Abstract
As few epidemiological studies have investigated the effect of lifestyle factors on hypertension in the very elderly population, we conducted a cross‐sectional study to examine the association of estimated salt intake and body weight with blood pressure in the very elderly population. We enrolled 288 participants aged 75 years or older who were residents of Sukagawa City, Fukushima Prefecture, Japan, who attended the health checkup conducted in 2015. Salt intake was estimated from spot urine samples using the Tanaka method. The mean values for age, estimated salt intake, and body weight of all participants were 79.7 years, 9.1 g/d (standard deviation 2.4 g), and 54.3 kg (standard deviation 10.2 kg), respectively. General linear models showed that salt intake and body weight were associated with higher systolic blood pressure (SBP) levels (per standard deviation higher level, adjusted difference 4.13 mm Hg [95% confidence interval 1.69‐6.57] and 5.34 mm Hg [95% confidence interval 2.12‐8.56], respectively). Body weight was associated with higher diastolic blood pressure (DBP) levels (per standard deviation higher level, 2.74 mm Hg [95% confidence interval 0.58‐4.90]). However, salt intake was not associated with higher diastolic blood pressure levels (per standard deviation higher level, 1.15 mm Hg [95% confidence interval −0.49 to 2.79]). Our findings suggest that higher SBP is associated with both salt intake and body weight and that higher DBP is associated with body weight in the very elderly population. This study provides a rationale for lifestyle modifications to prevent hypertension as a population approach.
Keywords: body weight, hypertension, non‐pharmacological approach, salt intake, very elderly
1. INTRODUCTION
Hypertension is a common and critical public health problem in the elderly population. Its prevalence among individuals older than 75 years exceeds 75% in America1 and exceeds 70% in Japan.2 In addition, hypertension is a major cause of death, stroke, myocardial infarction, congestive heart failure, and chronic renal disease.3, 4, 5, 6 Pharmacological treatments of hypertension can be effective in reducing mortality even in the very elderly population. In the Hypertension in the Very Elderly Trial study,7 which focused on very elderly individuals older than 80 years (mean age: 84 years), antihypertensive treatment reduced the risks of non‐fatal and fatal stroke and death from cardiovascular causes. Despite accumulating evidence on the effectiveness of a pharmacological approach for hypertension, evidence on the effectiveness of a non‐pharmacological approach for the very elderly population is limited.
Among white adults with hypertension, adjusting salt intake from high to below recommended levels resulted in effective decreases in SBP/DBP.8 Among younger elderly individuals aged 60‐80 (mean age: 66.5) years, clinical trials have demonstrated that both weight loss and sodium reduction are effective for the treatment of hypertension.9 In the very elderly population, a small double‐blind, placebo‐controlled, crossover trial (n = 7, mean age = 85) demonstrated a salt‐dependent increase in blood pressure among patients with borderline hypertension living in a long‐term care facility.10 However, in that study, the effect of sodium intake reduction was only evident in diastolic blood pressure (DBP), with no evidence of an effect on systolic blood pressure (SBP); this finding was probably due to the small sample size. In addition, previous studies on weight loss as a non‐pharmacological treatment for hypertension are scarce among the very elderly population. Thus, quantifying the separate contribution of salt intake and body weight on blood pressure among the very elderly population is helpful for physicians and public health professionals. In this study, we aimed to conduct a community‐based, cross‐sectional study to examine the association of both salt intake and body weight with blood pressure in the very elderly population (age: ≥75 years).
2. METHODS
2.1. Setting
The Sukagawa study is a community‐based observational study that started in 2015 in Sukagawa City, which is located in the center of Fukushima Prefecture, Japan, and has a population of 77 000. The percentage of residents aged ≥65 years in Sukagawa City is 27.1%, which is almost the same as the national average value. The central area of Sukagawa City was selected as the study setting. This district has an estimated population of 21 000 (ie, approximately one‐third of the total population of Sukagawa City), 2711 of which are elderly (age: ≥75 years).
