Abstract
The relative efficacy of antihypertensive treatment has been assessed primarily by randomized clinical trials (RCTs). The increasing availability of electronic medical records (EMR) allows results from RCT to be compared to data from actual clinical practice. EMR from TriNetX were used to compare patients starting and adhering to antihypertensive treatment on diuretics, beta blockers, angiotensin II or ACE inhibitors, or calcium channel blockers for at least 36 months. Cardiovascular (CV) events as defined by ICD‐10 codes were evaluated for an observation period of three years. Outcomes were assessed with and without propensity score matching for confounding factors. A total of 79 288 patients fulfilled the criteria for first‐line therapy and adherence (17.4% diuretics, 25.9% beta blockers, 45.1% inhibitors of the renin‐angiotensin system, and 11.6% calcium channel blockers). Differences in demography and comorbidities were consistent with expectations based on treatment guidelines. RAS blockers showed the best BP control (28.7% episodes of uncontrolled BP) and, together with diuretics, the lowest rate of CV events (diuretics, 5.2%; RAS blockers, 5.4%). Beta blockers were associated with the highest rate of uncontrolled BP (45.9%) and a high CV event rate (9.5%). These trends remained after matching the cohorts for confounding factors. EMR show that actual prescribing behavior for first‐line treatment of essential hypertension reflects treatment guidelines. Patients taking either RAS blockers or diuretics experienced the lowest CV event rates. Beta blockers, even when adjusted for pre‐existing cardiovascular conditions, do not seem to be as protective against CV events as the three other classes.
Keywords: first‐line treatment, hypertension, monotherapy, real‐world data
1. INTRODUCTION
With an estimated prevalence of 34%, about 86 million adults in the United States are believed to have high blood pressure (BP), hypertension.1
Hypertension is a major risk factor for stroke, due to both cerebral infarction and intracerebral hemorrhage,2 for coronary heart disease, renal impairment, and other end‐organ damage.
For primary prevention, reduction of BP is generally considered more important than the choice of specific antihypertensive agents, but some classes may offer more protective benefit than others.3 Extensive meta‐analyses and searches have been undertaken to evaluate the effect of antihypertensive treatment classes on morbidity and mortality. Treatment guidelines focus on individual risk factors like comorbidities and age, but do not generally favor one class over another for primary prevention.4 The most recent update of the Cochrane systematic database review came to the following conclusions: First‐line low‐dose thiazides reduced morbidity and mortality, first‐line ACE inhibitors and calcium channel blockers may be similarly effective, and first‐line high‐dose thiazides and first‐line beta blockers were inferior to first‐line low‐dose thiazides.5 Similar recommendations were also put forth by the Eighth Joint National Committee and published by the Journal of the American Medical Association: Initial treatment with a thiazide‐type diuretic was more effective than an ACE.6
Such analyses and recommendations are mostly based on results from randomized clinical trials (RCTs). Because of their sophisticated data collection and their robustness against confounding factors due to randomization, RCTs are generally considered the gold standard in clinical research. However, in RCTs multiple eligibility criteria lead to a highly selected patient population. Treatment protocols in RCTs rarely reflect reality in terms of compliance with medication, intensity of care, and motivation of patients.
The intense digitalization of health care and the accessibility of electronic medical records (EMR) now allow analyses of real‐world data (RWD) which were not possible years ago. It was therefore the objective of our analysis to describe the treatment of essential hypertension, the use of antihypertensive classes, and the incidence of cardiovascular events related to hypertension with EMR as representative of actual clinical practice.
2. METHODS
We used a subset of TriNetX, a global federated research network providing access to statistics on EMR (diagnoses, procedures, medications, laboratory values, and genomic information) from approximately 68 million patients in 56 large health care organizations predominately in the United States. TriNetX aggregates information directly from electronic medical records (EMR) systems on a continuous basis. Participating health care organizations include a mix of hospital, primary care, and specialty treatment providers spanning a wide range of geographies, age groups, and income levels. As a federated network, TriNetX received a waiver from Western IRB since only aggregated counts, statistical summaries of de‐identified information, but no protected health information is received, and no study‐specific activities are performed in retrospective analyses. Details of the network have been described elsewhere.7, 8 All analyses were done in the TriNetX “Analytics” network using the browser‐based real‐time analytics features, where not only patients but also health care organizations as data sources stay anonymous.
