Abstract
The burden of chronic kidney disease (CKD) is rapidly rising in developing countries due to astronomical increases in key risk factors including hypertension and diabetes. We sought to assess the burden and predictors of CKD among Ghanaians with hypertension and/or diabetes mellitus in a multicenter hospital‐based study. We conducted a cross‐sectional study in the Ghana Access and Affordability Program (GAAP) involving adults with hypertension only (HPT), hypertension with diabetes mellitus (HPT + DM), and diabetes mellitus only (DM) in 5 health facilities in Ghana. A structured questionnaire was administered to collect data on demographic variables, medical history, and clinical examination. Serum creatinine and proteinuria were measured, and estimated glomerular filtration rate derived using the CKD‐EPI formula. A multivariable logistic regression model was used to identify factors associated with CKD. A total of 2781 (84.4%) of 3294 participants had serum creatinine and proteinuria data available for analysis. The prevalence of CKD was 242 (28.5%) among participants with both DM and HPT, 417 (26.3%) among participants with HPT, and 56 (16.1%) among those with DM alone. Predictors of CKD were increasing age aOR 1.26 (1.17‐1.36), low educational level aOR 1.7 (1.23‐2.35), duration of HPT OR, 1.02 (1.01‐1.04), and use of herbal medications aOR 1.39 (1.10‐1.75). Female gender was protective of CKD aOR 0.75 (0.62‐0.92). Among patients with DM, increasing age and systolic blood pressure were associated with CKD. There is high prevalence of CKD among DM and hypertension patients in Ghana. Optimizing blood pressure control and limiting the use of herbal preparations may mitigate CKD occurrence in high cardiovascular risk populations in developing countries.
Keywords: ACE inhibitors, chronic kidney disease, diabetes, epidemiology, hypertension, chronic kidney disease, hypertension, diabetes mellitus, Ghana
Keywords: chronic kidney disease, hypertension, diabetes mellitus, Ghana
1. INTRODUCTION
Non‐communicable diseases (NCDs) are on the rise in low‐ and middle‐income countries (LMICs).1 This epidemiological transition is due to the adoption of western lifestyles with changes in nutrition, rapid urbanization, and aging population as a result of relatively improved health care systems.2 This has culminated in burgeoning epidemics of cardiovascular risk factors and diseases such as hypertension, type II diabetes mellitus (DM), obesity, dyslipidemia, and chronic kidney disease (CKD).3
CKD is increasing in incidence and prevalence worldwide and is estimated to be between 10% and 13% globally.4, 5 CKD has now emerged as a significant public health challenge in sub‐Saharan Africa with a reported prevalence of 13.9% in a meta‐analysis.6 The prevalence of CKD in Ghana has been shown to be 13.3% in a recent study.7 As a non‐communicable disease, CKD has not received the necessary attention in most countries in sub‐Saharan Africa including Ghana.8 CKD is associated with increasing morbidity and mortality and also known to impact negatively on quality of life.9 Progression of CKD to end‐stage kidney disease (ESKD) is an important burden to the health care system in most LMICs as most patients cannot afford renal replacement therapy.10 There is therefore the need to identify common causes of CKD and screen for CKD in patients with DM and hypertension, which are the major risk factors for the development of CKD.
Hypertension has a global burden of 26%, affecting 972 million people in 2000, 639 million of which live in LMICs.11 The prevalence of hypertension in Ghana varies widely from 4.5% to 54.6% according to the Ghana Demographic and Health Survey being highest among urban dwellers.12 CKD has been shown to occur in almost half of patients with hypertension in Ghana 13 and also an independent risk factor for the development of other NCD such as stroke, ischemic heart diseases, and hypertension.14 Hypertension, DM, and CKD in these settings are under‐recognized, untreated, or under‐treated due to a combination of factors in developing countries.15
DM is projected to rise from 171 million in 2000 to 366 million in 2030 with an estimated prevalence of 4.4% worldwide.16 The prevalence of DM in Ghana was 6.4% in a community‐based study.17 DM is known to be the most common cause of CKD worldwide 5 but the second most common cause in Ghana.18 When diagnosed early, most NCDs can be properly managed to decrease morbidity and mortality with lifestyle changes, dietary modifications and appropriate medicines when required.
Management of NCDs is, however, challenged by access to quality medicines due to unavailability and high costs in LMICs.19 Where medicines are available, they are often of generic brands and not quality assured. However, innovator brands of essential cardiovascular medicines are often expensive and not within the reach of many individuals living in resource‐limited settings. Hence, innovative strategies are urgently needed to improve access to quality‐assured cardiovascular medicines for the control of NCDs in under‐served populations of the world.
The Ghana Access and Affordability Program (GAAP) pilot study is a prospective cohort study in which Ghanaian adults with hypertension and or DM were enrolled to assess differential pricing as an intervention for improved access to innovator‐branded study medications. The prevalence of CKD among patients with hypertension and DM has not yet been assessed in a multicenter study in Ghana. We sought to narrow this knowledge gap by evaluating the prevalence and factors associated with occurrence of CKD among the GAAP study population.