2.2. Participants
Through the mail, we invited the residents of the model district who were aged 75 years or older, excluding bedridden residents who were care levels 4 and 5 in the Japanese care insurance, to participate in the Sukagawa study and in a special health checkup. The respondents underwent a special health checkup. Measurements of physical health, blood pressure, spot sodium excretion, hearing and cognitive impairment, and frailty were conducted. A self‐report questionnaire survey was also administered. Participants who underwent the special health checkup and submitted a self‐report questionnaire were included.
2.3. Measurement of body weight and salt intake
The main exposures were salt (NaCl) intake and body weight. Height and body weight were measured using an automatic height and weight scale guaranteed by the Japanese Industrial Standards, and body mass index (BMI, kg/m2) was calculated. Daily salt intake was calculated using the Tanaka method11; it was estimated based on the calculation of 24‐hour sodium and creatinine levels from spot urine samples. The Tanaka method is recommended in the hypertension guideline because of its convenience and reliability.12 Estimation of daily salt intake (g/d) was calculated using the following formula:
In this formula, sodium and creatinine concentrations were determined from random spot urine samples collected in the morning.
2.4. Measurement of blood pressure
The primary outcomes were SBP and DBP measured at rest. Blood pressure was measured after 5 minutes of seated rest by trained health professionals with an automated upper arm blood pressure monitor (OMRON HEM‐906) using an appropriate cuff size. Given that blood pressure was measured twice only when SBP in the first measurement exceeded 130 mm Hg, values of SBP and DBP in the first measurement were analyzed for primary analyses.
2.5. Measurement of confounding variables
Confounding variables included in this study were age, sex, height, smoking status including ex‐smoker, physical activity, comorbidities including a history of cardiovascular and cerebrovascular diseases, diabetes mellitus, dyslipidemia, alcohol intake, and medication usage including antihypertensive agents or diuretics. Data on smoking status, physical activity, alcohol intake, medications, and history of cardiovascular and cerebrovascular diseases were obtained from the self‐reported questionnaire. Smoking status was defined as current smoker, former smoker, or never smoker depending on whether the patients smoked more than one cigarette per day, did not smoke but had smoked more than one cigarette per day, or had never smoked, respectively. Regular physical activity was defined as an exercise habit of more than once a week. A history of cardiovascular disease was determined if they had a past history of angina pectoris or myocardial infarction or they were under treatment for their disease. A history of cerebrovascular disease was determined if they had a past history of stroke. A history of renal disease was determined if they answered “yes” to the question, “Have you been told that you have renal disease at present?” Regular alcohol intake was determined if they consumed more than 20 g of alcohol per day (equivalent to 500 mL beer) more than once a week. Medication usage was determined if they answered “yes” to the question “Are you currently taking antihypertensive medications including diuretics?”
Diabetes mellitus was defined as having glycosylated‐hemoglobin values ≥6.5% (National Glycohemoglobin Standardization Program) or taking medications for diabetes mellitus in the self‐report questionnaire. Dyslipidemia was defined as a fasting low‐density lipoprotein cholesterol level of ≥140 mg/dL, a fasting triglyceride level of ≥150 mg/dL, a fasting high‐density lipoprotein cholesterol level of ≤40 mg/dL, according to the dyslipidemia guideline,13 or taking antihyperlipidemic drugs. Participants were instructed to walk along a smooth and horizontal walkway, in which the segment consists of a 10‐m length walkway for measurement and sections from either end for acceleration and deceleration. Walking time in the 10‐m section was measured twice using a stopwatch and was converted to m/s. The average value among the two was used for subgroup analysis.
2.6. Statistical analysis
Participants with measured exposure variables (estimated salt intake and body weight) and outcome variables (SBP/DBP) were included in the primary analyses. Demographic characteristics, estimated daily salt intake, body weight, height, and SBP/DBP of the participants were described. Age, body weight, height, BMI, SBP/DBP, and salt intake were reported as continuous variables and shown as mean values and standard deviation (SD). Sex, smoking status, medication, alcohol intake, physical activity, diabetes mellitus, dyslipidemia, and comorbidities, including a history of cardiovascular, cerebrovascular, and renal diseases, were reported as categorical variables and shown as numbers and proportions. The distribution of estimated salt intake and body weight is shown in a histogram.