In November 2018, we analyzed the EMR of 5 746 151 patients who had essential hypertension (ICD‐10 code I10) and their first recorded instance of any cardiovascular medication after 12/31/2008. The four antihypertensive treatment classes were defined by VA National Formulary as follows:
Diuretics (V700), beta blockers (V100), blockers of the renin‐angiotensin system (angiotensin II inhibitors, V805, or ACE inhibitors, V800), and calcium channel blockers (V200). To ensure consistent use of the same treatment class over a sufficient period of time, it was required to have the same class documented in the EMR twice within a time frame of at least 36 months and no documentation of a medication from any of the respective three other classes during the same time.
The start date of the respective antihypertensive medication was used as the index event, and the observation period was defined as from 30 days to 1095 days (3 years) after the index event.
Cardiovascular events were defined by the following ICD‐10 codes:
First stroke (I63), first occurrence of any cerebrovascular episode (I60‐I69), first myocardial infarction (I21), first episode of any ischemic heart disease (I20‐I25), and first diagnosis of chronic kidney disease (I12, I13, N18). As the focus was on primary prevention, patients who had an event before the index date were excluded.
To compare antihypertensive treatment effects, the following criteria were used:
Uncontrolled BP episode, defined as incidences of BP measurement of ≥140 mm Hg systolic or ≥90 mm Hg diastolic, and mean systolic and diastolic BP values as patient's most recent value in the time window.
As laboratory value of interest, C‐reactive protein values were analyzed, too.
The results are shown as obtained before and after matching by propensity scores for the following potential confounding baseline factors, pre‐existing before the start of therapy:
Age, sex, race (white/black), ischemic heart disease, atrial fibrillation, diabetes mellitus, systolic and diastolic BP, and LDL cholesterol. The diuretics group was matched with beta blockers and the RAS blocker group with the CCB group.
3. RESULTS
3.1. Patients
There were 5 746 151 patients with essential hypertension in the TriNetX Analytics network, of whom 3 222 140 (56.1%) had a cardiovascular medication documented as starting after 12/31/2008. A total of 204 865 patients had a documentation with one of the four antihypertensive treatment classes for three or more years; 79 288 (39%) of them fulfilled the requirements for stability within one class for at least three years: 13 808 (17.4%) on diuretics, 20 538 (25.9%) on beta blockers, 35 110 783 (45.1%) on inhibitors of the renin‐angiotensin system, and 9159 (11.6%) on calcium channel blockers.
3.2. Documentation density
Documentation density in patients' electronic medical records (EMR) reached from 931 total facts per patient and 203 diagnosis facts per patient in the RAS blocker group up to 1422 and 279, respectively, in the diuretics group.
3.3. Demography, comorbidities, and baseline values
Before propensity matching, the four groups did not differ in a clinically meaningful way with regard to mean age at start of therapy (53‐58 years), LDL cholesterol values and blood pressure values before start of therapy, although a slightly higher systolic BP was to be seen in the CCB group (136.9 mm Hg).
Mean C‐reactive protein ranged from 6.9 mg/L (CCB) to 9.5 mg/L (diuretics).
There were more females (74%) in the diuretics group and more black patients in the diuretics and CCB group (each 24%).
The beta blocker group had the highest percentage of patients with concomitant ischemic heart disease (8%) and with atrial fibrillation (3%). Most patients with diabetes mellitus were to be found in the RAS blocker group (16%).
Unmatched baseline characteristics are listed in Table 1.
Table 1.
