Abstract
The role of uric acid (UA) on the arterial stiffness progression has been evaluated only in three studies. Our aim was to evaluate its role as a possible determinant of the pulse wave velocity (PWV) progression over a 3.7 ± 0.5 years follow‐up period in hypertensive patients. Specific sex analysis was done due to the well‐known sex interaction with UA levels. We enrolled 422 consecutive hypertensive outpatients. At baseline anamnestic, blood pressure (BP) and laboratory data as well as PWV were assessed. PWV was performed again at follow‐up examination. Hyperuricemia was defined as a UA > 6 mg/dL for women and > 7 mg/dL for men. Baseline age was 53.2 ± 13 years, 58% were males, systolic and diastolic BP (SBP/DBP) 141.7 ± 17.7/86.8 ± 10.8 mm Hg, UA 5.2 ± 1.4 mg/dL, and PWV 8.5 ± 1.9 m/s. At follow‐up, despite better BP values (−8.5 ± 24.6 for SBP and −7.5 ± 15.4 for DBP), PWV increases to 9.1 ± 2.3 m/s (P < 0.001) with mean ΔPWV of+ 0.5 ± 2.2 m/s. A total of 61 patients were hyperuricemic (14.4%), and they present higher PWV baseline (9.0 ± 2.5 vs 8.5 ± 1.8 m/s, P = 0.03) without significant differences in ΔPWV. Hyperuricemic female (6.2%, 11 patients) presents higher baseline PWV without significant differences in ΔPWV. No differences were found in arterial stiffness in hyperuricemic males (20.4%, 50 patients). UA showed association with baseline and ΔPWV in the whole population but it loses statistical significance at the linear regression model. Same figures were also for sex analysis. Our findings provide evidence that baseline UA levels are not determinants of PWV progression over a median follow‐up of 3.8 years’ in hypertensive patients.
Keywords: arterial hypertension, arterial stiffness, pulse wave velocity, pulse wave velocity progression, uric acid
1. INTRODUCTION
Arterial hypertension (HT) is the primary cardiovascular risk factor leading to an important increase in morbidity and mortality.1 Target organ damage (TOD) at various levels, that is pathological heart, vascular, and kidney involvement, is the linking between increased blood pressure (BP) values and the clinical events observed.
Arterial stiffness (a kind of vascular TOD) is determined by changes in vascular structure and function 2 that reflects the advancement of arteriosclerosis and is an independent predictor of cardiovascular and all‐cause mortality.3, 4, 5
Prior studies suggested that the principal determinants of arterial stiffening process are age and BP,6 but also a number of cardiovascular (CV) risk factors (as an example diabetes,7 chronic kidney disease,8 and inflammation 9). However, only age and BP were recognized as the prominent determinants of pulse wave velocity (PWV) progression in the few longitudinal studies published.10, 11, 12, 13
HT patients also frequently suffer from elevated uric acid (UA) levels that have been related to the occurrence of cardiovascular and renal events.14, 15, 16 UA has been also identified as a potential factor concurring in the development and/or progression of preclinical TOD. Regarding arterial stiffness, this association has been evaluated in numerous cross‐sectional studies; however, these studies frequently provided heterogeneous results.17, 18, 19, 20 Furthermore, to the best of our knowledge, only three published paper have evaluated the relationship between UA and PWV in prospective cohort.21, 22, 23
In the present study, we sought to determine the role of UA as a determinant of carotid‐femoral PWV progression over a medium‐term follow‐up period in treated hypertensive patients. Specific sex‐specific analysis was done due to the well‐known sex differences in UA levels.
2. METHODS
2.1. Study population
From September 2006 to October 2008, we enrolled 422 consecutive 18‐80 aged outpatients, followed by the Hypertension Unit of St. Gerardo Hospital (Monza, Italy) affected by essential hypertension. Exclusion criteria included an age less than 18 years old, pregnancy, atrial fibrillation, secondary hypertension (investigated by appropriate biochemical and instrumental assessment), chronic pulmonary disease, substance abuse, history of cancer, and history of a cardiovascular events in the 12 months preceding the evaluation (defined as myocardial infarction, angina pectoris, heart failure, stroke, transient cerebral ischemic attacks, and claudication).
From July 2010 to October 2012, we performed the follow‐up visit with a median follow‐up time of 3.85 (min 2.1 max 5.2 years, 5th and 95th percentiles: 3 and 4.85 years). The study protocol complies with the Declaration of Helsinki, and it was approved by the Ethics Committee of the Institutions involved. All participants provided informed written consent after being informed of its nature and purpose.
At baseline visits, we collected a comprehensive medical history and performed a complete physical examination in all patients. At follow‐up, patients’ medical history and physical examination were rechecked and BP was still measured twice. After BP measurements, PWV was recorded through the same techniques and using the same protocol.