We fitted a series of general linear models to examine the association of salt intake and body weight with SBP or DBP. For SBP and DBP, the models were fitted separately, and the two exposure variables and the confounders described above were simultaneously entered as explanatory variables. We estimated adjusted differences in SBP and DBP for 1 g difference in salt intake or 1 kg difference in body weight, respectively. In addition, to illustrate the magnitudes of the associations at the population level, the adjusted differences of SBP and DBP were redisplayed as those corresponding to a one SD increase in salt intake and body weight, respectively. Missing values of covariates, including smoking status, alcohol intake, physical activity, and comorbidity, were multiply imputed in the primary analysis, assuming that the values were missing at random.14 As a sensitivity analysis, we conducted a complete case analysis by excluding the cases without confounding variables. Further, among the primary analysis populations, we repeated analyses using all the blood pressure measurements (namely inclusion of the second blood pressure value) via generalized estimating equations. We also conducted ad hoc subgroup analyses by separately including interaction pairs (exposures [namely salt intake or body weight] × subgroup variable) to the original general linear models. Three subgroups were analyzed: (a) those taking antihypertensive drugs and those not taking antihypertensive drugs, (b) men and women, and (c) those with or without frailty determined by gait speed 1.0 m/s or lower. All statistical analyses were conducted using Stata®/SE version 15.0 (Stata Corp LP).
2.7. Ethical considerations
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Fukushima Medical University School of Medicine. Written consent to participate in the study was obtained from each participant.
3. RESULTS
3.1. Characteristics of the study participants
Two hundred ninety‐one residents responded and participated in the health checkup. We included 288 participants in the primary analysis after excluding three residents who lacked salt intake exposure (Figure 1). The number of participants with at least one missing covariate was 122, and the number of participants included in the sensitivity analysis was 166. Participant characteristics are shown in Table 1. The mean age of the patients was 79.7 (SD: 4.2) years, and 172 (59.7%) were women. The mean estimated salt intake was 9.1 g/d (SD 2.4 g/d, min: 2.8 g/d, max: 19.3 g/d), and the mean body weight was 54.3 kg (SD 10.2 kg) (men: 61.7 kg [SD 8.3 kg], women: 49.3 kg [SD 8.1 kg]). The mean BMI was 22.8 kg/m2 (SD 3.3 kg/m2), and the mean blood pressure was 144.4/75.4 mm Hg (SD 19.8/13.1 mm Hg). The prevalence of hypertension (defined as an SBP of ≥140 mm Hg or taking antihypertensive medications or diuretics) was 81.2% (234/288). The distributions of salt intake and body weight are shown in Figure 2. The proportion of participants with an estimated salt intake of <5 g/d, which is recommended by the World Health Organization,15 was 1.0% (3/288), and the proportion of those with an estimated salt intake of <8 g/d, which was recommended as a target value by the Japanese Ministry of Health and Welfare,16 was 33.7% (97/288). The proportion of those with a BMI ≥25 kg/m2 was 26.4% (76/288).
Figure 1.
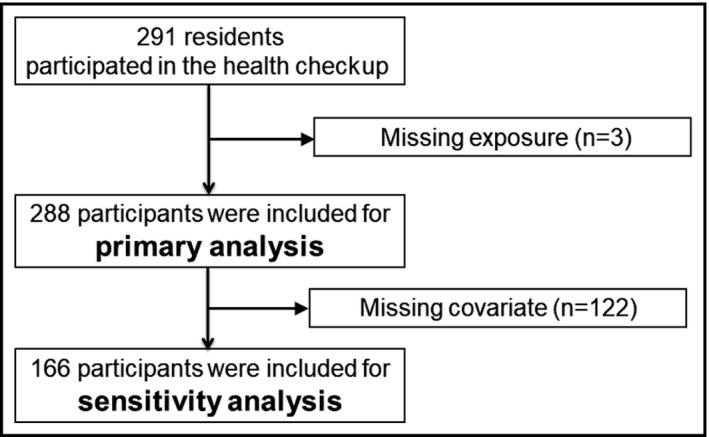
Flowchart of study participants
Table 1.