Patient numbers and characteristics before documented start of therapy (baseline)
| Starting antihypertensive medication after 12/31/2008 | ||||
|---|---|---|---|---|
| Diuretics | Beta blockers | RAS blockers | CCB | |
| Numbers | ||||
| n | 13 808 | 20 538 | 35 783 | 9159 |
| Percent (%) | 17.4 | 25.9 | 45.1 | 11.6 |
| Documentation density | ||||
| Avg facts per patient | 1422 | 1197 | 931 | 1129 |
| Diagnosis facts/patient | 279 | 266 | 203 | 231 |
| Demography | ||||
| Mean age | 54 | 56 | 53 | 58 |
| ± SD | 15 | 16 | 15 | 16 |
| Female (%) | 74 | 55 | 46 | 59 |
| White (%) | 68 | 82 | 78 | 62 |
| Black (%) | 24 | 8 | 9 | 24 |
| Unknown or other race (%) | 8 | 10 | 13 | 14 |
| Comorbidities | ||||
| Ischemic heart disease (I20‐I25) (%) | 2 | 8 | 2 | 3 |
| Diabetes (E08‐E13) (%) | 4 | 5 | 16 | 4 |
| Atrial fibrillation (I48) (%) | 1 | 3 | 0 | 2 |
| Laboratory values | ||||
| Mean C‐reactive protein, mg/L | 9.5 | 7.7 | 8.9 | 6.9 |
| ±SD | 29.5 | 25.1 | 32.0 | 25.7 |
| Mean systolic BP, mm Hg | 133.6 | 131.7 | 134.7 | 136.9 |
| ±SD | 17.9 | 18.2 | 17.3 | 18.8 |
| Mean diastolic BP, mm Hg | 80.2 | 78.2 | 81.0 | 80.9 |
| ±SD | 12.1 | 11.8 | 11.6 | 12.2 |
| Mean heart rate/min | 80.3 | 77.6 | 78.8 | 79.1 |
| ±SD | 15.6 | 16.0 | 13.7 | 14.9 |
| LDL cholesterol, mg/dL | 114.7 | 108.1 | 109.2 | 110.3 |
| ±SD | 37.4 | 38.8 | 38.7 | 31.7 |
Values before propensity matching. Therefore, differences across groups are partially caused by prescribing behavior following treatment guidelines.
3.4. Three‐year outcomes
9.5% (beta blockers), 7.6% (CCB), 5.4% (RAS blockers), and 5.2% (diuretics), respectively, had a documentation of at least one of the predefined clinical outcome criteria within three years after the defined start of therapy. The highest percentage of an individual component of the primary outcome was seen in the beta blocker group with 6.6% of patients having at least one episode in the set of ICD codes for ischemic heart diseases, followed by 4.1% of patients in the CCB group having any type of cerebrovascular outcome.
Using BP values below 140/90 mm Hg as treatment target, patients taking RAS blockers had the fewest documentations of uncontrolled hypertension (28.7%) and beta blockers the most (45.9%). Beta blockers also showed the smallest reduction in BP, with a change from baseline of −3.6/−2.2 mm Hg, compared to RAS blockers with the highest reduction (−5.8/−3.8 mm Hg).
C‐reactive protein fell the most (−1.2 mg/L) in the diuretics group and increased by 0.8 mg/L with beta blockers. For full results, see Table 2 and Figure 1.
Table 2.
Unmatched clinical outcomes and efficacy parameters after 3 y, as documented in patients' EMR
| Diuretics | Beta blockers | RAS blockers | CCB | |
|---|---|---|---|---|
| N | 13 808 | 20 538 | 35 783 | 9159 |
| Three‐y outcomes (30 until 1095 d after index); before propensity matching | ||||
| First stroke (I63) (%) | 0.7 | 1.3 | 0.8 | 1.3 |
| Any cerebrovascular outcome (I60‐I69) (%) | 2.5 | 3.9 | 2.4 | 4.1 |
| Myocardial infarction (I21) (%) | 0.2 | 0.9 | 0.2 | 0.3 |
| Any ischemic heart disease (I20‐I25) (%) | 2.8 | 6.6 | 2.6 | 4.1 |
| Chronic kidney disease (N18, I12, I13) (%) | 2.3 | 2.4 | 2.6 | 3.0 |
| Any of these CV outcomes (%) | 5.2 | 9.5 | 5.4 | 7.6 |
| Efficacy parameters | ||||
| LDL cholesterol, mg/dL | 114.7 | 108.1 | 109.24 | 110.32 |
| −2.9 | −4.4 | −6.1 | −3.3 | |
| Uncontrolled BP values, any episode (%) | 42.9 | 45.9 | 28.7 | 35.8 |
| Man systolic BP, most recent value, mm Hg | 128.7 | 128.1 | 128.9 | 132.2 |
| ±SD | 15.4 | 16.7 | 15.1 | 16.2 |
| Difference to baseline | −4.9 | −3.6 | −5.8 | −4.8 |
| Mean diastolic BP, most recent value, mm Hg | 77.1 | 76.0 | 77.2 | 77.7 |
| ±SD | 10.5 | 10.6 | 10.0 | 10.5 |
| Difference to baseline | −3.1 | −2.2 | −3.8 | −3.2 |
| Heart rate/min | 78.1 | 72.7 | 77.3 | 77.1 |
| Difference to baseline | −2.2 | −4.9 | −1.5 | −2.1 |
|
Mean C‐reactive protein, most recent value, mg/L, mean |
8.3 | 8.5 | 9.3 | 6.8 |
| Difference to baseline | −1.2 | 0.8 | 0.4 | −0.1 |
Figure 1.
