Abstract
High salt intake is known to increase blood pressure (BP) and also to be associated with carotid‐femoral pulse wave velocity (cf‐PWV). However, recent data showed a sex‐specific pattern in the salt‐induced rise of BP. Thus, we aimed to investigate whether the association between salt intake and arterial stiffness also has a sex‐specific pattern. A total of 7755 normotensive participants with a validated 12‐h overnight urine collection in which daily salt intake was estimated were included. cf‐PWV, as well as clinical and anthropometric parameters, was measured. Salt intake positively correlated with cf‐PWV, in which the linear regression was steeper in women than in men (0.0199 ± 0.0045 vs 0.0326 ± 0.0052 m/s per gram of salt, P < .05). cf‐PWV increases over the salt quartiles in men and women. However, after adjustment for confounders, the association remained significant only for men. In the path analysis, the direct path (men: 0.048 P < .001, women: 0.029 P = .028) was higher in men while that mediated by SBP (men: 0.020 P < .001, women: 0.034 P < .001) was higher in women. We clearly demonstrated that high salt intake has a direct and independent effect increasing arterial stiffness regardless of sex. Also, the association between salt intake and arterial stiffness is more dependent on BP in normotensive women than it is in normotensive men. These results highlight the need for a sex‐specific approach in the evaluation of cardiovascular risk associated with dietary habits.
Keywords: arterial stiffness, blood pressure, mediation, salt intake, sex differences
1. INTRODUCTION
Several epidemiological and experimental studies showed that high salt intake is associated with higher blood pressure (BP) levels.1, 2 The PURE study, for instance, showed in a large sample of participants from different countries that salt intake is associated with high BP levels and cardiovascular diseases, mainly in those consuming over 6 g of salt per day.3, 4 Also, high salt intake was linked to increased risk of cardiovascular events in hypertensive individuals.5
However, the high salt intake has been related to other deleterious effects on cardiovascular system regardless of its effect on BP. These effects include endothelial dysfunction, ventricular hypertrophy, arterial and ventricular stiffening, cardiac arrhythmias, and heart failure.2, 6 Clinical and experimental studies have consistently demonstrated that the increased salt intake impairs the endothelial function and changes the arterial structure.6, 7 A recent meta‐analysis evaluated eleven studies and showed that salt restriction decreases arterial stiffness. The authors hypothesized that such effect might be caused, at least partially, by the effect of salt increasing BP.8 However, beyond its direct effect on BP, increased dietary salt consumption can augment arterial stiffness independently of increases in BP levels.9, 10, 11
We have recently showed that changes in BP levels in response to salt intake are higher in women than in men.12 However, if this differential response to increased salt intake might also have a sex‐specific influence on arterial stiffness is still undetermined. Thus, we decided to investigate in a large sample of Brazilian normotensive adults whether the direct and the mediated effects of salt intake on arterial stiffness are different between men and women.
2. METHODS
2.1. Study and participants
The ELSA‐Brasil cohort included 15,105 adults, 35‐74 years old at baseline (2008‐2010), and all civil servants of public universities or research institutions in six Brazilian capitals. Detailed description on the study design and the main characteristics of the cohort was published elsewhere.13, 14 The ethic committees of the six study holding institutions approved the research protocol, and all participants signed written consent before data collection. For the present analysis, we included only normotensive participants (BP <140/90 mm Hg without use of BP‐lowering drug including diuretics) without self‐reported previous cardiovascular disease (myocardial infarction, stroke, congestive heart failure, coronary revascularization, including use of vascular stents). Salt consumption was estimated according to the overnight (12 hours) sodium excretion on the eve of BP and carotid‐to‐femoral pulse wave velocity (cf‐PWV) measurements.
2.2. Clinical assessment
After enrollment in the ELSA‐Brasil cohort, all participants were invited to a single visit to one of the six investigation centers responsible for data acquisition. Clinical and anthropometric data and laboratory tests were carried out in the morning period. Self‐reported medical history, medications under regular use, and sociodemographic information were recorded during an interview. The race/skin color of each participant was self‐declared according to the IBGE standards of white, black, brown, and others (indigenous [native Brazilian] or Asiatic). Laboratory examinations were performed in a venous blood sample collected in 10‐14 h fasting. Samples were processed by a central laboratory.15, 16
Bodyweight (Toledo Scale, Brazil) and height (Seca Stadiometer) were obtained by trained technicians, and the body mass index (BMI) was calculated as the ratio between weight and the squared height (kg/m2). Overweight was considered when BMI was 25‐29.9 kg/m2, and obesity was defined as BMI ≥30 kg/m2. Diabetes mellitus was defined as having at least one of the following: fasting blood glucose ≥126 mg/dL; 2‐hour postload plasma glucose ≥200 mg/dL or ≥6.5% of glycated hemoglobin; or the use of glucose‐lowering drugs. BP and heart rate were measured in the seated position after a 5‐min rest period. Three consecutive readings were obtained with an oscillometric device (Omron 765CP, Japan) with nearly 1‐min interval between readings in a quiet and temperature‐controlled (20‐24°C) room.15 The mean of the two last measures of BP and heart rate were calculated. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration formula.17
2.3. Pulse wave velocity assessment
Participants were positioned in the supine position, and cf‐PWV was measured using a validated automatic device (Complior; Artech Medicale) following standard protocols.18, 19 Briefly, with participants in the supine position, BP was recorded in the right arm using a validated oscillometric device (HEM Omron 705CP). Pulse waveforms were captured by sensors placed at the right carotid and femoral arteries. The direct distance from the suprasternal notch to the right femoral site where the pulse was recorded was measured with a metric tape. cf‐PWV was calculated by dividing the distance from the suprasternal notch to the femoral site by the time delay between the carotid and the femoral pulse waves, and it was expressed in m/s. The individual value was automatically recorded as the average of the measurements that were obtained in 10 consecutive cardiac cycles recorded under regular cardiac rhythm.