2. METHODS
2.1. Study design and participants
The Ghana Access and Affordability Program (GAAP) pilot study is a prospective cohort study involving adults with hypertension only (HPT), hypertension with diabetes mellitus (HPT + DM), and diabetes mellitus only (DM) at five health facilities situated in ecological zones of Ghana to capture rural, semi‐urban, and urban settings across the country. Participants at enrollment visit had serum creatinine and level of proteinuria measured to assess the burden of CKD. The GAAP pilot study is supported by three biopharmaceutical companies and the Bill and Melinda Gates Foundation to evaluate whether differential pricing of innovator brands of antihypertensive and antiglycemic medications in Ghana would lead to improved access and affordability. The health facilities included in the study were Agogo Presbyterian Hospital (APH), Atua Government Hospital, (AGH), Komfo Anokye Teaching Hospital, (KATH), Kings Medical center, (KMC), and the Tamale Teaching Hospital, (TTH). Ethical approval was obtained from all study sites before the commencement of the study. The study protocol has been published.20
2.2. Eligibility criteria
Participants were eligible for inclusion if they were aged 18 years and above with a known diagnosis of hypertension and or type II DM.
Hypertension was defined as the presence of a persistently elevated systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg in patients who were 18 years and above, and were on antihypertensive drugs at enrollment visit. Diabetes mellitus was defined as a fasting blood glucose ≥7 mmol/L on two or more occasions, a random blood glucose ≥11.1 mmol/L with symptoms, HbA1C of >6.5%, and on medications for treatment of type 2 diabetes mellitus.
Patients with known type I DM were excluded as well as those with hypertensive urgencies/emergencies, hyperglycemic, or hypoglycemic emergencies at enrollment visit.
2.3. Evaluation of study participants
Trained research assistants obtained a written informed consent from all participants before they were enrolled into the study. Demographic information including age, gender, educational attainment, employment status, and lifestyle behaviors such as alcohol use, cigarette smoking, level of physical activities, frequency and daily quantities of fruits and vegetable consumption as well as table added salt were assessed through interviews and responses collected on a questionnaire. A detailed medical history including duration of diagnosis of HPT or DM and doses of medications currently being taken were obtained.
Anthropometric evaluations including measurement of weight, height, and waist circumference were performed by trained study nurses. Body mass index (BMI) of each participant was then derived by dividing the weight in kilograms by the square of the height in meters. Individuals were classified as physically active if they were regularly involved in moderate exercise (walking, cycling, or gardening) or strenuous exercise (jogging, football, and vigorous swimming) for 4 hours or more per week. Alcohol use was categorized into current users (users of any form of alcoholic drinks) or never/former drinker, while alcohol intake was categorized as low drinkers (1‐2 drinks per day for female and 1‐3 drinks per day for male) and high drinker (>2 drinks per day for female and >3 drinks per day for male. 1 drink or 1 unit of alcohol = 8 g of alcohol). Smoking status was defined as current smoker (individuals who smoked any tobacco in the past 12 months) or never or former smoker. Vegetable and fruit intake was assessed based on number of daily servings per week. The Ghana Statistical Services defines urban residence as settlements with population >20 000, peri‐urban as settlements with population size between 5000 and 19 999, and rural residence as those with population <5000.
2.4. Laboratory measurements
To ensure standardization across all study sites, an International Organization for Standardization (ISO)‐certified and quality‐assured laboratory was contracted to run all biochemical panels which included serum creatinine and hemoglobin A1C for all participants with diabetes. Samples were transported to the laboratory by trained phlebotomists on the same day of collection often within 4 hours or where not feasible (KMC and AGH sites), and samples were stored in a freezer before transported to the laboratory the next day. Estimated glomerular filtration rate (eGFR) was determined from serum creatinine using CKD‐EPI formula.21 The CKD‐EPI creatinine equation is based on four variables and uses the 2‐slope spline to model the relationship between serum creatinine and estimated eGFR based on age, sex, and race. It has been shown to be more accurate than the Modification of Diet in Renal Disease (MDRD) Study equation for populations with GFR >60 mL/1.73 m2.21 CKD was defined as either eGFR <60 mL/min/1.73 m2 with or without trace proteinuria and above or eGFR ≥60 mL/min with proteinuria according to the Kidney disease Improving Global outcome (KDIGO).22
2.5. Statistical analysis
For baseline characteristics, means were compared using Student's t test for 2‐group comparisons and proportions were compared using chi‐squared tests or Fisher's exact test for proportions with subgroupings <5. A multivariable logistic regression model was fitted to identify factors associated with CKD defined as either eGFR <60 mL/min/1.73 m2 with or without trace proteinuria and above or eGFR ≥60 mL/min with proteinuria according to the Kidney disease Improving Global outcome (KDIGO).22 Independent variables evaluated included the following socio‐demographic factors: age, gender, location of residence, and employment status; lifestyle/behavioral factors: included previous cigarette smoking, current alcohol use, physical activity, table added salt, fruit and vegetable intake; health system factors: level of health care institution (primary, secondary, or tertiary); and patho‐biologic factors: central obesity, duration of hypertension or diabetes, number of antihypertensive medications, and baseline systolic and diastolic BP as well as baseline HBA1c. Variable selection was based on clinical and empirical significance of covariates in the model. Variables were included in the multivariate analyses upon meeting a P‐value cutoff of <0.05 in bivariate unadjusted regression analysis. In all analyses, two‐tailed P < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism version 7 and SPSS version 20.