At both visit, with the patient in the sitting position for at least 5 minutes and with the arm placed at heart level BP measurements were taken by a trained physician with a manual mercury sphygmomanometer (OMRON Healthcare Europe). BP was measured two times, and the mean of the two measurements was used for the calculation. Height and weight were obtained to calculate body mass index (BMI), and glomerular filtration rate was estimated (eGFR) by the Modification of Diet in Renal Disease (MDRD) Equation 24 Furthermore, diabetes was defined as a fasting plasma glucose > 126 mg/dL in two occasions or as the use of antidiabetic drugs while uncontrolled BP was defined as a systolic BP (SBP) ≥ 140 mm Hg and/or a diastolic BP (DBP) ≥ 90 mm Hg.
Finally, hyperuricemia was defined as a UA level > 6.0 mg/dL in women and > 7.0 mg/dL in men as previously reported.25 Patients taking UA‐lowering drugs have been excluded from the present analysis.
2.2. Laboratory analyses
They were performed only at the baseline evaluation on the automatic analyzer Modular Analytics SWA (Roche Diagnostics) with the following methods: enzymatic colorimetric (GOD‐PAP) for glucose, enzymatic colorimetric test with.
uricase for uric acid, enzymatic colorimetric (CHOD‐PAP) for total cholesterol, enzymatic colorimetric without pretreatment (3rd gen) for HDL cholesterol, enzymatic colorimetric (GPO‐PAP) for triglycerides, and LDL‐cholesterol was estimated according to the Friedewald equation, enzymatic method standardized against ID‐MS for creatinine, and immunoturbidimetric for albuminuria.
2.3. Pulse wave velocity
Aortic stiffness was evaluated by pulse wave velocity (PWV) between the carotid and the femoral artery of the same side with the patient in the supine position. The pressure pulse waveforms were simultaneously obtained at the two arterial sites on the right side using an automatic device (Complior, Colson; Alam Medical), and their distance calculated by taking the distance between hip and neck via a rigid ruler. Measurements were corrected by a 0.8 factor accordingly to the PWV measurement methods consensus documents which indicate the use of the subtraction methods instead of the direct one when assessing the distance between the two measurements points.6 Two measurements were obtained in each patient, and the mean was used for the analysis. In our laboratory, the intra‐session within‐ and between‐operator variability of PWV amounted to a coefficient of variation, respectively, of the mean value of 2% and 4%. The corresponding value for the inter‐session between‐operator variability was 4%. Arterial stiffness was defined as a PWV measurement higher than 10 m/s accordingly to current guidelines.6
2.4. Statistical analysis
Data obtained in each subject were averaged, and individual data were summed and expressed as means and standard deviations (SD).
We created a new variable (ΔPWV), which represented the difference between the measurement at follow‐up and at baseline, so its positive value means an increase in PWV, while negative value indicated a decrease in PWV. In addition, ΔSBP and ΔDBP represent the differences between the measurement at follow‐up and at baseline. Therefore, their positive value means an increase in BP values, while negative value indicated a decrease.
The change of blood pressure and PWV during follow‐up was evaluated by a paired t test, while the change in drug intake was evaluated by the McNemar test for each particular drug class. The study population was divided into normal and hyperuricemic groups according to baseline UA levels separately in males and females. Between‐group differences were assessed by Student's t test, Mann‐Whitney test and chi‐square test (or Fisher's exact test when needed) for normally distributed, non‐normally distributed, and categorical variables, respectively.
In order to investigate the association between UA and baseline PWV, a linear regression model was performed. A multivariable linear model was fitted including age, sex, BMI, and SBP. Owing to the importance of sex in this context, we also implemented stratified models on sex.
In order to investigate the association between UA and the variation in PWV during follow‐up, a longitudinal linear regression model was applied with follow‐up PWV as dependent variable and adjusting for baseline PWV. The longitudinal multivariable model included the time of follow‐up, age, sex, BMI, SBP, and SBP variation during follow‐up. The same model was also performed in patients without B‐blockers and diuretics (as they are well‐known agents that could act on UA). As sensitivity analyses, we also applied multivariable models including HDL cholesterol, glucose, triglycerides, eGFR, and antihypertensive therapies. STATA v14 was used for the statistical analyses, and a first type error of 0.05 was set.
3. RESULTS
3.1. Population characteristics
Table 1 shows baseline and follow‐up data of hypertensive treated population. At baseline, the mean age was 53.2 ± 13 years, and SBP was 141.7 ± 17.7 mm Hg, while DBP was 86.8 ± 10.8 mm Hg. As a result, 61.2% of patients had uncontrolled blood pressure defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg. The mean UA levels were 5.2 ± 1.4 mg/dL, while PWV was 8.5 ± 1.9 m/s and the 17% of the individuals had a value of PWV greater than 10 m/s. Regarding laboratory data, mean serum creatinine was 0.9 ± 0.2 mg/dL (with an eGFR of 92.9 ± 20.4 mL/min), also mean levels of lipids (triglycerides, total and fractioned cholesterol) were within the normal range. The proportion of patients with an impairment of kidney function (eGFR < 60 mL/min) was 4.5%.