2.4. Urine collection
Participants were previously contacted and received oral and written instructions about the procedures for the 12‐h overnight urine collection. The collection kit included a large, wide‐opened bottle (2 liters), an instruction form, and a handling bag. Participants were asked to void in the toilet at approximately 7:00 pm and then to collect all the urine in the bottle thereafter until the next morning. The last urine collection should be obtained approximately 12 hours after the last void in the toilet. Participants were asked to record in a form the exact times indicating the beginning and end of urine collection and to report any potential urine loss during the collecting period. They were also instructed to keep the collected urine in a refrigerator and to bring it to the investigation center within 2 hours after the last urine collection. Some participants collected the last void just before BP and anthropometric measurements and blood collection. Upon arriving at the investigation center, the participants were questioned about the exact time of the beginning and end of the collection period. Aliquots were sampled in sterile vials and stored at −80°C until evaluation for the concentrations of creatinine (Jaffé method), sodium and potassium (selective electrodes). Validation of the 12‐h urine collection was based on three criteria as described elsewhere.12 The urinary loss of creatinine, sodium, and potassium was adjusted to 12 hours. Based on a validation study in adults and assuming that all excreted sodium derives from NaCl intake, the 24‐h sodium excretion was calculated using the following equation: Na24‐h (mg) = 1614.1 + 1.39 × Na12‐h night (mg), indicating that the 12‐h night sodium excretion corresponds to nearly 47% of the 24‐h sodium excretion.20, 21
2.5. Statistical analysis
Data are described as the mean ± standard deviations. The adequacy to a normal distribution was evaluated with the Kolmogorov‐Smirnov test. Frequencies and proportions were compared with chi‐squared test. Comparisons between two means were performed with the unpaired Student t test. Analysis of variance (ANOVA) was used to compare differences among quartiles of estimated salt intake, and in case of a significant F test, individual differences were assessed applying Bonferroni's post hoc correction. Analysis of covariance (ANCOVA) was used to test the association between estimated salt intake and cf‐PWV. Different models were created based on progressive inclusion of covariables and were followed by Bonferroni's post hoc correction.
Multivariate path analysis was used to test a hypothetical model (Figure 1) linking estimated salt intake to cf‐PWV directly or by mediating paths through systolic BP (SBP) or eGFR. The arrows represent a direct effect which are independent of the other variables in the path model, or indirect effects mediating associations. The model was further adjusted by age, BMI, uric acid, K+ excretion, albuminuria, and fasting glycemia based on their proved association with cf‐PWV.22, 23, 24, 25, 26, 27 For parameter estimation, we used the maximum‐likelihood procedure and a root mean square error of approximation (RMSEA) <0.03 indicated a very good model fit in the proposed model.
Figure 1.
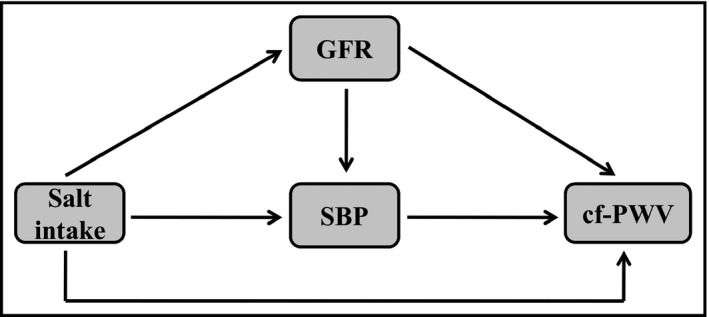
Hypothetical model to explain the association between salt intake and cf‐PWV through direct or mediating paths
Statistical analysis was performed using R software (version 3.5.1) with semPlot (path diagrams and visual analysis of various SEM packages' output, v. 1.1) and Lavaan (latent variable analysis, v. 0.6‐3) packages for path analysis procedures. Statistical significance was set at P < .05.
3. RESULTS
3.1. General characteristics
A total of 7755 normotensive participants with validated urine collection and cf‐PWV measurement were included in the present analysis. The sample comprised 55.7% of women, with mean age of 49.8 ± 8.4 years. Except for BMI and LDL cholesterol, all other clinical and anthropometric variables were different between men and women (Table 1). Diabetes was more prevalent in men than in women (13.5% vs 8.2%, P < .001), while obesity was more frequent in women (12.6% vs 16.2%, P < .001). Daily estimated sodium and potassium excretion were higher in men than in women. Also, the estimated daily salt intake was significantly higher in men than in women (12.3 ± 5.5 vs 9.0 ± 4.0 g/day, P < .05). Table 1 also shows that the hemodynamic parameters were higher in men than in women and that arterial stiffness assessed by cf‐PWV was also higher in men (9.2 ± 1.5 vs 8.5 ± 1.4 m/s, P < .05).
Table 1.