3. RESULTS
3.1. Prevalence of CKD in the study population
The study included 3294 participants from all the 5 study sites. A total of 2781 (84.4%) participants had serum creatinine and proteinuria data at baseline available for analysis. Among those with serum creatinine and/or proteinuria data, 1585 (57.0%) participants had hypertension, 348 (12.5%) had DM only, and 848 (30.5%) had both DM and hypertension. From the entire study cohort, CKD was found in 715 (25.7%). Out of 715 participants with renal impairment, 15 (2.1%) had stage 5 CKD, 35 (4.9%) had stage 4 CKD, 358 (50.1%) had stage 3 CKD, and 307 (42.9%) had eGFR ≥60 mL/min but with significant proteinuria of trace or higher.
The prevalence of CKD was 242 (28.5%) among participants with both DM and hypertension, 417 (26.3%) among participants with hypertension, and 56 (16.1%) among those with DM alone. The majority, 2108 (84.0%) had no proteinuria, 4.4% had trace proteinuria, 8.6% had 1 + proteinuria, and 3.1% had proteinuria of 2 + or more.
3.2. Demographic and clinical characteristics of participants according to CKD status
The mean age in years of participants with CKD was higher as compared to those without CKD 60.5 ± 12.3 versus 56.2 ± 12.4; (P < .0001). There were significantly less proportion of females with CKD 527 (73.7%) as compared to participants without CKD 1627 (78.8%) P = .005. The mean duration of hypertension in years was higher in patients with CKD as compared to participants without CKD (8.9 ± 8.2 versus 7.4 ± 7.0, P < .0001). Mean systolic blood pressure was higher in participants with CKD than those without 145.6 ± 23.2 versus 139.3 ± 21.5, P < .0001 but mean diastolic pressures were not significantly different. The mean duration of DM in years was also higher among participants with CKD as compared to those without CKD 10.1 ± 7.6 versus 8.8 ± 6.5, P = .0043. In addition, study participants with CKD compared with those without CKD were involved in less regular physical activity 426 (59.6) versus 1327 (64.2), P = .03, and used more herbal medications in the past six months 131 (18.3) versus 288 (13.9), P = .0048. Comparisons between the two groups are depicted in Table 1.
Table 1.
Comparison of demographic and clinical characteristics of study participants with chronic kidney disease (CKD) and those without
| Characteristic | Participants with CKD (N = 715) | Participants without CKD (N = 2066) | P‐value |
|---|---|---|---|
| Age, mean ± SD | 60.5 ± 12.3 | 56.2 ± 12.4 | <.0001 |
| Female, n (%) | 527 (73.7) | 1627 (78.8) | .005 |
| Location of residence, n (%) | .48 | ||
| Urban | 277 (38.7) | 844 (40.9) | |
| Semi‐urban | 163 (22.8) | 473 (66.2) | |
| Rural | 275 (38.5) | 744 (36.0) | |
| Highest educational status | .002 | ||
| No formal education | 297 (41.5) | 728 (35.2) | |
| Primary level | 104 (14.5) | 377 (18.2) | |
| Secondary level | 250 (35.0) | 709 (34.3) | |
| Tertiary level or more | 64 (9.0) | 250 (12.1) | |
| Level of health institution | <.0001 | ||
| Tertiary level | 349 (48.8) | 1139 (55.1) | |
| Secondary level | 360 (50.3) | 829 (40.0) | |
| Primary level | 6 (0.9) | 98 (4.7) | |
| Hypertension alone, n (%) | 417 (58.3) | 1168 (56.5) | .39 |
| Diabetes alone, n (%) | 56 (7.8) | 292 (14.1) | <.0001 |
| Hypertension and Diabetes, n (%) | 242 (33.8) | 606 (39.3) | .02 |
| Lifestyle/behavioral factors | |||
| Current alcohol use, n (%) | 58 (8.1) | 166 (8.0) | .95 |
| Current cigarette smoking, n (%) | 1 (0.1) | 11 (0.5) | .17 |
| Previous cigarette use, n (%) | 47 (6.6) | 136 (6.6) | .99 |
| Regular Physical activity, n (%) | 426 (59.6) | 1327 (64.2) | .03 |
| Body Mass Index, mean ± SD | 26.4 ± 5.4 | 26.6 ± 5.5 | .32 |
| Waist Circumference, mean ± SD | 96.0 ± 12.8 | 95.7 ± 13.2 | .65 |
| Laboratory data | |||
| HBA1C, mean ± SD | 8.5 ± 2.5 | 8.8 ± 2.6 | .08 |
| Fasting blood glucose, mean ± SD | 9.3 ± 4.4 | 9.3 ± 4.1 | .83 |
| Serum total cholesterol, mean ± SD | 5.5 ± 1.3 | 5.4 ± 1.4 | .30 |
| LDL cholesterol, mean ± SD | 3.5 ± 1.2 | 3.5 ± 1.2 | .91 |
| HDL cholesterol, mean ± SD | 1.3 ± 0.5 | 1.3 ± 0.5 | .45 |
| Triglyceride, mean ± SD | 1.8 ± 1.1 | 1.5 ± 0.8 | .0004 |
| Use of renoprotective medication | .12 | ||
| ACE‐I alone, n (%) | 272 (38.0) | 802 (38.8) | |
| ARB alone, n (%) | 192 (26.8) | 467 (22.6) | |
| ARB + ACE‐I, n (%) | 12 (1.7) | 39 (1.9) | |
| Neither use of ARB nor use of ACE‐I, n (%) | 239 (33.4) | 758 (36.7) | |
| Use of herbal treatments over the past 6 months | 131 (18.3) | 288 (13.9) | .0048 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; SD, standard deviation.