Characteristics of the population of the SUKAGAWA study
| Total | Missing, n | |
|---|---|---|
| N = 288 | ||
| Age, y | 79.7 (4.2) | |
| Female, n (%) | 172 (59.7) | |
| Body weight, kg | 54.3 (10.2) | |
| Height, cm | 153.9 (9.3) | |
| Body mass index, kg/m2 | 22.8 (3.3) | |
| Systolic blood pressure, mm Hg | 144.4 (19.8) | |
| Diastolic blood pressure, mm Hg | 75.4 (13.1) | |
| Salt intake, g/d | 9.1 (2.4) | |
| Comorbidities, n (%) | ||
| Dyslipidemia | 61 (31.6) | 95 |
| Diabetes | 23 (11.9) | 95 |
| History of cardiovascular disease | 43 (15.8) | 16 |
| History of cerebrovascular disease | 18 (6.6) | 16 |
| History of renal disease | 13 (4.5) | |
| Smoking, n (%)a | 91 (36.8) | 41 |
| Prescription of antihypertensive agents or diuretics, n (%) | 168 (58.3) | |
| Alcohol intake more than 20 g/d, n (%) | 74 (28.2) | 26 |
| Physical activity (regular exercise more than once a week), n (%) | 236 (89.1) | 23 |
| Gait speed more than 1 m/s, n (%) | 213 (74.7) | 3 |
Continuous variables are summarized as mean (standard deviation).
Current or former smoker (more than one cigarette per day)
Figure 2.
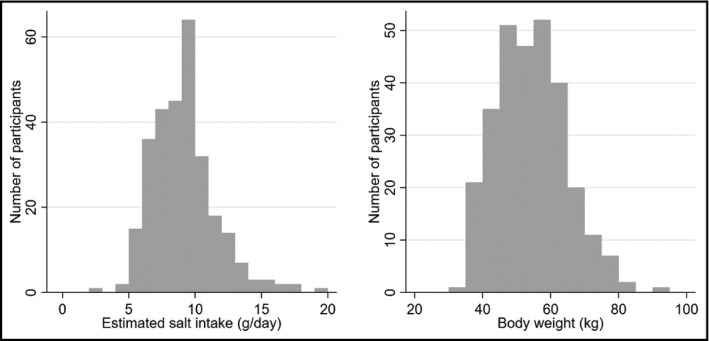
Distribution of estimated salt intake (g/d) and body weight (kg)
3.2. Association of estimated salt intake and body weight with blood pressure
One‐unit higher values in estimated salt intake (per g/d) and body weight (per kg) were associated with a higher SBP (adjusted difference 1.73 mm Hg [95% confidence interval {CI} 0.71‐2.76] and 0.52 mm Hg [95% CI 0.21‐0.84], respectively; Table 2). A one‐unit higher value in body weight (per kg) was also associated with a higher DBP (adjusted difference 0.27 mm Hg [0.06‐0.48]). However, a one‐unit higher value in salt intake was not associated with higher DBP (0.48 mm Hg [95% CI −0.21 to 1.17]). One SD higher values in estimated salt intake and body weight were associated with a higher SBP (adjusted difference 4.13 mm Hg [95% CI 1.69‐6.57] and 5.34 mm Hg [95% CI 2.12‐8.56], respectively; Table 2). A one SD higher value in body weight was also associated with a higher DBP (adjusted difference 2.74 mm Hg [95% CI 0.58‐4.90]), but a one SD higher value in salt intake was not associated with a higher DBP (1.15 mm Hg [95% CI −0.49 to 2.79]). In a complete case analysis, one‐unit higher values in estimated salt intake (per g/d) and body weight (per kg) were associated with a higher SBP (adjusted difference 2.3 mm Hg [95% CI 0.85‐3.75] and 0.64 mm Hg [95% CI 0.15‐1.13], respectively; see Table S1). A one‐unit higher value in body weight (per kg) was also associated with a higher DBP (adjusted difference 0.46 mm Hg [0.14‐0.78]), but a one‐unit higher value in salt intake was not associated with a higher DBP (0.31 mm Hg [95% CI −0.63 to 1.26]). The additional analyses using all the blood pressure measurements are shown in Table S2. The magnitudes of the associations are slightly smaller than those presented in Table 2.