Table 1.
Demographic, clinical, and target organ damage characteristics of the whole population at baseline and follow‐up
| Characteristic |
Mean (standard deviation)/number (%) N = 422 |
|---|---|
| Male sex (%) | 245 (58%) |
| Age (y) | 53.2 (13.0) |
| Systolic BP (mm Hg) | 141.7 (17.7) |
| Δ Systolic BP (mm Hg) | ‐9.2 (19.3) |
| Diastolic BP (mm Hg) | 86.8 (10.8) |
| Δ Diastolic BP (mm Hg) | ‐ 7.9 (12.2) |
| Heart Rate (bpm) | 66.1 (11.1) |
| BMI (Kg/m2) | 26.9 (4.1) |
| Smoking (%) | 62 (15%) |
| Diabetes (%) | 28 (7%) |
| Glucose (mg/dL) | 88.1 (19.6) |
| Tryglicerides (mg/dL) | 122.7 (73.7) |
| Total cholesterol (mg/dL) | 196.6 (33.9) |
| HDL cholesterol (mg/dL) | 53.1 (13.6) |
| LDL‐cholesterol (mg/dL) | 118.9 (31.4) |
| Uric Acid (mg/dL) | 5.2 (1.4) |
| Creatinine (mg/dL) | 0.9 (0.2) |
| Glomerular Filtration Rate <60 mL/min (%) | 19 (4.5%) |
| Pulse wave velocity (m/s) | 8.5 (1.9) |
| Pulse wave velocity >10 m/s (%) | 71 (17%) |
| Δ Pulse wave velocity (m/s) | 0.6 (1.9) |
Data are shown as means (standard deviation) or number(percentage). ΔPWV, ΔSBP, and ΔDBP represented the difference between the measurement at follow‐up and at baseline. Median follow‐up time was 3.85 y.
Abbreviations: BP, blood pressure; BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
After a median time of 3.85 (5th and 95th percentiles: 3 and 4.85) years, the population was reviewed, and mean BP values improved with a mean decrease of −9.2 ± 19.3 mm Hg for SBP and of −7.9 ± 12.2 mm Hg for DBP (P < 0.001 for baseline vs follow‐up SBP and DBP values). Therefore, we found a significant improvement in the blood pressure control (from 38.8% to 67.5%) with a greater proportion of patients taking antihypertensive drugs (from 82.2% to 94.2% at baseline and follow‐up respectively). Despite this, PWV increased significantly over that period (8.5 ± 2.1 m/s at baseline vs. 9.1 ± 2.3 m/s at follow‐up, P < 0.001) with an average ΔPWV of 0.6 ± 1.9 m/s.
Regarding specific drug classes, there was a significant increase in ACE Inhibitors, angiotensin receptor blockers (ARB), beta‐blockers, calcium channel antagonist, and statins use (Table 2).
Table 2.
Drug classes of the whole population at baseline and follow‐up
|
Baseline N(%) |
Follow‐up N(%) |
P (McNemar test) | |
|---|---|---|---|
| ACE inhibitors | 122 (29%) | 155 (37%) | 0.001 |
| Angiotensin receptor blockers | 116 (27%) | 184 (44%) | <0.001 |
| Beta‐blockers | 94 (22%) | 119 (28%) | 0.0011 |
| Calcium channel antagonist | 134 (32%) | 163 (39%) | 0.0032 |
| Alpha‐blockers | 50 (12%) | 55 (13%) | 0.4751 |
| Diuretics | 130 (31%) | 156 (37%) | 0.0116 |
| Statins | 50 (12%) | 92 (22%) | <0.001 |
Data are shown as number and percentage. Median follow‐up time was 3.85 y.
ACE, angiotensin‐converting enzyme.
3.2. Normouricemic versus hyperuricemic patients
Sex‐related analysis was done, and when males were classified according to their UA levels in normal and hyperuricemic individuals (50 patients, 20.4%), we found that the latter group was older (57.2 ± 12.1 vs 51.6 ± 12.6 years, P = 0.005) and displayed a higher values of triglycerides and a higher proportion of patients with a GFR < 60 mL/min (12% vs 3%, P = 0.011) (Table 3). No difference was seen both in baseline SBP values (143.3 ± 17.7 vs 142.0 ± 17.3 mm Hg, P = 0.62) and ΔSBP (−11.6 ± 20.7 vs −7.5 ± 16.8 mm Hg, P = 0.14). Regarding PWV (Figure 1, panel B and E), no difference was found for baseline values (9.0 ± 2.6 vs 8.8 ± 1.9 m/s, P = 0.44) and ΔPWV (0.6 ± 1.9 vs 0.5 ± 1.8 m/s, P = 0.73) between hyperuricemic males, when compared with normouricemic individuals. Regarding drugs, hyperuricemic males used more frequently alpha‐blockers, diuretics, and statins.