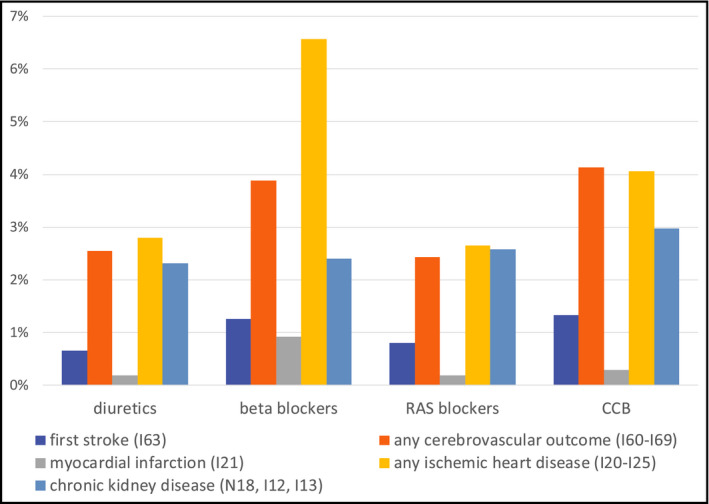
Percentage of patients with new documentation of respective outcome after 3 y, results before propensity matching. RAS, renin‐angiotensin system; CCB, calcium channel blockers
3.5. Propensity matching
Pairwise matching of propensity scores for potentially confounding demographic and clinical baseline factors further reduced the number of patients in the analyses and achieved well‐balanced pairs (diuretics vs beta blockers and RAS blockers vs CCB) for sex, race, and comorbidities, except age (RAS blockers 51.6 and CCB 57.8 years). Matching did not change the overall outcome results, with beta blockers still having the highest rate of any clinical CV outcome (9.0%), mainly driven by ischemic heart disease (6.0%), and CCB showing the highest rate of a cerebrovascular outcome (4.1%). The details of baseline and outcome data after propensity matching are listed in Table 3 and Figure 2.
Table 3.
Baseline characteristics and outcome results after pairwise propensity matching (diuretics vs beta blockers and RAS blockers vs CCB)
| Baseline characteristics after propensity matching | Diuretics | Beta blockers | RAS blockers | CCB |
|---|---|---|---|---|
| Number of patients | 11 766 | 11 766 | 8887 | 8887 |
| Number of patients excluded due to propensity matching | 2042 | 8772 | 26896 | 272 |
| In percent (%) | 15 | 43 | 75 | 3 |
| Mean age at start of therapy | 54.9 | 54.8 | 51.6 | 57.8 |
| SD | 14.8 | 15.9 | 16.1 | 15.9 |
| Female (%) | 70.4 | 70.8 | 58.1 | 58.2 |
| White (%) | 77.5 | 77.5 | 62.2 | 62.3 |
| Black (%) | 12.6 | 12.7 | 23.0 | 22.9 |
| Pre−existing conditions | ||||
| Ischemic heart disease (I20‐I25) (%) | 1.9 | 1.9 | 2.6 | 2.5 |
| Diabetes (E08‐E13) (%) | 4.5 | 4.8 | 4.4 | 4.4 |
| Atrial fibrillation (I48) (%) | 1.0 | 0.9 | 1.1 | 1.2 |
| Three‐y outcomes of the matched cohorts | ||||
| First stroke (I63) (%) | 0.7 | 1.1 | 0.7 | 1.3 |
| Any cerebrovascular outcome (I60‐I69) (%) | 2.7 | 3.6 | 2.4 | 4.1 |
| Myocardial infarction (I21) (%) | 0.2 | 0.7 | 0.1 | 0.3 |
| Any ischemic heart disease (I20‐I25) (%) | 2.8 | 6.0 | 2.3 | 3.9 |
| Chronic kidney disease (N18, I12, I13) (%) | 2.5 | 2.4 | 2.6 | 2.9 |
| Any of these CV outcomes (%) | 5.4 | 9.0 | 5.3 | 7.6 |
Figure 2.