Clinical and anthropometric characteristics of normotensive individuals stratified by sex
|
Men (n = 3431) |
Women (n = 4324) |
P value |
All (n = 7755) |
|
|---|---|---|---|---|
| Age (y) | 49.6 ± 8.6 | 50.0 ± 8.2 | .064 | 49.8 ± 8.4 |
| Weight (kg) | 77.7 ± 12.6 | 66.0 ± 12.2 | <.001 | 71.2 ± 13.7 |
| Height (cm) | 172.7 ± 7.0 | 159.6 ± 6.4 | <.001 | 165.4 ± 9.3 |
| BMI (kg/m2) | 26.0 ± 3.7 | 25.9 ± 4.4 | .270 | 25.9 ± 4.1 |
| Uric acid (mg/dL) | 6.2 ± 1.3 | 4.5 ± 1.0 | <.001 | 5.3 ± 1.4 |
| Glucose (mg/dL) | 111.3 ± 27.3 | 103.2 ± 17.6 | <.001 | 106.8 ± 22.8 |
| Cholesterol (mg/dL) | 213.0 ± 41.6 | 215.9 ± 40.9 | .003 | 214.6 ± 41.2 |
| HDL (mg/dL) | 51.1 ± 12.0 | 62.9 ± 14.5 | <.001 | 57.6 ± 14.7 |
| LDL (mg/dL) | 133.0 ± 34.4 | 131.2 ± 34.2 | .019 | 132.3 ± 34.3 |
| TG (mg/dL) | 149.9 ± 114.7 | 109.8 ± 74.9 | <.001 | 127.5 ± 96.7 |
| GFR (mL/min per 1.73 m2) | 84.3 ± 14.1 | 103.6 ± 11.4 | <.001 | 95.1 ± 15.9 |
| Na+ excretion (mg/d) | 4839 ± 2159 | 3534 ± 1563 | <.001 | 4111 ± 1961 |
| K+ excretion (mg/d) | 2599 ± 1062 | 2132 ± 836 | <.001 | 2338 ± 971 |
| Salt intake (g/d) | 12.3 ± 5.5 | 9.0 ± 4.0 | <.001 | 10.5 ± 5.0 |
| cf‐PWV (m/s) | 9.2 ± 1.5 | 8.5 ± 1.4 | <.001 | 8.8 ± 1.4 |
| SBP (mm Hg) | 118.3 ± 10.1 | 111.2 ± 11.4 | <.001 | 114.3 ± 11.4 |
| DBP (mm Hg) | 74.9 ± 7.6 | 70.8 ± 8.0 | <.001 | 72.6 ± 8.1 |
| HR (bpm) | 68.6 ± 10.1 | 71.3 ± 9.3 | <.001 | 70.1 ± 9.7 |
| Diabetes (n, %) | 465 (13.5) | 357 (8.2) | <.001 | 822 (10.6) |
| Overweight (n, %) | 1476 (43.0) | 1462 (33.8) | <.001 | 2938 (37.9) |
| Obesity (n, %) | 434 (12.6) | 703 (16.2) | <.001 | 1137 (14.6) |
| Race (n, %) | ||||
| White | 1900 (55.4) | 2453 (56.7) | .242 | 4353 (56.1) |
| Brown | 1004 (29.3) | 1086 (25.1) | <.001 | 2090 (26.9) |
| Black | 371 (10.8) | 585 (13.5) | <.001 | 956 (12.3) |
| Other | 156 (4.5) | 200 (4.6) | .913 | 356 (4.6) |
Abbreviations: BMI, body mass index; cf‐PWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TG, triglycerides.
3.2. Association between estimated salt intake and cf‐PWV
When clinical and anthropometric characteristics were stratified by estimated salt intake quartiles, hemodynamic variables changed significantly from the lowest to the highest quartile of estimated salt intake. Also, cf‐PWV increased over the estimated salt intake quartiles (Table 2). We detected a positive significant correlation between estimated salt intake and cf‐PWV, regardless of sex (Figure 2, A for men and C for women), in which the slope of the linear regression was steeper in women than in men (Men: 0.0199 ± 0.0045 vs Women: 0.0326 ± 0.0052 m/s per gram of salt intake, P < .05; All participants: 0.0440 ± 0.0033). We also observed that cf‐PWV increases as the estimated salt intake quartiles increase in men and women. After adjusting for age and SBP, the association was reduced mainly in women (Table 3). However, after fully adjustment for covariates, the association was kept significant only for men (Figure 2, B for men and D for women, Table 3). We also tested the association between estimated salt intake and cf‐PWV stratified by race. After pertinent adjustment for confounders, no racial differences were found. Also, the correlation between cf‐PWV and salt intake had similar slope regardless of race (data not shown).
Table 2.