Table 2.
Differences in systolic and diastolic blood pressure per unit per SD increase in salt intake and body weight (n = 288)
| SBP (mm Hg) | DBP (mm Hg) | |||||
|---|---|---|---|---|---|---|
| Mean difference | (95% CI) | P‐value | Mean difference | (95% CI) | P‐value | |
| Salt intake per 1 g | 1.73 | (0.71 to 2.76) | 0.001 | 0.48 | (−0.21 to 1.17) | 0.168 |
| per 1 SD gram | 4.13 | (1.69 to 6.57) | 1.15 | (−0.49 to 2.79) | ||
| Body weight per 1 kg | 0.52 | (0.21 to 0.84) | 0.001 | 0.27 | (0.06 to 0.48) | 0.013 |
| per 1 SD kg | 5.34 | (2.12 to 8.56) | 2.74 | (0.58 to 4.90) | ||
Estimated by general linear models adjusted for age, sex, height, smoking status, physical activity, comorbidity (cardiovascular, cerebrovascular, and renal diseases), diabetes mellitus, dyslipidemia, alcohol intake, and medication (antihypertensive agents and diuretics). Missing values were multiply imputed.
Bold value indicates significance at P < 0.05.
Abbrevaitions: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
The associations among participants with or without antihypertensive medications are shown in Table S3. The associations were similar between those with and without antihypertensive medications, as the interactions were not significant. The associations among the participants by participants' sex are shown in Table S4. The magnitude of the associations between body weight and SBP differed by sex; however, all point estimates of the associations between the exposures and SBP/DBP showed the same directions regardless of sex. The associations among participants with or without frailty determined by gait speed are shown in Table S5. The associations were similar between those with and without frailty, as the interactions were not significant.
4. DISCUSSION
Our findings suggested that higher values of both estimated salt intake and body weight gain in the very elderly population were associated with higher SBP values. The present findings are consistent with those reported in previous studies involving middle‐aged individuals or approximately 60‐70‐year‐old individuals. According to a global epidemiologic study targeting adults aged 55‐70 years in 18 countries,17 the increments in SBP and DBP for every 1 g of salt intake were 1.17 and 0.47 mm Hg, both of which were lower than increments observed in our study. A study of the Japanese adult population also showed lower increments in SBP and DBP for every 1 g of salt intake (1.52 and 0.35 mm Hg) compared to our findings.18 The greater increments in our study might be explained by the age‐dependent elevation in blood pressure in response to salt intake as was suggested in a meta‐analysis.19 Among the very elderly population, only one small, double‐blind, placebo‐controlled, crossover trial (n = 7, mean age = 85 years)10 examined the effects of salt reduction on lowering blood pressure. Despite the large difference in the amount of salt (equivalent of 7.8 g), the intervention led to a significant difference in only DBP. The failure to detect differences in SBP was probably owing to the small sample size. In contrast, we could show differences in SBP associated with the difference in salt intake but not differences in DBP. High aortic stiffness among our very elderly population may have caused the low range of DBP distribution, and owing to the small variability in DBP, associations between DBP and salt intake may be absent.
With regard to higher blood pressure being associated with a higher body weight, our study showed that a 1‐kg higher value in body weight was associated with a 0.5/0.2‐mm Hg higher value of SBP/DBP. This finding is in accordance with a previous study showing a 0.5 to 2‐mm Hg reduction in DBP for every 1 kg of weight loss among patients who were 30‐54 years old and were between 115% and 165% of their desired body weight.20 Considering the above‐mentioned results, the simultaneous presence of higher salt intake and body weight rather than salt intake or body weight alone might be associated with higher blood pressure levels. The blood pressure lowering effect of a combination of salt reduction and weight reduction was shown in the TONE trial targeting the younger elderly population.9 As our study differs from the TONE trial in terms of study design (observational) and target population (very elderly vs. younger elderly), the effect estimates among the studies are not comparable. Further study is warranted to determine whether the combination of salt intake and weight reduction has an additive effect on blood pressure among the very elderly population.