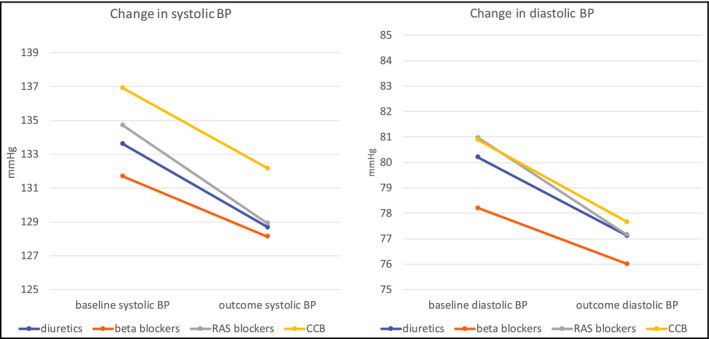
Changes in systolic and diastolic blood pressure over 3 y as documented in patients' electronic medical records. BP, blood pressure; RAS, renin‐angiotensin system; CCB, calcium channel blockers
4. DISCUSSION
4.1. Background
This study was intended to describe treatment patterns of first‐line therapy for essential hypertension and to evaluate relevant patient outcomes over the first 3 years after the start of therapy. No hypothesis confirming comparison was intended, but rather a descriptive evaluation was conducted across the four major antihypertensive treatment classes: diuretics, beta blockers, inhibitors of the renin‐angiotensin system (ACE inhibitors or angiotensin II inhibitors), or calcium channel blockers.
Randomized clinical trials (RCTs) are complex, lengthy, and costly; conducted in a highly selected patient population; and follow an experimental artificial treatment protocol. They are subject to a noteworthy placebo or Hawthorne effect,9 and their representativity for the general patient population may be limited. In contrast to RCT, EMR originate directly from actual medical practice and may therefore better represent real world, especially for the evaluation of long‐term outcomes.
4.2. Methods
Cardiovascular risk is impacted by adherence to treatment and compliance over a long time. The Cochran Systematic Review5 used studies with a minimum duration of 1 year, while our analysis required a treatment with the same antihypertensive class for at least 3 years.
Our requirement to have two documentations of the same treatment class over at least three years, but no documentation of any other antihypertensive class during the same time, was intended to ensure a sufficiently long observation period and to exclude dilution of the cohorts by patients who changed therapy from one to another class. These requirements led to a significant reduction in patient data available for analysis, to a similar extent as eligibility criteria for clinical trials. However, selection criteria for RCTs usually change the patient population according to certain conditions or risks (be it for safety or efficacy), while the criteria in this real‐world analysis were applied in order to improve the completeness and quality of the data, while still achieving sufficient sample sizes in each group to detect and evaluate clinical events.
4.3. Patient cohorts
There were some noteworthy differences in the baseline characteristics across the four treatment classes. The diuretic and CCB classes had the highest percentages of African American patients. Recommendations from JNC 8, issued in 2014, suggest that diuretics and CCB are most effective for that population.6 The diuretics class had a much higher percentage of female patients (74%) despite there being no specific recommendation suggesting diuretics as a preferred antihypertensive class in women.10 However, the lowest percentage of female patients in the RAS blocker class (46%) can well be explained by the contraindication for women who are or intend to become pregnant. The high fraction of patients with ischemic heart disease (8%) and with atrial fibrillation (3%) in the beta blocker class reflects general treatment practices for these conditions.4 Considering that beta blockers are not recommended as first‐line agents, the percent of patients on beta blockers seems high, but is consistent with the higher percentage of these pre‐existing cardiac conditions. The highest portion of patients with diabetes mellitus was to be found in the RAS blocker class (16%), which can also be explained by recommendations to consider ACE inhibitors or ARBs, especially in the presence of diabetes with albuminuria.11, 12
Overall, the distribution of comorbidities across the four treatment classes is at least in part consistent with influence of prescribing practices by treatment recommendations. In comparative effectiveness studies, it is standard practice to correct for imbalances in the baseline characteristics if the intention is to compare only the drugs (compounds) and to exclude all of the other factors which may influence a treatment decision, like treatment guidelines, preferences and prescribing behavior, formulary recommendation, pharmaceutical marketing, or a product's reputation. In real world, however, these factors, although not directly related to the chemical mechanism of action of the individual molecule, can nevertheless be properties of the product, influence a prescribing decision, and should also be taken into consideration. We performed both analyses, with and without propensity score matching, which revealed similar results.