3.3. Predictors of CKD among Ghanaians with DM and or hypertension
Predictors of CKD in the entire cohort of DM and hypertension reported as adjusted odds ratio (aOR) include increasing age, aOR of 1.26 (1.17‐1.36), P < .001, low educational status, aOR of 1.70 (1.23‐2.35), P = .001, increase duration of hypertension in years, aOR of 1.02 (1.01‐1.04), P = 0001, and reported use of herbal preparations aOR of 1.39 (1.10‐1.75), P = .006 as shown in Table 2. Female gender was, however, protective of CKD (aOR of 0.75 (0.61‐0.93), P = .008). The use of ACE‐I and ARBs was not significantly protective of CKD.
Table 2.
Factors associated with chronic kidney disease among Ghanaians with hypertension and/or diabetes mellitus
| Predictor | Unadjusted OR (95% CI) | P‐value | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| Age, each 10 year older | 1.34 (1.22‐1.43) | <.0001 | 1.26 (1.17‐1.36) | <.0001 |
| Female gender | 0.76 (0.62‐0.92) | 0.006 | 0.75 (0.61‐0.93) | .008 |
| Educational level | ||||
| Primary/none | 1.41 (1.05‐1.90) | 0.02 | 1.47 (1.07‐2.02) | .02 |
| Secondary | 1.39 (1.02‐1.89) | 0.04 | 1.70 (1.23‐2.35) | .001 |
| Tertiary | 1.00 | 1.00 | ||
| Duration of hypertension | 1.03 (1.02‐1.05) | <.0001 | 1.02 (1.01‐1.04) | .0001 |
| Duration of diabetes mellitus | 1.01 (0.99‐1.02) | 0.24 | — | — |
| Regular exercise | ||||
| Yes | 1.00 | 1.00 | ||
| No | 1.24 (1.04‐1.48) | 0.02 | 1.18 (0.98‐1.41) | .07 |
| BMI, each 5 kg/m2 rise | 0.96 (0.89‐1.04) | 0.31 | — | — |
| WC, each 5 cm rise | 1.02 (0.95‐1.08) | 0.65 | — | — |
| Use of ACE‐I and or ARB | ||||
| None | 1.00 | 1.00 | ||
| ACE‐I | 1.08 (0.88‐1.31) | 0.48 | 0.94 (0.76‐1.16) | .56 |
| ARB | 1.32 (1.06‐1.65) | 0.01 | 1.09 (0.86‐1.38) | .47 |
| ARB + ACE‐I | 0.87 (0.45‐1.66) | 0.66 | 0.79 (0.40‐1.58) | .51 |
| Use of herbal preparations | 1.38 (1.10‐1.74) | 0.005 | 1.39 (1.10‐1.75) | .006 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; SD, standard deviation; WC, waist circumference.
Among participants with only hypertension, factors associated with CKD included increasing age, aOR of 1.14 (1.04‐1.26), P = .006, secondary level as highest level of education, aOR of 2.0 (1.28‐+++3.14), P = .002, increase in systolic blood pressure for each 10 mm Hg rise, aOR of 1.08, (1.02‐1.14), P = .004, and the use of herbal preparations, 1.38, (1.02‐1.86), P = .03 as shown in Table 3.
Table 3.
Factors associated with chronic kidney disease among Ghanaians with only hypertension
| Predictor | Unadjusted OR (95% CI) | P‐value | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| Age, each 10 years older | 1.19 (1.09‐1.30) | 0.0001 | 1.14 (1.04‐1.26) | .006 |
| Female gender | 0.74 (0.57‐0.95) | 0.02 | 0.76 (0.57‐1.00) | .05 |
| Educational level | ||||
| Primary/none | 1.48 (0.99‐2.23) | 0.06 | 1.49 (0.96‐2.32) | .07 |
| Secondary | 1.69 (1.11‐2.59) | 0.01 | 2.00 (1.28‐3.14) | .002 |
| Tertiary | 1.00 | 1.00 | ||
| Duration of hypertension | 1.02 (1.01‐1.04) | 0.004 | 1.01 (1.00‐1.03) | .08 |
| Systolic BP, each 10 mm Hg | 1.10 (1.05‐1.16) | 0.0003 | 1.08 (1.02‐1.14) | .004 |
| Regular exercise | ||||
| Yes | 1.24 (0.99‐1.56) | 0.06 | — | — |
| No | 1.00 | |||
| BMI, each 5 kg/m2 increase | 0.97 (0.88‐1.07) | 0.49 | — | — |
| Use of ACE‐I and or ARB | ||||
| None | 1.00 | 1.00 | ||
| ACE‐I | 1.08 (0.84‐1.40) | 0.55 | 0.98 (0.74‐1.28) | 0.86 |
| ARB | 1.45 (1.08‐1.95) | 0.01 | 1.24 (0.91‐1.70) | 0.18 |
| Use of herbal preparations | 1.44 (1.07‐1.93) | 0.01 | 1.38 (1.02‐1.86) | 0.03 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; SD, standard deviation.