There are several clinical implications for clinicians and those involved in public health, such as policy makers and health professionals working in the community. First, the impact of blood pressure reduction and body weight reduction, even by a few numbers, is enormous in the perspective of a population approach. In the meta‐analysis of the effects of lowering blood pressure, each 5‐mm Hg reduction in blood pressure was associated with a 20% lower risk of coronary heart disease and a 29% lower risk of stroke in the population approach.19 With regard to the importance of body weight reduction, the overall mortality rose by 30% for each 5‐kg/m2 increase in a study pooling 57 prospective studies.21 Second, dietary sodium restriction is a non‐pharmacological approach applicable not only at the individual level but also at the community and national levels. A non‐pharmacological approach can be adopted not only in hospitals and clinics but also in communities or homes. For example, the effects of cooking classes in a community focusing on salt reduction could be effective in reducing the salt intake of housewives and their families.22 At the national level, one attempt to reduce the salt intake of a population was successfully made in the UK.23 The UK strategy involving voluntary agreement with the food industry to reduce salt content in processed foods, improvement in food labeling, and a public awareness campaign.24 This program was followed by a reduced salt intake and a decrease in blood pressure among the population from 2003 to 2011.25 Third, in the very elderly population, non‐pharmacological treatments are preferred over pharmacological treatments because of the high possibility of pharmacological adverse effects,26 low adherence due to polypharmacy,27 and the cost‐effectiveness.28 The American College of Cardiology/American Heart Association suggests that lifestyle modification may be all that is needed to treat milder forms of hypertension in elderly patients.29 However, reducing salt intake for reduction of blood pressure may be detrimental among frail elderly individuals. Indeed, one cohort study showed that among elderly individuals who could not complete a walking test, higher rather than lower blood pressure was associated with reduced mortality.30 Weight reduction is also regarded as a modifiable factor and the first line of treah obesity.31, 32, 33 In the TONE trial,9 a regimen of diminished caloric intake and increased physical activity was associated with a persistent weight loss of 4.7 kg, which led to a reduction in the SBP/DBP by 3.2/0.3 mm Hg as a sole effect of weight intervention. However, as the very elderly are prone to loss of muscle volume and function associated with body weight loss, further studies to examine the safety and feasibility of weight loss interventions are warranted among this population.
This study has several strengths. First, it analyzed a very elderly population (75‐90 years old) living in a community and was not limited to patients with hypertension. Inclusion of patients without hypertension allowed us to provide a basis for considering a preventive strategy for hypertension at the population level. Second, to our knowledge, this study is the first to show the magnitude of the associations of both salt intake and body weight with blood pressure among the very elderly population. In terms of similar magnitudes of higher blood pressure associated with one SD higher values of the two exposures, our study suggests that both salt intake and body weight are important targets for preventing hypertension at the population level.
Several limitations of the study must be mentioned. First, this was a cross‐sectional study; thus, it cannot attribute causality from the association of estimated salt intake and body weight with blood pressure. Second, although 24‐hour collection of urine is the gold standard to calculate daily salt intake, we estimated the salt intake by using the Tanaka formula as an alternative indicator of 24‐hour salt intake. However, many large epidemiological studies have adopted estimation methods using spot urine samples, such as the Tanaka method34 and the Kawasaki method17 because of the ease of urine collection and participant recruitment. In addition, the Tanaka method has been validated in other observational studies and is a less biased method than other methods.35 Finally, Japanese hypertension