4.4. Outcomes
Patients in the group taking RAS blockers for at least three years had the largest BP reduction and the best BP control, and‐together with patients on diuretics‐experienced the lowest rate of the predefined clinical outcome events within the 3‐year observation period. Previous observations have suggested that antagonism of the renin‐angiotensin system may improve cardiovascular outcome beyond blood pressure control, by reducing vascular inflammation and remodeling.13 We have not seen significant changes in CRP; however, <10% of patients actually had CRP values. Thus, this sample may not be sufficiently representative to draw robust conclusions on the impact of antihypertensive treatment on C‐reactive protein levels. The low event rate in the diuretics group confirms findings of numerous meta‐analyses.14 Guidelines drawn from collective analysis of RCT also suggest thiazide‐type diuretics as a first‐line treatment with fewer cardiovascular events than CCBs or RAS blockers, and RAS blockers more effective than CCBs.6
The highest percentage of patients who experienced any of the cardiovascular events was found in the beta blocker group. This was mainly driven by myocardial infarction or any type of ischemic heart disease. In addition, the worst BP control was seen with beta blockers. The higher event rate may not necessarily be attributed to their lack of a protective effect but rather to pre‐existing conditions where patients with ischemic heart disease were more likely to receive beta blockers. However, the difference remained, compared to diuretics, even after balancing for pre‐existing ischemic heart disease. Therefore, these data support guidelines which do not recommend beta blockers as first‐line monotherapy, especially without pre‐existing cardiac condition. The reduction in heart rate in the beta blocker group is to be expected and can serve as internal validation of cohort separation.
CCBs had the highest event rates of cerebrovascular events and a slightly higher rate of newly documented chronic kidney disease. Since amlodipine was the major representative of CCB in this class and it is specifically recommended for prevention of cardiovascular events,15 no robust conclusions about a CCB class effect can be drawn from these data. The small difference observed could be due to background noise.
Outcomes without and with propensity matching are summarized in Tables 2 and 3.
4.5. Limitations
Information about the actual dose taken is usually not available in EMR or claims data, and therefore, compliance cannot be assessed.
Real‐world analyses lack randomization and are therefore subject to potentially confounding factors, for example, pre‐existing conditions that play a role in a physician's prescribing decision, which can lead to imbalance of risk factors. We did not intend to compare the isolated effect of compounds, but rather to describe the use of treatment paradigms in reality. Although we applied an analysis with and without bias corrections, that is, propensity scoring, which did not change the overall outcomes, a certain component of confounding by indication for the effect of beta blockers cannot be completely ruled out. Our data reflect and describe the actual use of antihypertensives including the influence of comorbidities and should not be understood as tool for efficacy comparisons like in a RCT. It is also noteworthy that the prevalence of comorbidity in our study population was overall lower than one would expect. This can be explained by the less strict documentation of secondary versus primary diagnoses in the “real world” or by selection of patients with relatively mild hypertension due to the requirement of a monotherapy.
The selection of patients taking an antihypertensive medication from a specific class for three years was done to achieve a “clean” cohort for each class and to reduce background noise from “switchers.” However, under consideration of guidelines, many patients may not be on monotherapy for 3 years.
Smoking status, obesity, renal impairment, and Hispanic ethnicity were not documented well enough in the EMR to be used as a factor for propensity scoring without introducing a new confounder.
Data completeness expressed as documentation density in average facts per patient can explain differences in the detection of clinical events. Indeed, the density of diagnoses per patient was lower in the RAS group, but the data density did not correlate with the percentage of events across the groups, so it could not explain the difference alone. In addition, cardiovascular outcomes are significant medical events which can be assumed to be documented well in patients' EMR and in a consistent manner across all four classes.
Data collection at the health care providers occurs as patient information is entered into the record by the provider and translated into ICD‐10 code by medical coders. Consequently, the potential for errors in coding or data entry is always present.
BP values in RCTs are usually measured by 24‐hour ambulatory blood pressure monitoring or expressed as a mean of a series of individual office BP values obtained by manual or automated readings. As the BP values for our analysis were taken from EMR, they may be more variable and less precise than taken within the study protocol of a RCT. Therefore, this analysis has to accept more background noise and a higher standard deviation, eventually leading to a smaller effect size than usually seen in RCTs.