Among patients with DM, increasing age (aOR 1.49 (1.30‐1.70) P < .0001) and 10 mm Hg rise in systolic blood pressure (aOR 1.09 (1.02‐1.16) were the predictors of CKD.
3.4. Factors associated with proteinuria
The presence of proteinuria in the entire cohort was associated with no/primary educational level, aOR of 1.82 (1.22 ‐ 2.73); P = .004, secondary educational level, aOR of 1.57 (1.02 ‐ 2.39); P = .04 as compared with tertiary educational attainment as a referent group. In addition, each 10 mm Hg increase in systolic blood pressure was associated with an aOR of 1.08 (1.04‐1.14), P = .0005 for proteinuria. The use of herbal medication did not predict proteinuria in the cohort, and the use of ACE‐I and or ARBs was not significantly protective of proteinuria in the entire cohort (Table 4). However, specifically among the subgroup with evidence of renal impairment with data on urinalysis, the percentage with no proteinuria was highest among those on dual blockade with ACE inhibitor and ARB, followed by use of ARB, then ACE‐I, and finally none of the two RAAS modulators, P = .008 by chi‐squared analysis (Figure 1).
Table 4.
Predictors of proteinuria among Ghanaians with hypertension and or diabetes Mellitus
| Predictor | Unadjusted OR (95% CI) | P‐value | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| Age, each 10 years older | 1.05 (0.96‐1.14) | 0.32 | — | — |
| Female gender | 0.94 (0.73‐1.21) | 0.64 | — | — |
| Educational level | ||||
| Primary/none | 1.85 (1.23‐2.77) | 0.003 | 1.82 (1.22‐2.73) | 0.004 |
| Secondary | 1.58 (1.04‐2.41) | 0.03 | 1.57 (1.02‐2.39) | 0.04 |
| Tertiary | 1.00 | 1.00 | ||
| Duration of hypertension | 1.00 (0.99‐1.02) | 0.73 | — | — |
| Duration of diabetes | 0.98 (0.96‐1.01) | 0.18 | — | — |
| Regular exercise | ||||
| Yes | 1.15 (0.92‐1.43) | 0.22 | — | — |
| No | 1.00 | |||
| Systolic BP, each 10 mm Hg | 1.10 (1.05‐1.15) | 0.0001 | 1.08 (1.04‐1.14) | 0.0005 |
| HBA1C > 7% | 1.19 (0.81‐1.73) | 0.38 | — | — |
| BMI > 30 kg/m2 | 0.81 (0.63‐1.05) | 0.12 | — | — |
| Use of ACE‐I and or ARB | ||||
| None | 1.00 | — | — | |
| ACE‐I | 0.91 (0.71‐1.16) | 0.43 | ||
| ARB | 0.86 (0.66‐1.16) | 0.36 | ||
| ARB + ACE‐I | 0.59 (0.23‐1.51) | 0.27 | ||
| Use of herbal preparations | 1.20 (0.90‐1.61) | 0.21 | — | — |
Interaction between educational attainment and mean systolic blood pressure, adjusted OR (95% CI) = 1.01 (0.94‐1.09), P = .79.
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; HbA1C, glycated hemoglobin concentration; SD, standard deviation.
Figure 1.