guidelines endorse the Tanaka method for clinical use.12 Third, our findings were obtained from the attendees of the health checkup and may be subject to selection bias. However, we believe that our participants are representative of elderly residents living in local cities in Japan to some degree, considering the results that summary measures of exposure and outcome among the participants were similar to those of the national survey in Japan. According to the national survey of the Japanese Ministry of Health and Welfare, the mean salt intake estimated from the dietary record was 10.8 and 9.3 g/d for men and women aged 70 years or older, respectively.36 The mean salt intake (9.1 g/d) in our study was similar to the values from the national survey. Regarding summary measures for the outcomes, means and SDs of the SBP and DBP were 146.6 mm Hg (SD: 19.6) and 79.6 mm Hg (SD: 11.9), respectively, in the community (≥70 years old) in the national survey,37 whereas they were 144.4 mm Hg (SD: 19.8) and 75.4 mm Hg (SD: 13.1), respectively, in our study. The results of this study may be applicable to the relatively healthy elderly population who can independently perform activities of daily living. Fourth, despite the high prevalence of individuals taking antihypertensive medications, information on antihypertensive medication was inclusively collected along with that on diuretics via a questionnaire. We think some antihypertensive drugs with natriuretic properties could be a confounder, but other antihypertensive drugs could not be confounders (please note that a confounder requires correlation with exposure [namely salt intake in this study]).38 Expected bias due to failure of complete adjustment of diuretics is underestimation of the association between salt intake and blood pressure in this study because those with higher salt excretion in the urine are more likely to be taking antihypertensive drugs with natriuretic properties, and these contribute to lowering of blood pressure levels. Therefore, if data on the class of antihypertensive drugs and their dose are collected separately and are completely adjusted, the magnitude of the association between salt intake and blood pressure may be intensified compared to that presented in this study. Fifth, renal function was not obtained. Although we adjusted for participants who answered to “yes” to the question, “Have you been told that you have renal disease at present?”, some participants may have had mild undiagnosed impairment in renal function.
5. CONCLUSIONS
Our findings suggested that higher estimated salt intake levels and higher weight are associated with higher SBP. The study provided evidence and rationale for lifestyle modification not only for hypertensive management but also for hypertension prevention at the population level. However, the actual efficacy of lifestyle modification is unknown. Further research is needed to clarify whether reduction of salt intake and body weight lowers blood pressure among this population.
CONFLICT OF INTEREST
There is no conflict of interest to declare.
AUTHOR CONTRIBUTION
H.I. and N.K. conceived and designed this study, conducted statistical analysis, interpreted data, and drafted the article. S.T., S.S., H.N., K.O., N.Y., S.K., K.N., T.N., and R.T. acquired data, interpreted data, and revised the article. S.Fukuma, T.H., and S.Fukuhara interpreted data and revised the article critically for important intellectual content. All the authors approved this study finally.
Supporting information
ACKNOWLEDGMENTS
We thank the participants of the Sukagawa study, the government officers in the Department of Healthcare and Welfare, Sukagawa City, who conducted the health checkups, and other members of the Sukagawa Study Group including Susumu Kobayashi, Kakuya Niihata, Toru Naganuma, and Ryoji Tominaga. Full details of the members are provided in the Supplemental Text S1.
Iida H, Kurita N, Takahashi S, et al; The Sukagawa Study Group . Salt intake and body weight correlate with higher blood pressure in the very elderly population: The Sukagawa study. J Clin Hypertens. 2019;21:942–949. 10.1111/jch.13593
Iida and Kurita equally contributed to this work.
Funding information
The health checkup was funded by the government of Sukagawa City. This work was supported by JSPS KAKENHI (Grant Number: JP15K00877).
Contributor Information
Noriaki Kurita, Email: kuritanoriaki@gmail.com.