4.6. Conclusions
Real‐world data (RWD) represent the actual medical practice and allow long‐term follow‐up periods in a more efficient way than randomized controlled trials (RCTs).
However, the need for complete long‐term data on patients with sufficient medication adherence requires eligibility criteria which can reduce usable sample sizes to a similar extent as RCT.
RWD of actual prescribing patterns for first‐line treatment of essential hypertension correlate to treatment guidelines for patients with the relevant characteristics, for example, beta blockers preferred for patients with coronary heart disease or RAS blockers in diabetes.
With and without propensity score matching to control for these comorbidities, RAS blockers showed the best BP control and, together with diuretics, the lowest cardiovascular event rates. The data support guidelines which do not recommend beta blockers as first‐line monotherapy, especially without pre‐existing cardiac condition.
To our knowledge, this is the first real‐world analysis comparing first‐line treatment of the four antihypertensive classes with an observation period of 3 years.
CONFLICT OF INTEREST
MS is Chief Medical Officer of TriNetX Inc, the data network and analytics platform used for this publication. SH was employee of TriNetX at the time of analysis and manuscript preparation. MS owns stocks from Merck and Allergan, stemming from previous employments. Neither of these, nor any other pharmaceutical company was involved or had any influence in the design of the study; the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
AUTHOR CONTRIBUTIONS
Manfred Stapff developed the scientific concept, performed literature search, drafted the study design, developed the data querying concept, interpreted the results, prepared the scientific discussion, drafted the manuscript, and addressed reviewer comments. Sarah Hilderbrand contributed to the literature search, refined and executed the data queries, performed the quality checks, created tables and figures, and collaborated in the interpretation of the results, preparation of the discussion, and response to reviewer comments.
Stapff M, Hilderbrand S. First‐line treatment of essential hypertension: A real‐world analysis across four antihypertensive treatment classes. J Clin Hypertens. 2019;21:627–634. 10.1111/jch.13531
REFERENCES
- 1. Benjamin EJ, Nasir K, Virani SS, et al. Heart disease and stroke statistics—2018 Update. Circulation. 2018;137:e67‐e492. [DOI] [PubMed] [Google Scholar]
- 2. Meschia JF, Bushnell C, Boden‐Albala B, et al. Guidelines for the primary prevention of stroke. Stroke. 2014;45:3754‐3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravenni R, Jabre JF, Casiglia E, Mazza A. Primary stroke prevention and hypertension treatment: which is the first‐line strategy? Neurol Int. 2011;3(e12):45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. JACC. 2018;71:e127‐248. [DOI] [PubMed] [Google Scholar]
- 5. Wright JM, Musini VM, Gill R. First‐line drugs for hypertension. Cochrane Database Syst Rev. 2018;18(4):CD001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. James PA, Oparil S, Carter BL, et al. 2014 Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311(5):507‐520. [DOI] [PubMed] [Google Scholar]
- 7. Stacey J, Mehta M. Using EHR data extraction to streamline the clinical trial process. Clinical Researcher. April. 2017; 10.14524/CR‐17‐0004.
- 8. Stapff M. Use of electronic health data in clinical development. Pharm Ind. 2017;79(2):204‐210. [Google Scholar]
- 9. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Igho Pemu P, Ofili E. Hypertension in Women. J Clin Hypertens. 2008;10(5):406‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of hypertension and the international society of hypertension. J Hypertens. 2014;32:3‐15. [DOI] [PubMed] [Google Scholar]
- 12. Rubenfire M. 2017 Guideline for high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 13. Savoia C, Schiffrin EL. Reduction of C‐reactive protein and the use of anti‐hypertensives. Vasc Health Risk Manag. 2007;3(6):975‐983. [PMC free article] [PubMed] [Google Scholar]
- 14. Olde Engberink RHG, Frenkel WJ, van den Bogaard B, Brewster LM, Vogt L, van den Born B‐J. Effects of thiazide‐type and thiazide‐like diuretics on cardiovascular events and mortality. Hypertension. 2015;65:1033‐1040. [DOI] [PubMed] [Google Scholar]
- 15. Fares H, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Amlodipine in hypertension: a first‐line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016;3:e000473. [DOI] [PMC free article] [PubMed] [Google Scholar]