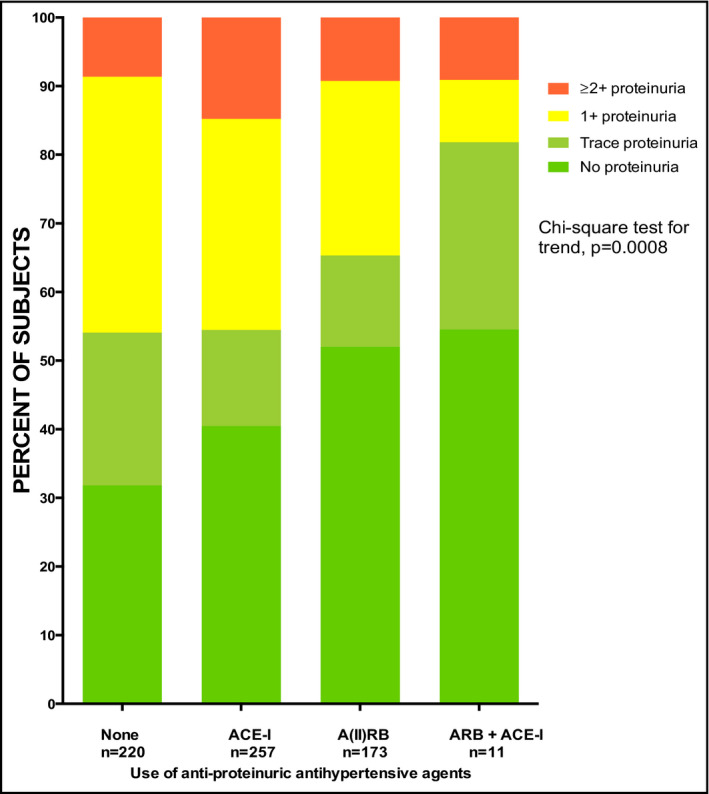
Use of antiproteinuric antihypertensive medications on frequency of proteinuria among renally impaired Ghanaian patients with hypertension and or diabetes. ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers
4. DISCUSSION
This is the first multicenter study in Ghana to describe CKD among patients with type II DM, hypertension, and those with both hypertension and type II DM. As expected, we found a highest prevalence of CKD among patients with both type II DM and hypertension. The prevalence of hypertension in Ghana is up to 54.6% according to the Ghana Demographic and Health Survey.12 Urban dwellers have the highest prevalence as compared to rural dwellers.23 The prevalence of CKD among patients with hypertension was 26.3% which is less than that reported in an earlier single‐center study in Ghana showing a prevalence of CKD of 46.9% among hypertensive patients.13 Hypertension has also been shown to be the most common cause of CKD in some studies across Africa.24, 25 Hypertension is both a presentation and a cause of CKD because the kidney plays a significant role in the control of blood pressure and may predict underlying kidney disease.26 Poorly controlled hypertension also leads to rapid deterioration in renal function culminating in end‐stage kidney disease (ESKD). Hypertension was, however, found to be the third most common causes of CKD in a single‐center study in Ghana.18
The prevalence of CKD among participants with both type II DM and hypertension was 28.5%. DM is a known cause of hypertension in the context of diabetic kidney disease.27 DM is the second most common cause of CKD in Ghana as shown in a single‐center study.18 This is contrary to studies from developed countries where DM is the most common cause of CKD accounting for the majority of patients on renal replacement therapy globally.5 The total number of people with DM worldwide is projected to rise from 171 million in 2000 to 366 million in 2030 with an estimated prevalence of 4.4% from 2.8% in 2000.16 In Ghana, the prevalence of DM was found to be 6.4% in a community‐based study 17 which is higher than the estimated global prevalence in 2030.16
Participants with CKD were significantly older than those without CKD in our study. Glomerular filtration rate decreases by 0.75 mL/min/year after the third decade in normal individuals.28 There is progressive deterioration of kidney function with aging. This has been attributed to decrease in renal mass and increased incidence of sclerotic glomeruli in normal individuals with age. This will be further worsened with acquired conditions causing kidney disease such as hypertension or DM.29 Age has also been shown to be an independent predictor of CKD.30 Those with CKD were also mainly males as female gender was protective. There is evidence suggesting that males have a higher predilection for CKD. Male gender is also associated with faster progression of CKD.31 Increasing duration of hypertension and DM was also associated with CKD as progression of CKD requires time in its pathogenesis especially in type I DM.32
Those involved in less physical activity were also at increased risk of developing CKD. Decreased physical activity leads to obesity. Obesity is associated with diseases such as hypertension and DM. Increased BMI has been shown to lead to CKD through DM and hypertension and through other pathophysiology such as hyperfiltration leading to focal segmental glomerulosclerosis which presents with heavy proteinuria.33 Physical inactivity has also been associated with the occurrence of incident stroke in the present cohort 34 and indeed among West Africans.35
Chronic use of herbal medications increases risk of kidney disease. The use of herbs has been associated with acute kidney injury, tubular dysfunctions, hypertension, chronic kidney disease, renal papillary necrosis, and even urothelial carcinomas.36 There is evidence worldwide and in Africa to implicate the use of herbal medications as a cause of kidney disease.37
The use of ACE‐I among the cohort was protective of neither CKD nor proteinuria. This contrasts major guideline suggesting the benefits with the use of ACE‐I or ARBs in decreasing proteinuria and preventing progression of kidney disease.38 Most patients are on low or conventional doses of ACE‐I or ARBs but high doses have been shown to improve proteinuria and prevent CKD progression.39 They may not be compliant with their medications as expected. Non‐compliance has been shown to be as high as 93% among patients with hypertension in Ghana with unaffordability as a major reason.40 The concomitant use of other medications such as herbal medications that may be worsening their renal functions cannot be ruled out. ACE‐I or ARBs have also been associated with acute kidney injury.41 The use of ACE‐I and or ARBs can also be associated with CKD by decreasing glomerular filtration rate more especially with the concomitant use of NSAIDs which are very common in the cohort as most patients are prescribed NSAIDS for the musculoskeletal pains or buy them over the counter. ACE inhibitors can also cause CKD in a short run as a nephrotoxin and may account for the non‐protection in our cohort. However, among patients with renal impairment with available urinalysis data, dual blockade with ARB and ACE‐I was most protective against proteinuria than ACE‐I or ARBs alone as shown in literature.42 Dual blockade has, however, been shown in a meta‐analysis of randomized control trials to be associated with hyperkalemia, hypotension, and worsening renal failure 43 therefore limiting its use in clinical settings among clinicians.