Table 3.
Demographic, clinical, and target organ damage characteristics of normal versus hyperuricemic among males and females
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Normal SUA | Hyperuricemic | P‐value | Normal SUA | Hyperuricemic | P‐value | |
| Number (n) | 195 | 50 | 166 | 11 | ||
| Age (y) | 51.6 (12.6) | 57.2 (12.1) | 0.005 | 53.2 (13.3) | 62.5 (10.9) | 0.026 |
| Systolic BP (mm Hg) | 142.0 (17.3) | 143.3 (17.7) | 0.62 | 140.9 (18.3) | 142.0 (16.5) | 0.84 |
| Δ Systolic BP (mm Hg) | ‐7.5 (16.8) | ‐11.6 (20.7) | 0.14 | ‐11.1 (20.6) | 0.2 (28.2) | 0.088 |
| Diastolic BP (mm Hg) | 87.6 (10.9) | 87.3 (10.4) | 0.86 | 86.1 (10.7) | 80.0 (11.0) | 0.067 |
| Δ Diastolic BP (mm Hg) | ‐6.9 (12.0) | ‐8.8 (11.1) | 0.31 | ‐9.0 (12.6) | ‐4.0 (13.5) | 0.20 |
| Heart Rate (bpm) | 65.1 (11.5) | 65.9 (11.4) | 0.66 | 67.3 (10.4) | 66.3 (13.9) | 0.76 |
| BMI (Kg/m2) | 27.2 (3.5) | 27.5 (3.2) | 0.61 | 26.2 (4.7) | 31.0 (5.6) | 0.001 |
| Smoking (%) | 35 (18%) | 4 (8%) | 0.084 | 23 (14%) | 0 (0%) | 0.36* |
| Diabetes (%) | 13 (7%) | 3 (6%) | 1.00* | 8 (5%) | 4 (36%) | 0.003* |
| Glucose (mg/dL), median (IQR) | 86 (79, 94) | 86 (82, 95) | 0.63** | 82.0 (76.0, 89.0) | 95.0 (80.0, 124.0) | 0.032** |
| Triglycerides (mg/dL), median (IQR) | 105 (79, 143) | 152 (93, 233) | <0.001** | 91.0 (71.0, 126.0) | 175.0 (107.0, 280.0) | <0.001** |
| Total cholesterol (mg/dL) | 191.6 (33.6) | 200.9 (34.1) | 0.099 | 200.3 (34.3) | 206.2 (24.7) | 0.58 |
| HDL cholesterol (mg/dL) | 49.2 (11.1) | 45.7 (12.3) | 0.077 | 59.8 (13.7) | 47.1 (12.8) | 0.003 |
| LDL‐cholesterol (mg/dL) | 118.2 (30.3) | 118.5 (34.6) | 0.96 | 119.6 (32.2) | 121.0 (27.4) | 0.89 |
| Creatinine (mg/dL) | 0.9 (0.2) | 1.0 (0.2) | 0.001 | 0.7 (0.1) | 0.9 (0.2) | 0.007 |
| Glomerular Filtration Rate <60 mL/min (%) | 6 (3%) | 6 (12%) | 0.011 | 5 (3%) | 2 (18%) | 0.016 |
| Drugs (%): | ||||||
| ACE inhibitors | 57 (29%) | 19 (38%) | 0.23 | 41 (25%) | 5 (45%) | 0.16* |
| Angiotensin receptor blockers | 59 (30%) | 15 (30%) | 0.97 | 39 (23%) | 3 (27%) | 0.72* |
| Beta‐blockers | 37 (19%) | 10 (20%) | 0.87 | 39 (23%) | 8 (73%) | 0.001* |
| Calcium channel antagonist | 73 (37%) | 17 (34%) | 0.65 | 41 (25%) | 3 (27%) | 1.00* |
| Alpha‐blockers | 19 (10%) | 10 (20%) | 0.045 | 19 (11%) | 2 (18%) | 0.62* |
| Diuretics | 56 (29%) | 22 (44%) | 0.039 | 44 (27%) | 8 (73%) | 0.003* |
| Statins | 22 (11%) | 12 (24%) | 0.020 | 15 (9%) | 1 (9%) | 1.00* |
ΔSBP and DBP represented the difference between the measurement at follow‐up and at baseline. Median follow‐up time was 3.85 y.
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; BP, blood pressure; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein.
Fisher's exact test.
Wilcoxon rank‐sum test.
Figure 1.