Clinical and anthropometric characteristics displayed by quartiles of salt intake in the entire sample
| Estimated salt intake (n = 7755) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Age (y) | 49.3 ± 8.4 | 49.9 ± 8.4 | 50.3 ± 8.4* | 49.7 ± 8.2 | .001 |
| Weight (kg) | 67.6 ± 12.6 | 70.1 ± 13.3* | 72.0 ± 13.4* , ** | 75.7 ± 14.2* , ** , *** | <.001 |
| Height (cm) | 164.9 ± 9.2 | 165.4 ± 9.3 | 165.3 ± 9.4 | 166.2 ± 9.4* , ** , *** | <.001 |
| BMI (kg/m2) | 24.8 ± 3.8 | 25.5 ± 3.9* | 26.3 ± 4.1* , ** | 27.3 ± 4.3* , ** , *** | <.001 |
| Uric acid (mg/dL) | 5.3 ± 1.4 | 53.2 ± 1.4 | 5.3 ± 1.4 | 5.3 ± 1.4 | .786 |
| Glucose (mg/dL) | 104.6 ± 16.3 | 105.0 ± 19.2 | 107.9 ± 25.9* , ** | 110.4 ± 28.2* , ** , *** | <.001 |
| Cholesterol (mg/dL) | 213.9 ± 40.2 | 214.9 ± 40.0 | 214.7 ± 41.8 | 214.9 ± 43.2 | .862 |
| HDL (mg/dL) | 58.1 ± 14.7 | 57.9 ± 15.1 | 57.3 ± 14.2 | 57.1 ± 14.4 | .105 |
| LDL (mg/dL) | 132.7 ± 34.4 | 132.5 ± 34.1 | 132.0 ± 34.6 | 130.6 ± 34.1 | .257 |
| TG (mg/dL) | 117.7 ± 71.0 | 123.6 ± 77.0 | 129.5 ± 89.0* | 141.4 ± 140.1* , ** , *** | <.001 |
| GFR (mL/min per 1.73 m2) | 95.2 ± 16.0 | 95.0 ± 15.9 | 94.7 ± 16.0 | 95.3 ± 15.7 | .707 |
| K excretion (mg/d) | 1961 ± 842 | 2194 ± 843* | 2454 ± 949* , ** | 2819 ± 1049* , ** , *** | <.001 |
| cf‐PWV (m/s) | 8.7 ± 1.4 | 8.8 ± 1.4* | 8.9 ± 1.4* | 9.0 ± 1.4* , ** | <.001 |
| SBP (mm Hg) | 112.7 ± 11.3 | 113.9 ± 11.4* | 114.9 ± 11.2* , ** | 115.9 ± 11.3* , ** | <.001 |
| DBP (mm Hg) | 71.5 ± 8.1 | 72.2 ± 8.0* | 72.9 ± 8.0* , ** | 74.0 ± 7.9* , ** , *** | <.001 |
| HR (bpm) | 70.0 ± 10.1 | 69.7 ± 9.5 | 70.3 ± 9.6 | 70.6 ± 9.8** | .039 |
Abbreviations: BMI, body mass index; cf‐PWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TG, triglycerides.
P < .05 vs Q1.
P < .05 vs Q2.
P < .05 vs Q3.
Figure 2.
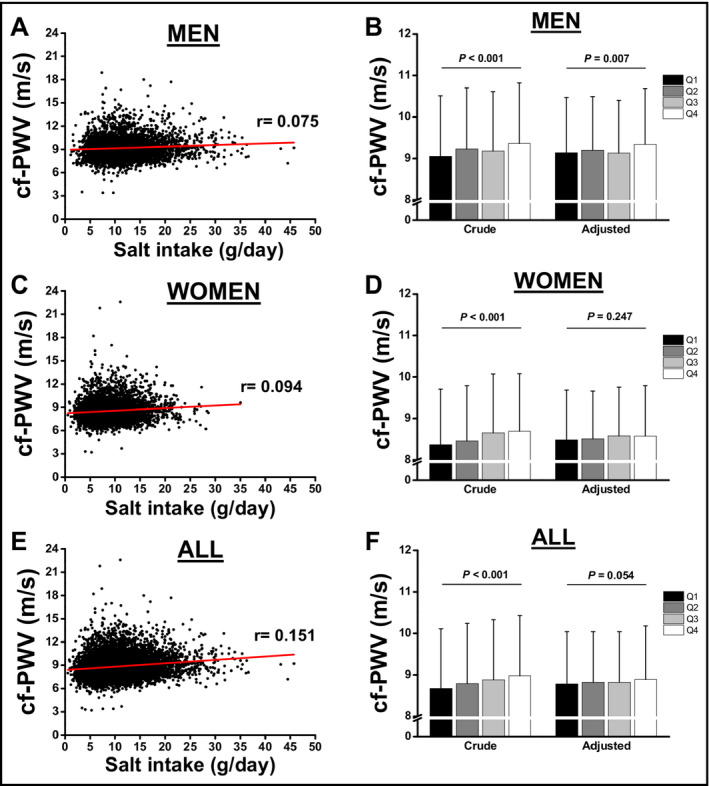
Pearson correlation analysis between salt intake and cf‐PWV in men (A) and women (C). All participants are represented at panel E and F. Analysis of covariance for the association between cf‐PWV according to quartiles (Q) of salt intake in men (B) and women (D). Association was adjusted for age, SBP, BMI, eGFR, fasting glucose, uric acid, albuminuria, and K+ excretion
Table 3.
Analysis of covariance for the association between cf‐PWV according to quartiles of salt intake in men and women
| Men | P value | Women | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| Crude | 9.05 ± 1.46 | 9.23 ± 1.47 | 9.18 ± 1.43 | 9.36 ± 1.45 | ≤.001 | 8.37 ± 1.34 | 8.46 ± 1.33 | 8.65 ± 1.42 | 8.69 ± 1.39 | ≤.001 |
| Model 1 | 9.13 ± 1.30 | 9.19 ± 1.29 | 9.14 ± 1.27 | 9.35 ± 1.30 | .002 | 8.47 ± 1.16 | 8.50 ± 1.19 | 8.58 ± 1.18 | 8.59 ± 1.19 | .043 |
| Model 2 | 9.12 ± 1.30 | 9.19 ± 1.29 | 9.14 ± 1.30 | 9.36 ± 1.30 | .001 | 8.49 ± 1.19 | 8.51 ± 1.15 | 8.57 ± 1.18 | 8.57 ± 1.19 | .232 |
| Model 3 | 9.14 ± 1.33 | 9.20 ± 1.29 | 9.13 ± 1.27 | 9.34 ± 1.35 | .007 | 8.48 ± 1.19 | 8.51 ± 1.15 | 8.57 ± 1.18 | 8.57 ± 1.22 | .247 |
Model 1 was adjusted by age and SBP; Model 2 was adjusted by Model 1 + BMI and uric acid; Model 3 was adjusted by Model 2 + fasting glucose, eGFR, and K excretion.