4.1. Limitations
Although multicenter in nature with good sample size, our study had a number of limitations. Because of the cross‐sectional study design, no causal inferences can be drawn between the observed factors associated with CKD. Only single measurements of serum creatinine and urine protein were performed, and hence, acute, transient derangements in these parameters cannot be ruled out. We used semiquantitative methods for assessing proteinuria instead of urine albumin‐to‐creatinine ratio as suggested by KDIGO. We suggest further studies to follow‐up on patients with hypertension and DM to ascertain progression of renal progression with time.
4.2. Implications
Our study has identified potentially modifiable factors for remediation to avert CKD among hypertensive and diabetic patients in developing countries. Among patients with hypertension and DM under routine care, follow‐up measurements of serum creatinine and urinalysis at least once a year would be cost‐saving measure to detect renal impairment, given the high prevalence of CKD in this cohort. Patients should also be advised to avoid herbal medications and ensure blood pressure is controlled to target with antihypertensive medications and adherence to therapeutic lifestyle interventions. We have previously reported that blood pressure 44 and glycemic control are poor among Ghanaians seeking health care in medical facilities.45 We have also shown earlier that CKD independently predicts incident stroke among Ghanaians with diabetes and hypertension 46 and that this increased risk may have genetic underpinnings.47, 48 In this regard, interventions that would enhance adherence to medications such as use of m‐health may be useful to explore in our settings.49, 50 Proteinuria should be treated with high‐dose ACE‐I or ARBs but with extreme caution in combination due to high‐risk hyperkalemia, hypotension, and worsening renal function.43 Clinicians should therefore screen aggressively for and manage CKD among patients with DM and hypertension to halt its progression.
5. CONCLUSION
There is high burden of CKD among patients with type II DM and hypertension in Ghana. Increasing age, systolic blood pressures, low educational status, increase duration of hypertension, and reported use of herbal preparations were significantly associated with CKD. Optimizing blood pressure control and limiting the use of herbal preparations are interventions that may mitigate the rising burden of CKD in high cardiovascular risk populations in developing countries.
DISCLOSURES
None.
Tannor EK, Sarfo FS, Mobula LM, Sarfo‐Kantanka O, Adu‐Gyamfi R, Plange‐Rhule J. Prevalence and predictors of chronic kidney disease among Ghanaian patients with hypertension and diabetes mellitus: A multicenter cross‐sectional study. J Clin Hypertens. 2019;21:1542–1550. 10.1111/jch.13672
Funding information
Funding for this study was provided by MSD, Novartis, Pfizer, Sanofi, and the Bill and Melinda Gates Foundation (collectively, the Funders) through the New Venture Fund (NVF). The funders had no role in study design, data collection, data analysis, or in study report writing.
REFERENCES
- 1. Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low‐income and middle‐income countries. Lancet. 2007;370(9603):1929‐1938. [DOI] [PubMed] [Google Scholar]
- 2. Popkin BM. The nutrition transition in low‐income countries: an emerging crisis. Nutr Rev. 1994;52(9):285‐298. [DOI] [PubMed] [Google Scholar]
- 3. Miranda JJ, Kinra S, Casas JP, Davey Smith G, Ebrahim S. Non‐communicable diseases in low‐and middle‐income countries: context, determinants and health policy. Tropical Med Int Health. 2008;13(10):1225‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seck SM, Diallo IM, Diagne S. Epidemiological patterns of chronic kidney disease in black African elders: a retrospective study in West Africa. Saudi J Kidney Dis Transpl. 2013;24(5):1068. [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038‐2047. [DOI] [PubMed] [Google Scholar]
- 6. Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub‐Saharan Africa: a systematic review and meta‐analysis. Lancet Global Health. 2014;2(3):e174‐e181. [DOI] [PubMed] [Google Scholar]
- 7. Adjei DN, Stronks K, Adu D, et al. Chronic kidney disease burden among African migrants in three European countries and in urban and rural Ghana: the RODAM cross‐sectional study. Nephrol Dial Transplant. 2018;33(10):1812‐1822. [DOI] [PubMed] [Google Scholar]
- 8. Tannor EK. Chronic kidney disease‐The ‘neglected’ Non‐Communicable Disease in Ghana. Afr J Curr Med Res. 2018;2:1. [Google Scholar]
- 9. Tannor EK, Norman BR, Adusei KK, Sarfo FS, Davids MR, Bedu‐Addo G. Quality of life among patients with moderate to advanced chronic kidney disease in Ghana‐a single centre study. BMC Nephrol. 2019;20(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Batlle D, Ramadugu P, Soler M. Progress in retarding the progression of advanced chronic kidney disease: grounds for optimism. Kidney Int. 2006;70:S40‐S44. [Google Scholar]
- 11. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 12. Service GS, Research NMIfM, MEASURE/DHS+ OM . Ghana demographic and health survey, 2003: Ghana Statistical Service; 2004.