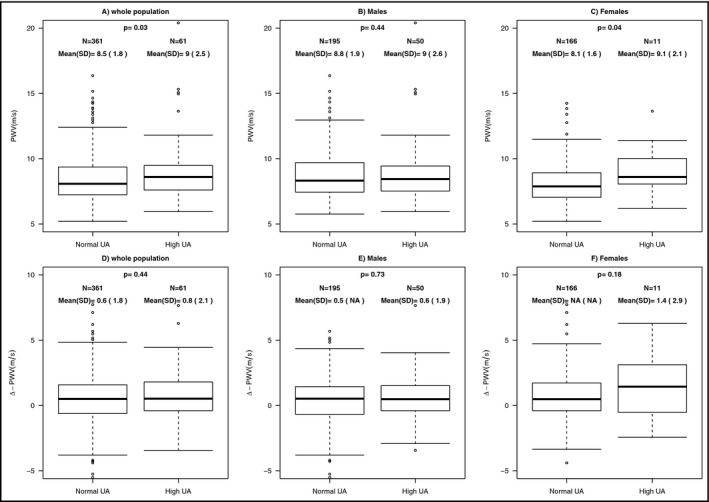
PWV and ΔPWV in normouricemic vs hyperuricemic patients in the whole population (panel A and D) and divided according to sex (panel B and E for males and panel C and F for females). PWV, pulse wave velocity; UA, uric acid. Vertical lines show 5th and 95th percentiles, boxes show 25th and 75th percentiles, and horizontal lines show 50th percentile
When females were classified according to their UA levels in normal and hyperuricemic individuals (11 patients, 6.2%), we found that the latter group was older (62.5 ± 10.9 vs 53.2 ± 13.2 years, P = 0.026) and displayed higher values of BMI, glucose, triglycerides with lower values of HDL cholesterol and a higher proportion of patients with a GFR < 60 mL/min (18% vs 3%, P = 0.016) as well as a higher prevalence of diabetes (Table 2). The two group showed similar baseline SBP values (142.0 ± 16.5 vs 140.9 ± 18.3 mm Hg, P = 0.84) with a trend for an increase in ΔSBP for hyperuricemic patients that did not reach the statistical significance (0.2 ± 28.2 vs −11.1 ± 20.6 mm Hg, P = 0.088). Regarding PWV (Figure 1, panel C and F), hyperuricemic patients, when compared with the normouricemic ones, showed significantly higher baseline values (9.1 ± 2.1 vs 8.1 ± 1.6 m/s, P = 0.04) with a trend for a higher increase in ΔPWV that did not reach the statistical significance (1.4 ± 2.9 vs 0.6 ± 1.8 m/s, P = 0.18). Regarding drugs, hyperuricemic patients use more frequently diuretics and B‐blockers.
Figure 1 also shows PWV data for the whole population, in which hyperuricemic patients displayed higher baseline values (9.0 ± 2.5 vs 8.5 ± 1.8 m/s, P = 0.03) with similar ΔPWV (0.8 ± 2.1 vs 0.6 ± 1.8 m/s, P = 0.44).
3.3. Regression analysis
Table 4 shows association coefficients of the linear regression analysis. At unadjusted analysis, in the whole population UA was significantly associated with baseline PWV (β = 0.22, 95% CI: [0.09; 0.35], P = 0.0013) and ΔPWV (β = 0.15, 95% CI: [0.03;0.27], P = 0.016). When sex analysis was performed, no significant association between uric acid and baseline PWV was found while association remain significant only for ΔPWV in females (β = 0.34, 95% CI: [0.10; 0.57], P = 0.0047).
Table 4.
Serum acid uric coefficient estimate from multiple linear regression on baseline PWV and delta PWV
| N | Baseline PWV(m/s) | p‐value | N | Δ Pulse wave velocity (m/s) | p‐value | ||
|---|---|---|---|---|---|---|---|
| Estimate [95% CI] | Estimate [95% CI] | ||||||
| Unadjusted | Adjusted for baseline PWV | ||||||
| Total | 422 | 0.22 [0.09,0.35] | 0.0013 | Total | 417 | 0.15 [0.03,0.27] | 0.0160 |
| Males | 245 | 0.13 [−0.07,0.34] | 0.205 | Males | 244 | 0.07 [−0.10,0.23] | 0.4257 |
| Females | 177 | 0.16 [−0.05,0.37] | 0.130 | Females | 173 | 0.34 [0.10,0.57] | 0.0047 |
| Adjusted for age, sex, BMI and SBP | Adjusted for baseline PWV, age, sex, BMI, SBP, ΔSBP, follow‐up time | ||||||
| Total | 419 | 0.01 [−0.12,0.14] | 0.8834 | Total | 414 | 0.02 [−0.11,0.14] | 0.791 |
| Males | 242 | ‐0.04 [−0.22,0.14] | 0.6823 | Males | 241 | ‐0.04 [−0.19,0.11] | 0.6134 |
| Females | 177 | 0.08 [−0.11,0.27] | 0.4125 | Females | 173 | 0.12 [−0.12,0.36] | 0.3238 |
ΔPWV and ΔSBP represented the difference between the measurement at follow‐up and at baseline. Median follow‐up time was 3.85 y.