3.3. Path analysis for direct and mediating effects
We next investigated the direct and indirect (mediated) pathways by which increased salt intake would contribute to stiffen large arteries. A hypothetical model was established based on previous reports 22, 23, 24, 25, 26, 27, 28 and is shown in Figure 1. Based on this hypothetical model, estimated salt intake could increase cf‐PWV by a direct path, and three other mediating paths (through SBP, eGFR, or through eGFR and SBP). The final model was adjusted by age, BMI, uric acid, K+ excretion, albuminuria, and fasting blood glucose. For men (Figure 3A) and women (Figure 3B), the direct path and that mediated by SBP were statistically significant at the final adjusted model, and those nonsignificant mediated pathways were kept in the model but are not shown in Figure 3. As observed in Figure 3, the total effect (direct and mediated effects) was similar between men and women (0.068 vs 0.063). However, when we compared the magnitude of the direct and the mediated effects using nonstandardized estimates, the indirect effect of estimated salt intake on arterial stiffness mediated by BP was higher in women than in men (Figure 4). The direct effect was higher in men, but it did not reach statistical significance. The summary of direct and mediating effects is presented in Table 4.
Figure 3.
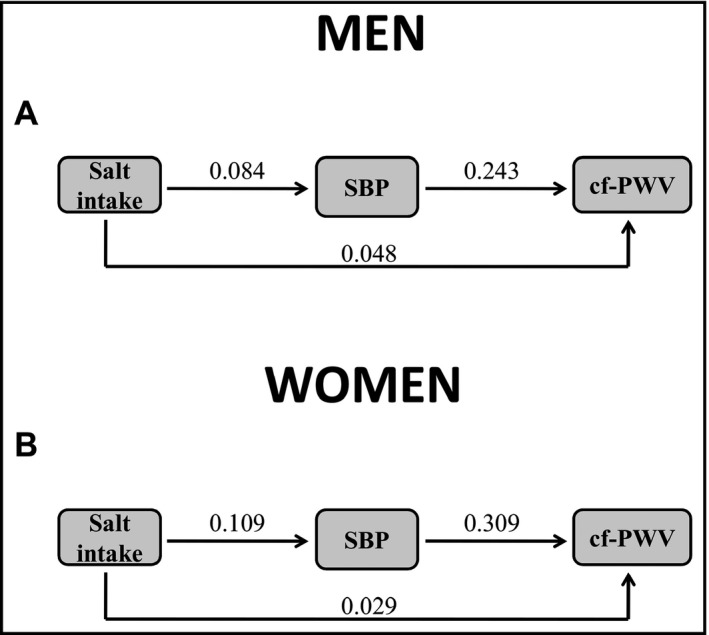
Path models for the direct and mediating effects of salt intake on cf‐PWV in men (A), women (B). The values on the arrows represent statistical significant standardized coefficients
Figure 4.
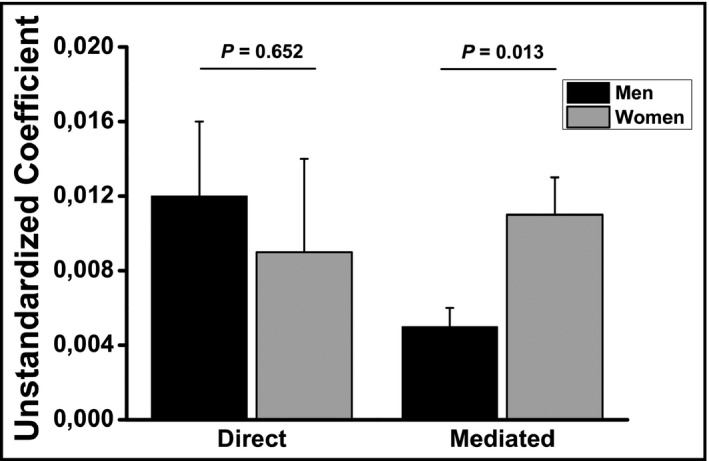
Unstandardized coefficients for the direct and mediated effects of salt intake on arterial stiffness in normotensive individuals
Table 4.
Summary of direct, mediated, and total effects for the association between salt intake and cf‐PWV in men and women
| Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | Mediated | Total | Direct | Mediated | Total | ||||||
| Std. Coef. | P | Std. Coef. | P | Std. Coef. | P | Std. Coef. | P | Std. Coef. | P | Std. Coef. | P |
| 0.048 | <.001 | 0.020 | <.001 | 0.068 | <.001 | 0.029 | .028 | 0.034 | <.001 | 0.063 | <.001 |
To test whether the results would be influenced by age strata, we performed path analysis stratified by age intervals (<50 or ≥50 years). Both direct and mediated effects increase in men and women, regardless of age group. However, in women, the direct effect increased more than the mediated effect, even that the mediated effect in women was higher than in men (data not shown).
