Norway CYP4A genes on to the Dahl salt‐sensitive background restores vascular function in SS‐5(BN) consomic rats. Clin Sci. 2013;124:333‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez‐Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt‐sensitive hypertension. Hypertens Res. 2010;33:975‐980. [DOI] [PubMed] [Google Scholar]
- 25. Simon G, Illyes G, Csiky B. Structural vascular changes in hypertension: role of angiotensin II, dietary sodium supplementation, blood pressure, and time. Hypertension. 1998;32:654‐660. [DOI] [PubMed] [Google Scholar]
- 26. Simon G, Jackel M, Illyes G. Role of angiotensin II, sympathetic stimulation and salt in the development of structural vascular changes in rat kidney. Clin Exp Pharmacol Physiol. 2003;30:476‐481. [DOI] [PubMed] [Google Scholar]
- 27. Zhu J, Mori T, Huang T, Lombard JH. Effect of high‐salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol. 2004;286:H575‐H583. [DOI] [PubMed] [Google Scholar]
- 28. Boegehold MA. Microvascular changes associated with high salt intake and hypertension in Dahl rats. Int J Microcirc Clin Exp. 1993;12:143‐156. [PubMed] [Google Scholar]
- 29. Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574‐581. [DOI] [PubMed] [Google Scholar]
- 30. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525‐1530. [DOI] [PubMed] [Google Scholar]
- 31. Rorije N, Olde Engberink R, Chahid Y, et al. Microvascular permeability after an acute and chronic salt load in healthy subjects: a randomized open‐label crossover intervention study. Anesthesiology. 2018;128:352‐360. [DOI] [PubMed] [Google Scholar]
- 32. Cavka A, Jukic I, Ali M, et al. Short‐term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J Hypertens. 2016;34:676‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56:253‐259. [DOI] [PubMed] [Google Scholar]
- 34. Govoni V, Sanders T, Reidlinger DP, et al. Compliance with dietary guidelines affects capillary recruitment in healthy middle‐aged men and women. Eur J Nutr. 2017;56:1037‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jablonski KL, Racine ML, Geolfos CJ, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle‐aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engelen L, Soedamah‐Muthu SS, Geleijnse JM, et al. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: the EURODIAB prospective complications study. Diabetologia. 2014;57:2315‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekinci Ei, Thomas G, Thomas D, et al. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care. 2009;32:1398‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han SY, Hong JW, Noh JH, et al. Association of the estimated 24‐h urinary sodium excretion with albuminuria in adult Koreans: the 2011 Korea national health and nutrition examination survey. PLoS ONE. 2014;9:e109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whelton PK, He J. Health effects of sodium and potassium in humans. Curr Opin Lipidol. 2014;25:75‐79. [DOI] [PubMed] [Google Scholar]
- 40. Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens. 2003;17:471‐480. [DOI] [PubMed] [Google Scholar]
- 41. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agabiti‐Rosei E, Heagerty AM, Rizzoni D. Effects of antihypertensive treatment on small artery remodelling. J Hypertens. 2009;27:1107‐1114. [DOI] [PubMed] [Google Scholar]
- 43. Hwang Jh, Chin Hj, Kim S, et al. Effects of intensive low‐salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: a single‐blinded randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9:2059‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fox Cs, Larson Mg, Hwang S‐j, et al. Cross‐sectional relations of serum aldosterone and urine sodium excretion to urinary albumin excretion in a community‐based sample. Kidney Int. 2006;69:2064‐2069. [DOI] [PubMed] [Google Scholar]
- 45. Konta T, Hao Z, Abiko H, et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata Study. Kidney Int. 2006;70:751‐756. [DOI] [PubMed] [Google Scholar]
- 46. Aaron KJ, Campbell RC, Judd SE, Sanders PW, Muntner P. Association of dietary sodium and potassium intakes with albuminuria in normal‐weight, overweight, and obese participants in the Reasons for geographic and racial differences in stroke (REGARDS) study. Am J Clin Nutr. 2011;94:1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakabe K, Fukui M, Ushigome E, et al. Low daily salt intake is correlated with albuminuria in patients with type 2 diabetes. Hypertens Res. 2012;35:1176‐1179. [DOI] [PubMed] [Google Scholar]
- 48. Freitas F, Estato V, Reis P, et al. Acute simvastatin treatment restores cerebral functional capillary density and attenuates angiotensin II‐induced microcirculatory changes in a model of primary hypertension. Microcirculation. 2017;24:(8). [DOI] [PubMed] [Google Scholar]