Abbreviations: BMI, body mass index; PWV, pulse wave velocity; SBP, systolic blood pressure.
When adjusting for relevant covariates in a multivariable linear regression, the association loses its statistical significance for both baseline PWV and ΔPWV in the whole population and also in sex‐specific analyses.
The same model was also performed in patients without B‐blockers and diuretics (as they are well‐known agents that could act on UA). On a total of 240 patients, results were similar to the absence of statistical association for both baseline PWV and ΔPWV, also in sex‐specific analyses.
A further model including also biochemical variables (glucose, triglycerides, HDL cholesterol, and eGFR) as well as drug classes was fitted resulting in consistent results.
4. DISCUSSION
In our essential hypertensive patients, although UA levels were significantly related to PWV, the association lost the significance in multivariable analyses including the principal confounding factors. As already stated, only three previous papers investigated the relationship between UA and arterial stiffness in prospective studies.
UA levels could be associated with arterial stiffness through a variety of mechanisms. Principally, UA contributes to the arterial stiffening process due to its capacity for determining oxidative stress and due to its pro‐inflammatory effects. Furthermore, it activates the local renin‐angiotensin system that, together with the two previously cited effects, is able to determine thickening of the vascular wall via promoting proliferation and differentiation of smooth muscle cells and endothelial dysfunction.26, 27, 28, 29 Those are important determinants of arterial stiffness and give us the physiopathological basis for this relationship. Finally, hyperuricemia has been related to a higher prevalence of metabolic syndrome (MS) and UA levels well correlates with all of its components.30, 31 Whether UA contributes to the development of the metabolic derangement or is only a marker of MS is still a matter of debate. However, since MS has been related to an increase in arterial stiffness 32, 33 this could be a further mechanism for the link between UA and PWV.
Despite physiopathological basis, previous works focused on the relationship between UA and PWV present heterogeneous results. In fact, one work clearly relates UA with arterial stiffness,23 one did not found this relationship,21 and the latter found only a borderline connection.22 Methodological and population differences could help explain these differences. Regarding the methodological issues in the first study,23 arterial stiffness has been evaluated with brachial‐ankle PWV (ba‐PWV) while the other two (and our study) used carotid‐femoral PWV (cf‐PWV). Although ba‐PWV is a well validated measurement of arterial stiffness, strongly related to CV events,34, 35 they are not interchangeable and cf‐PWV is still the gold‐standard evaluation as stated by the ESH 2018 guidelines.1 In fact, they represent different measures since in the evaluation of ba‐PWV also a large part of medium‐sized and resistive arteries is included in the measurement while in cf‐PWV only elastic aorta is assessed. Up to now, no data are available on the effects of UA on different types of arteries, so this remains a strong confounding factor. Some differences exist also between our and the two previous studies that use cf‐PWV. In fact, they use the subtracting methods to assess distances 21 or does not correct for 0.8 factor22 as we do following the last methodological consensus on PWV assessment.
In respect to the population differences, all the three cited studies21, 22, 23 are based on a community cohort that is strongly different from our hypertensive population. Furthermore, follow‐up time is very different, ranging from 521, 22 to 10 years,23 once again very different from our follow‐up time of nearly 4 years.
As previously mentioned, in hypertensive patients BP values have been found to be, together with age, the stronger determinants of arterial stiffness with the possibility of overshadow weaker determining factor such as UA.
Finally, as we evaluated hypertensive patients we have to deal also with possible influences of antihypertensive therapies. When we add therapies, divided by drug class, to the model results did not changes showing the absence of relation with this further confounding factor. Furthermore, when the analysis was repeated in a subset of patients (a total of 240) without B‐blockers and diuretics, as they are well‐known agents that could act on UA, same results were found.
A further finding of our study deserves to be mentioned, namely the difference among sex in the relationship between UA and PWV. As already known by previous studies when UA analysis was conducted according to sex, some results were confirmed only in males or in females.36, 37, 38 In our study, females showed a higher baseline PWV when hyperuricemia was detected although no differences were found on follow‐up levels. The most likely explanation of these findings is that UA metabolism is genetically controlled, and sex differences exist in gene function 10 as well as hormones influences.39
Finally, we already discuss the possible relationship between UA and MS as well as between the latter and PWV. This is a possible confounding factor, particularly for hyperuricemic females in which the metabolic cluster is clearly affected. This is why we perform regression analysis correcting it for MS components further confirming the absence of relation between UA and PWV.
Our study has some limitations. The principal one is the small sample size. Furthermore, despite the total number of patients in the whole population some subgroup presents a low number of patients (ie, hyperuricemic females): This sample size can limit our power to detect important associations or findings. Particularly in this group, also menopausal status could be an important information. Unfortunately, we did not collect data on menopausal status leading to the impossibility to perform such an analysis in sex‐related outcome. Another one is that we examined hypertensive patients that were treated, and some specific classes of drugs (ie, diuretics) may have affected the study results. Thirdly, while serial measurements of PWV could be an element of high accuracy in dynamic assessment, it could determine logistic and economic difficulties in large population. Finally, no UA data were available at follow‐up examination as well as for others biochemical variables.