4. DISCUSSION
In the present study, we showed that increased estimated salt intake is associated with arterial stiffness in normotensive men and women. However, the pathway by which increased salt consumption stiffens large arteries seems to have a sex‐specific pattern, being arterial stiffness in women more dependent on BP levels than in men. To the best of our knowledge, this is the first report showing the sex‐specific pattern in the association between estimated salt intake and arterial stiffness using a mediating analysis.
We and other authors have previously demonstrated a progressive elevation in BP according to increasing salt intake.1, 3, 12 Based on this well‐established association, we decided to investigate if the increased estimated salt intake would influence arterial stiffness once it acts directly increasing BP levels. It is noteworthy that the relationship between BP and arterial stiffness works as a vicious cycle in which increases in BP levels lead to arterial stiffening, which in turn contributes to elevate BP even more, creating a self‐generated cycle toward vascular remodeling and hypertension.29 Our data showed that increased estimated salt intake contributes to a progressive elevation in cf‐PWV, corroborating previous reports attesting the influence of salt intake on arterial stiffening.30 A meta‐analysis gathering 431 participants from 11 studies showed that a mean reduction of 2 g/day of sodium intake led to approximately 3% reduction in arterial stiffness.8 This effect, however, was partially explained by the direct reduction in BP caused by reduction in salt intake. However, this issue is still under debate as Liu et al31 showed a significant inverse association between sodium excretion and carotid and aortic augmentation indexes, and Redelinghuys et al32 observed that association between Na+/K + ratio and central pulse pressure cannot be explained by arterial stiffness.
Besides the well‐known direct effect of salt on BP, the BP‐independent effect of increased sodium intake on the vascular wall has been reported in other experimental and clinical studies.7, 33 In fact, the association between sodium ion and BP was thought to be exclusive based on the increase in blood flow and increasing vascular resistance. However, several experimental models and clinical studies have proved the BP‐independent effect of a high salt diet.34 Experimental studies have reported apoptosis, cellular senescence, oxidative stress, and inflammatory activation mediated by high sodium intake in the endothelium and in the vascular wall.2 Clinical studies have also described some BP‐independent salt action, such as ventricular hypertrophy, endothelial dysfunction, and arterial stiffness,35, 36 mainly caused by renin‐angiotensin‐aldosterone system activation.37
As the association between salt intake and arterial stiffness could be influenced by some other variables, we controlled for several confounding variables (including SBP). After appropriated adjustment, the association between salt intake and arterial stiffness remained statistically significant only for men. This is likely explained by the fact that women have a steeper increase in BP with rising salt intake than that observed in men, as we have recently demonstrated.12 To properly address this issue, we used the multivariate path analysis to define the direct and mediating effects that could build the complete (direct and mediating effects) association between salt and arterial stiffness. We showed that the association between salt and arterial stiffness has a strong dependency on the mediating effect of BP in women than in men. A recent study by Siriopol et al38 has addressed this issue. However, there are important differences between Siriopol's study and ours. Contrary to the use of both normotensive and hypertensive participants as in the Siriopol's study, we decided to use only normotensive participants as the cross‐sectional design would difficult to establish the cause‐and‐effect relationship between PWV and hypertension. Also, based on the large sample size of our study, it was possible to make analyses stratified by sex to reveal sex‐specific differences which would be abrogated after controlling for sex.
Our path analysis showed that neither estimated GFR nor albuminuria were associated with estimated salt intake, thus a mediated pathway through them does not explain the association between salt intake and cf‐PWV. A divergent data were presented by Triantafyllou et al39 in which salt intake primarily affect the kidney than other vascular bed. A growing body of evidence has shown a potential influence of race in the association between salt, BP, and arterial stiffness. Strauss et al40 have recently showed a racial difference in the association between salt intake and arterial stiffness that also corroborates the previous report by He and cols.41 in which a modest salt reduction was associated with reduced cf‐PWV only in blacks. In our study, after controlling for confounders (SBP included), we found no evidence that the effect of estimated salt intake on arterial stiffness varies by race in men or in women. This result corroborates a previous study by our group in which racial differences in cf‐PWV were blunted after controlling for BP levels.18
Only a few studies have addressed the sex differences in the response of BP to a high salt intake. However, to the best of our knowledge, there is no study to date investigating sex‐specific differences in the association between salt intake and arterial stiffness. Authors attributed the influence of sex hormones as a possible explanation for these sex differences.42, 43, 44 Conversely, this hypothesis has not been confirmed yet. In our study, we adjusted all associations by age to avoid any influence of aging and, as stated as limitation, at certain point would indicate menopausal status. Although the mechanisms for this sex‐specific effect are not well established, clinical and experimental studies have proposed associations between sex hormones. The GenSalt study 45 showed that BP responses to high salt intake were greater in women over 45 years and those with a higher baseline BP level. Our data showed that women over 50 years have increased both direct and indirect effects on arterial stiffness, indicating that aging has an important effect on vascular structure in women. The influence of age on the association of BP and salt intake was reported by Myers and Morgan46 showing that salt intake restriction causes a more pronounced reduction in BP in participants over 50 years, but no sex difference was detected. We showed that there is a strong association between salt and BP, being the main pathway by which salt increases cf‐PWV in women, but not in men. Another possible explanation might lay on the fact that women have lower BP than men, and the rise in BP by an increased salt consumption would be more intense in women, starting the vascular remodeling and contributing to stiffen large arteries in women.