The Sukagawa Study Group:
Susumu Kobayashi, Kakuya Niihata, Toru Naganuma, and Ryoji Tominaga
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29‐e322. [DOI] [PubMed] [Google Scholar]
- 2. Miura K, Nagai M, Ohkubo T. Epidemiology of hypertension in Japan: where are we now? Circ J. 2013;77:2226‐2231. [DOI] [PubMed] [Google Scholar]
- 3. Havas S, Roccella EJ, Lenfant C. Reducing the public health burden from elevated blood pressure levels in the United States by lowering intake of dietary sodium. Am J Public Health. 2004;94:19‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritz E, Koleganova N, Piecha G. Role of sodium intake in the progression of chronic kidney disease. J Ren Nutr. 2009;19:61‐62. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937‐952. [DOI] [PubMed] [Google Scholar]
- 6. O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112‐123. [DOI] [PubMed] [Google Scholar]
- 7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887‐1898. [DOI] [PubMed] [Google Scholar]
- 8. Graudal NA, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839‐846. [DOI] [PubMed] [Google Scholar]
- 10. Palmer RM, Osterweil D, Loon‐Lustig G, Stern N. The effect of dietary salt ingestion on blood pressure of old‐old subjects. A double‐blind, placebo‐controlled, crossover trial. J Am Geriatr Soc. 1989;37:931‐936. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97‐103. [DOI] [PubMed] [Google Scholar]
- 12. Kawano Y, Tsuchihashi T, Matsuura H, et al. Report of the working group for dietary salt reduction of the japanese society of hypertension: (2) Assessment of salt intake in the management of hypertension. Hypertens Res. 2007;30:887‐893. [DOI] [PubMed] [Google Scholar]
- 13. Teramoto T, Sasaki J, Ishibashi S, et al. Diagnostic criteria for dyslipidemia. J Atheroscler Thromb. 2013;20:655‐660. [DOI] [PubMed] [Google Scholar]
- 14. Sterne J, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO . Salt reduction. WHO. http://www.who.int/mediacentre/factsheets/fs393/en/. Accessed March 18, 2018.
- 16. https://www.mhlw.go.jp/stf/houdou/0000041733.html (In Japanese).
- 17. Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601‐611. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free‐living population‐based dietary modification study in Japan. J Hypertens. 2006;24:451‐458. [DOI] [PubMed] [Google Scholar]
- 19. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the trials of hypertension prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849‐858. [PubMed] [Google Scholar]
- 21. Collaboration PS, Whitlock G, Lewington S, et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takada T, Imamoto M, Fukuma S, et al. Effect of cooking classes for housewives on salt reduction in family members: a cluster randomized controlled trial. Public Health. 2016;140:144‐150. [DOI] [PubMed] [Google Scholar]
- 23. He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363‐384. [DOI] [PubMed] [Google Scholar]
- 24. Millett C, Laverty AA, Stylianou N, Bibbins‐Domingo K, Pape UJ. Impacts of a national strategy to reduce population salt intake in England: serial cross sectional study. PLoS ONE. 2012;7:e29836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He FJ, Pombo‐Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014;4:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population‐based study in the elderly. Am J Hyperten. 1997;10:697‐704. [DOI] [PubMed] [Google Scholar]
- 27. Pasina L, Brucato AL, Falcone C, et al. Medication non‐adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging. 2014;31:283‐289. [DOI] [PubMed] [Google Scholar]
- 28. Webb M, Fahimi S, Singh GM, et al. Cost effectiveness of a government supported policy strategy to decrease sodium intake: global analysis across 183 nations. BMJ. 2017;356:i6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037‐2114. [DOI] [PubMed] [Google Scholar]
- 30. Odden Michelle C, Peralta Carmen A, Haan Mary N, Covinsky Kenneth E. Rethinking the association of high blood pressure with mortality in elderly adults. Arch Intern Med. 2012;172(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. [DOI] [PubMed] [Google Scholar]
- 32. Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205S–213S. [DOI] [PubMed] [Google Scholar]
- 33. Masuo K, Mikami H, Ogihara T, Tuck ML. Differences in mechanisms between weight loss-sensitive and -resistant blood pressure reduction in obese subjects. Hypertens Res. 2001;24:371–376. [DOI] [PubMed] [Google Scholar]
- 34. Jensen PN, Bao TQ, Huong T, et al. The association of estimated salt intake with blood pressure in a Viet Nam national survey. PLoS ONE. 2018;13:e0191437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mill JG, Rodrigues SL, Baldo MP, Malta DC, Szwarcwald C. Validation study of the Tanaka and Kawasaki equations to estimate the daily sodium excretion by a spot urine sample. Rev Bras Epidemiol. 2015;18(Suppl 2):224–237. [DOI] [PubMed] [Google Scholar]
- 36. http://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou.pdf (in Japanese).
- 37. http://www.mhlw.go.jp/toukei/kouhyo/data-kou18/data12/junkan-h12-3.pdf (in Japanese).
- 38. VanderWeele TJ, Shpitser I. On the definition of a confounder. Ann Stat. 2013;41:196–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