5. CONCLUSIONS
In conclusion, our findings provide evidence that baseline UA levels are not determinants of PWV progression over a median follow‐up time of 3.8 years’ in hypertensive patients. Further studies with longer follow‐up could help us to determine whether age and BP remain the principal determinants of PWV in hypertension and whether they are able to overshadow other risk factor (such UA).
CONFLICT OF INTEREST
This work was funded by the European Community Seventh Framework Programme (FP7/2007‐2013) Grant Agreement no. 278249 and by the Italian Ministry of University and Research (MIUR)—Department of Excellence project PREMIA (PREcision MedIcine Approach: bringing biomarker research to clinic). PR was supported by the grant SIR RBSI14LOVD of the Italian Ministry of Education, University and Research. The authors report no other specific funding in relation to this research and no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Alessandro Maloberti: Substantial contributions to the conception and design of the work, contributions in the data acquisition and its interpretation. Substantial contributions in drafting the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Paola Rebora: Substantial contributions to the data analysis and its interpretation. Substantial contributions in drafting the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Anita Andreano: Substantial contributions to the data analysis and its interpretation. Substantial contributions in the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Paola Vallerio: Substantial contributions to data interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Benedetta De Chiara: Substantial contributions to data interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Stefano Signorini: Substantial contributions to data acquisition and interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Marco Casati: Substantial contributions to data acquisition and interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Silvia Besana: Substantial contributions to data acquisition and interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Michele Bombelli: Substantial contributions to data interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Guido Grassi: Substantial contributions to data interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Maria Grazia Valsecchi: Substantial contributions to data interpretation. Substantial contributions the critical revision of the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Cristina Giannattasio: Substantial contributions to the conception and design of the work, contributions in the data acquisition and its interpretation. Substantial contributions in drafting the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Maloberti A, Rebora P, Andreano A, et al. Pulse wave velocity progression over a medium‐term follow‐up in hypertensives: Focus on uric acid. J Clin Hypertens. 2019;21:975–983. 10.1111/jch.13603
REFERENCES
- 1. Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 2. Safar M, Levy BI, Struijker‐Boudier H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation. 2003;107:2864‐2869. [DOI] [PubMed] [Google Scholar]
- 3. Laurent Stéphane, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 4. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 patients. J Am Coll Cardiol. 2014;63:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55(13):1318‐1327. [DOI] [PubMed] [Google Scholar]
- 6. The Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giannattasio C, Failla M, Capra A, et al. Increased arterial stiffness in normoglycemic normotensive offspring of type 2 diabetic parents. Hypertension. 2008;51(2):182‐187. [DOI] [PubMed] [Google Scholar]
- 8. Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end‐stage renal disease. Kidney Int. 2003;63(5):1852‐1860. [DOI] [PubMed] [Google Scholar]
- 9. Maloberti A, Meani P, Vallerio P, et al. Annexin A5 in treated hypertensive patients and its association with target organ damage. J Hypertens. 2017;35(1):154‐161. [DOI] [PubMed] [Google Scholar]
- 10. Benetos A, Adamopoulos C, Bureau J‐M, et al. Determinants of accelerated progression of arterial stiffness in normotensive patients and in treated hypertensive patients over a 6‐year period. Circulation. 2002;105(10):1202‐1207. [DOI] [PubMed] [Google Scholar]
- 11. AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ait‐Oufella H, Collin C, Bozec E, et al. Long‐term reduction in aortic stiffness: a 5.3‐year follow‐up in routine clinical practice. J Hypertens. 2010;28(11):2336‐2341. [DOI] [PubMed] [Google Scholar]
- 13. Meani P, Maloberti A, Sormani P, et al. Determinants of carotid‐femoral pulse wave velocity progression in hypertensive patients over a 3.7 years follow‐up. Blood Press. 2018;27(1):32‐40. [DOI] [PubMed] [Google Scholar]
- 14. Viazzi F, Leoncini G, Ratto E, Pontremoli R. Serum uric acid as risk factor for cardiovascular and renal disease: an old controversy revived. J Clin Hypertens. 2006;5:510‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agabiti Rosei E, Grassi G. Uric acid and cardiovascular diseases. Curr Med Res Opin. 2013;29(Suppl 3):33‐39. [DOI] [PubMed] [Google Scholar]