We can list some strengths of our study. The first one is the large sample size of normotensive individuals (n = 7755) that allowed subgroup analyses. Also, our theoretical model was constructed taking into consideration the physiological aspects involved in the relationship between salt intake and arterial stiffening, and the majority of the studies support the same directions for the proposed association. Moreover, some limitations of our study should not be ruled out. We used a 12‐h urine collection instead of using the gold‐standard method to estimate the daily sodium intake (a 24‐h urine collection). Also, due to the cross‐sectional design of this study, results cannot provide any direct causal relationship. Thus, our decision to use only normotensive participants was based on the difficult to establish the causal direction between a pre‐established hypertension and arterial stiffness. Another limitation lays on the absence of any other variable to indicate menopausal status. Finally, although our multivariate model was adjusted for confounders, some missing confounders might have influence on the relationship between salt and PWV.
In conclusion, our data clearly demonstrated that the association between high salt intake and arterial stiffness has a higher dependency of BP increases in normotensive women when compared to normotensive men. Also, high salt intake has a direct and independent effect increasing the arterial stiffness regardless of sex, confirming experimental studies in which excessive sodium consumption leads to vascular remodeling. These results highlight the need for a sex‐specific approach in the evaluation of cardiovascular risk associated to dietary habits.
CONFLICT OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
AUTHOR CONTRIBUTIONS
MPB, LCCB, RSC, and MCBM participated in the analysis and interpretation of data, and drafting the manuscript article; RHG, SMB, PAL, IMB and JGM participated to the conception and design, analysis and interpretation of data, and the final approval of the version to be published; MPB, RHG, SMB, PAL, IMB and JGM participated in the conception and design, analysis and interpretation of data, drafting of the article and critical revision for important intellectual content, and the final approval of the version to be published.
ACKNOWLEDGMENTS
We would like to thank all ELSA‐Brasil participants for their valuable contribution to this study.
Baldo MP, Brant LCC, Cunha RS, et al. The association between salt intake and arterial stiffness is influenced by a sex‐specific mediating effect through blood pressure in normotensive adults: The ELSA‐Brasil study. J Clin Hypertens. 2019;21:1771–1779. 10.1111/jch.13728
Funding information
The ELSA‐Brasil study was supported by the Brazilian Ministry of Health (Department of Science and Technology) and Ministry of Science, Technology and Innovation (FINEP, Financiadora de Estudos e Projetos), grants no. 01 06 0010.00, 01 06 0212.00, 01 06 0300.00, 01 06 0278.00, 01 06 0115.00, and 01 06 0071.00 and CNPq (the National Council for Scientific and Technological Development).
REFERENCES
- 1. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldo MP, Rodrigues SL, Mill JG. High salt intake as a multifaceted cardiovascular disease: new support from cellular and molecular evidence. Heart Fail Rev. 2015;20:461‐474. [DOI] [PubMed] [Google Scholar]
- 3. Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601‐611. [DOI] [PubMed] [Google Scholar]
- 4. O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612‐623. [DOI] [PubMed] [Google Scholar]
- 5. Mente A, O'Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465‐475. [DOI] [PubMed] [Google Scholar]
- 6. Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;24:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kusche‐Vihrog K, Schmitz B, Brand E. Salt controls endothelial and vascular phenotype. Pflugers Arch. 2015;467:499‐512. [DOI] [PubMed] [Google Scholar]
- 8. D'Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta‐analysis of the randomized controlled trials. J Hypertens. 2018;36:734‐743. [DOI] [PubMed] [Google Scholar]
- 9. Safar M, Laurent S, Safavian A, Pannier B, Asmar R. Sodium and large arteries in hypertension. Effects of indapamide. Am J Med. 1988;84:15‐19. [PubMed] [Google Scholar]
- 10. Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202‐210. [DOI] [PubMed] [Google Scholar]
- 11. Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O'Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166‐169. [DOI] [PubMed] [Google Scholar]
- 12. Mill JG, Baldo MP, Molina M, et al. Sex‐specific patterns in the association between salt intake and blood pressure: the ELSA‐Brasil study. J Clin Hypertens. 2019;21:502‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aquino EM, Barreto SM, Bensenor IM, et al. Brazilian longitudinal study of adult health (ELSA‐Brasil): objectives and design. Am J Epidemiol. 2012;175:315‐324. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt MI, Duncan BB, Mill JG, et al. Cohort profile: longitudinal study of adult health (ELSA‐Brasil). Int J Epidemiol. 2015;44:68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mill JG, Pinto K, Griep RH, et al. Medical assessments and measurements in ELSA‐Brasil. Rev Saude Publica. 2013;47(Suppl 2):54‐62. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt MI, Griep RH, Passos VM, et al. Strategies and development of quality assurance and control in the ELSA‐Brasil. Rev Saude Publica. 2013;47(Suppl 2):105‐112. [DOI] [PubMed] [Google Scholar]
- 17. Veronese FV, Gomes EC, Chanan J, et al. Performance of CKD‐EPI equation to estimate glomerular filtration rate as compared to MDRD equation in South Brazilian individuals in each stage of renal function. Clin Chem Lab Med. 2014;52:1747‐1754. [DOI] [PubMed] [Google Scholar]
- 18. Baldo MP, Cunha RS, Ribeiro ALP, et al. Racial differences in arterial stiffness are mainly determined by blood pressure levels: results from the ELSA‐Brasil study. J Am Heart Assoc. 2017;6:e005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baldo MP, Cunha RS, Molina M, et al. Carotid‐femoral pulse wave velocity in a healthy adult sample: The ELSA‐Brasil study. Int J Cardiol. 2018;251:90‐95. [DOI] [PubMed] [Google Scholar]
- 20. Mill JG, Silva AB, Baldo MP, Molina MC, Rodrigues SL. Correlation between sodium and potassium excretion in 24‐ and 12‐h urine samples. Braz J Med Biol Res. 2012;45:799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues SL, Souza Junior PR, Pimentel EB, et al. Relationship between salt consumption measured by 24‐h urine collection and blood pressure in the adult population of Vitoria (Brazil). Braz J Med Biol Res. 2015;48:728‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baena CP, Lotufo PA, Mill JG, Cunha Rde S, Bensenor IJ. Serum uric acid and pulse wave velocity among healthy adults: baseline data from the Brazilian longitudinal study of adult health (ELSA‐Brasil). Am J Hypertens. 2015;28:966‐970. [DOI] [PubMed] [Google Scholar]
- 23. Brunner EJ, Shipley MJ, Ahmadi‐Abhari S, et al. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension. 2015;66:294‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high‐normal glucose levels and in subjects with pre‐diabetes. Cardiovasc Diabetol. 2011;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Y, Byon CH, Yang Y, et al. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight. 2017;2(19): 94920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wen W, Luo R, Tang X, et al. Age‐related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis. 2015;238:147‐152. [DOI] [PubMed] [Google Scholar]
- 27. Grupper A, Schwartz D, Berliner S, et al. Normal‐range albuminuria in healthy subjects increases over time in association with hypertension and metabolic outcomes. J Am Soc Hypertens. 2018;12:759‐767. [DOI] [PubMed] [Google Scholar]
- 28. Hermans MM, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the hoorn study. J Am Soc Nephrol. 2007;18:1942‐1952. [DOI] [PubMed] [Google Scholar]
- 29. AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore longitudinal study of aging. Hypertension. 2013;62:934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polonia J, Maldonado J, Ramos R, et al. Estimation of salt intake by urinary sodium excretion in a Portuguese adult population and its relationship to arterial stiffness. Rev Port Cardiol. 2006;25:801‐817. [PubMed] [Google Scholar]
- 31. Liu YP, Thijs L, Kuznetsova T, et al. Central systolic augmentation indexes and urinary sodium in a white population. Am J Hypertens. 2013;26:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Redelinghuys M, Norton GR, Scott L, et al. Relationship between urinary salt excretion and pulse pressure and central aortic hemodynamics independent of steady state pressure in the general population. Hypertension. 2010;56:584‐590. [DOI] [PubMed] [Google Scholar]
- 33. Grigorova YN, Wei W, Petrashevskaya N, et al. Dietary sodium restriction reduces arterial stiffness, vascular TGF‐beta‐dependent fibrosis and marinobufagenin in young normotensive rats. Int J Mol Sci. 2018;19(10): E3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frohlich ED, Varagic J. The role of sodium in hypertension is more complex than simply elevating arterial pressure. Nat Clin Prac Cardiovasc Med. 2004;1:24‐30. [DOI] [PubMed] [Google Scholar]
- 35. Safar ME, Temmar M, Kakou A, Lacolley P, Thornton SN. Sodium intake and vascular stiffness in hypertension. Hypertension. 2009;54:203‐209. [DOI] [PubMed] [Google Scholar]
- 36. Burnier M, Phan O, Wang Q. High salt intake: a cause of blood pressure‐independent left ventricular hypertrophy? Nephrol Dial Transplant. 2007;22:2426‐2429. [DOI] [PubMed] [Google Scholar]
- 37. Safar ME, Benetos A. Factors influencing arterial stiffness in systolic hypertension in the elderly: role of sodium and the renin‐angiotensin system. Am J Hypertens. 2003;16:249‐258. [DOI] [PubMed] [Google Scholar]
- 38. Siriopol D, Covic A, Iliescu R, et al. Arterial stiffness mediates the effect of salt intake on systolic blood pressure. J Clin Hypertens. 2018;20:1587‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Triantafyllou A, Anyfanti P, Gkaliagkousi E, et al. Association of urinary sodium excretion with vascular damage: a local kidney effect, rather than a marker of generalized vascular impairment. Int J Hypertens. 2018;2018: 7620563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strauss M, Smith W, Kruger R, van der Westhuizen B, Schutte AE. Large artery stiffness is associated with salt intake in young healthy black but not white adults: the African‐PREDICT study. Eur J Nutr. 2018;57:2649‐2656. [DOI] [PubMed] [Google Scholar]
- 41. He FJ, Marciniak M, Visagie E, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482‐488. [DOI] [PubMed] [Google Scholar]
- 42. Kawada T. Salt intake, overweight, and high blood pressure, with special reference to sex difference. Global Heart. 2017;12:266‐267. [DOI] [PubMed] [Google Scholar]
- 43. Ogola BO, Zimmerman MA, Clark GL, et al. New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol. 2018;315:H1073‐H1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu SL, Bajpai A, Hawthorne EA, et al. Cardiovascular protection in females linked to estrogen‐dependent inhibition of arterial stiffening and macrophage MMP12. JCI insight. 2019;4(1): 122742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He J, Gu D, Chen J, et al. Gender difference in blood pressure responses to dietary sodium intervention in the gensalt study. J Hypertens. 2009;27:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Myers J, Morgan T. The effect of sodium intake on the blood pressure related to age and sex. Clin Exp Hypertens. 1983;5:99‐118. [DOI] [PubMed] [Google Scholar]