- 16. Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729‐1741. [DOI] [PubMed] [Google Scholar]
- 17. Maloberti A, Maggioni S, Occhi L, et al. Sex‐related relationships between uric acid and target organ damage in hypertension. J Clin Hypertens (Greenwich). 2018;20(1):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomez‐Marcos MA, Recio‐Rodriguez JI, Patino‐Alonso MC, et al. Vasorisk group. Relationship between uric acid and vascular structure and function in hypertensive patients and sex‐related differences. Am J Hypertens. 2013;26(5):599‐607. [DOI] [PubMed] [Google Scholar]
- 19. Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D. Association of Uric Acid With Vascular Stiffness in the Framingham Heart Study. Am J Hypertens. 2015;28(7):877‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulè G, Riccobene R, Castiglia A, et al. Relationships between mild hyperuricaemia and aortic stiffness in untreated hypertensive patients. Nutr Metab Cardiovasc Dis. 2014;24(7):744‐750. [DOI] [PubMed] [Google Scholar]
- 21. Canepa M, Viazzi F, Strait JB, et al. Longitudinal Association Between Serum Uric Acid and Arterial Stiffness: Results From the Baltimore Longitudinal Study of Aging. Hypertension. 2017;69(2):228‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding X‐H, Wang X, Cao R, et al. A higher baseline plasma uric acid level is an independent predictor of arterial stiffness: A community‐based prospective study. Medicine (Baltimore). 2017;96(6):e5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagano S, Takahashi M, Miyai N, et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive patients. Hypertens Res. 2017;40(6):620‐624. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. [DOI] [PubMed] [Google Scholar]
- 25. Desideri G, Virdis A, Casiglia E, Borghi C. Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension. Exploration into Uric and Cardiovascular Disease: Uric Acid Right for heArt Health (URRAH) Project, A Study Protocol for a Retrospective Observational Study. High Blood Press Cardiovasc Prev. 2018;25(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 26. Kotliar C, Kempny P, Gonzalez S, et al. Lack of RAAS inhibition by high‐salt intake is associated with arterial stiffness in hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2014;15:498‐504. [DOI] [PubMed] [Google Scholar]
- 27. Madlala HP, Maarman GJ, Ojuka E. Uric acid and transforming growth factor in fructose‐induced production of reactive oxygen species in skeletal muscle. Nutr Rev. 2016;74:259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kruger R, Mothae M, Smith W. Yia 03–07 reactive oxygen species adversely relates to early vascular changes and arterial stiffness in black normotensive smokers: the African‐Predict Study. J Hypertens. 2016;34:e205‐e206. [Google Scholar]
- 29. Kang D‐H, Park S‐K, Lee I‐K, Johnson RJ. Uric acid‐induced C‐reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553‐3562. [DOI] [PubMed] [Google Scholar]
- 30. Cibičková Ľ, Langová K, Vaverková H, Kubíčková V, Karásek D. Correlation of uric acid levels and parameters of metabolic syndrome. Physiol Res. 2017;66(3):481‐487. [DOI] [PubMed] [Google Scholar]
- 31. Fu YQ, Yang H, Zheng JS, et al. Positive association between metabolic syndrome and serum uric acid in Wuhan. Asia Pac J Clin Nutr. 2017;26:343‐350. [DOI] [PubMed] [Google Scholar]
- 32. Safar ME, Thomas F, Blacher J, et al. Metabolic syndrome and age‐related progression of aortic stiffness. J Am Coll Cardiol. 2006;47(1):72‐75. [DOI] [PubMed] [Google Scholar]
- 33. Tomiyama H, Hirayama Y, Hashimoto H, et al. The effects of changes in the metabolic syndrome detection status on arterial stiffening: a prospective study. Hypertens Res. 2006;29(9):673‐678. [DOI] [PubMed] [Google Scholar]
- 34. Fortier C, Desjardins MP, Agharazii M. Aortic‐Brachial Pulse Wave Velocity Ratio: A Measure of Arterial Stiffness Gradient Not Affected by Mean Arterial Pressure. Pulse (Basel). 2018;5:117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial‐Ankle PWV: current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb. 2016;23(2):128‐146. [DOI] [PubMed] [Google Scholar]
- 36. Baena CP, Lotufo PA, Mill JG, Cunha Rde S, Benseñor IJ. Serum uric acid and pulse wave velocity among healthy adults: baseline data from the brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Am J Hypertens. 2015;28:966‐970. [DOI] [PubMed] [Google Scholar]
- 37. Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population based study. Ann Rheum Dis. 2010;69:1162‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuwahata SO, Hamasaki S, Ishida S, et al. Effect of uric acid on coronary microvascular endothelial function in women: association with eGFR and ADMA. J Atheroscler Thromb. 2010;17:259‐269. [DOI] [PubMed] [Google Scholar]
- 39. Yu S, Yang H, Guo X, Zheng L, Sun Y. Hyperuricemia is independently associated with left ventricular hypertrophy in post‐menopausal women but not in pre‐menopausal women in rural Northeast China. Gynecol Endocrinol. 2015;31(9):736‐741. [DOI] [PubMed] [Google Scholar]