- 13. Osafo C, Mate‐Kole M, Affram K, Adu D. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail. 2011;33(4):388‐392. [DOI] [PubMed] [Google Scholar]
- 14. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629‐636. [DOI] [PubMed] [Google Scholar]
- 15. Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047‐1053. [DOI] [PubMed] [Google Scholar]
- 17. Amoah AG, Owusu SK, Adjei S. Diabetes in Ghana: a community based prevalence study in Greater Accra. Diabetes Res Clin Pract. 2002;56(3):197‐205. [DOI] [PubMed] [Google Scholar]
- 18. Amoako YA, Laryea DO, Bedu‐Addo G, et al. Clinical and demographic characteristics of chronic kidney disease patients in a tertiary facility in Ghana. Pan Afr Med J. 2014;18(274). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kankeu HT, Saksena P, Xu K, Evans DB. The financial burden from non‐communicable diseases in low‐and middle‐income countries: a literature review. Health Res Policy Syst. 2013;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mobula LM, Sarfo S, Arthur L, et al. A multi‐center prospective cohort study to evaluate the effect of differential pricing and health systems strengthening on access to medicines and management of hypertension and diabetes in Ghana: a study protocol. Gates Open Res. 2018;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Eckardt K‐U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089‐2100. [DOI] [PubMed] [Google Scholar]
- 23. Bosu WK. Epidemic of hypertension in Ghana: a systematic review. BMC Public Health. 2010;10(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arogundade FA, Barsoum RS. CKD prevention in Sub‐Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis. 2008;51(3):515‐523. [DOI] [PubMed] [Google Scholar]
- 25. Naicker S. End‐stage renal disease in sub‐Saharan and South Africa. Kidney Int. 2003;63:S119‐S122. [DOI] [PubMed] [Google Scholar]
- 26. Ritz E. Hypertension and kidney disease. Clin Nephrol. 2010;74:S39‐43. [PubMed] [Google Scholar]
- 27. Ritz E, Rychlík I, Locatelli F, Halimi S. End‐stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34(5):795‐808. [DOI] [PubMed] [Google Scholar]
- 28. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278‐285. [DOI] [PubMed] [Google Scholar]
- 29. Anderson S, Brenner BM. Effects of aging on the renal glomerulus. Am J Med. 1986;80(3):435‐442. [DOI] [PubMed] [Google Scholar]
- 30. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 31. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease a meta‐analysis. J Am Soc Nephrol. 2000;11(2):319‐329. [DOI] [PubMed] [Google Scholar]
- 32. Andersen A, Christiansen JS, Andersen J, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin‐dependent) diabetes: an epidemiological study. Diabetologia. 1983;25(6):496‐501. [DOI] [PubMed] [Google Scholar]
- 33. Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 34. Sarfo FS, Mobula LM, Plange‐Rhule J, Ansong D, Ofori‐Adjei D. Incident stroke among Ghanaians with hypertension and diabetes: a multicenter, prospective cohort study. J Neurol Sci. 2018;395:17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarfo FS, Ovbiagele B, Gebregziabher M, et al. Stroke among young West Africans: evidence from the SIREN (Stroke Investigative Research and Educational Network) large multisite case‐control study. Stroke. 2018;49(5):1116‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barnes PM, Bloom B,Nahin RL.Complementary and alternative medicine use among adults and children: United States, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Hyattsville, MD; 2008. [PubMed]
- 37. Bagnis CI, Deray G, Baumelou A, Le Quintrec M, Vanherweghem JL. Herbs and the kidney. Am J Kidney Dis. 2004;44(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 38. Locatelli F, Nissenson AR, Barrett BJ, et al. Clinical practice guidelines for anemia in chronic kidney disease: problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2008;74(10):1237‐1240. [DOI] [PubMed] [Google Scholar]
- 39. Hollenberg NK, Parving H‐H, Viberti G, et al. Albuminuria response to very high‐dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25(9):1921‐1926. [DOI] [PubMed] [Google Scholar]
- 40. Buabeng KO, Matowe L, Plange‐Rhule J. Unaffordable drug prices: the major cause of non‐compliance with hypertension medication in Ghana. J Pharm Pharmaceut Sci. 2004;7(3):350‐352. [PubMed] [Google Scholar]
- 41. Navis G, Faber HJ, de Zeeuw D, de Jong PE. ACE inhibitors and the kidney. Drug Saf. 1996;15(3):200‐211. [DOI] [PubMed] [Google Scholar]
- 42. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin‐angiotensin system in patients with hypertension, microalbuminuria, and non‐insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin‐angiotensin system: meta‐analysis of randomised trials. BMJ. 2013;346:f360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarfo FS, Mobula LM, Burnham G, et al. Factors associated with uncontrolled blood pressure among Ghanaians: Evidence from a multicenter hospital‐based study. PLoS ONE. 2018;13(3):e0193494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mobula LM, Sarfo FS, Carson KA, et al. Predictors of glycemic control in type‐2 diabetes mellitus: evidence from a multicenter study in Ghana. Transl Metab Syndr Res. 2018;1:1‐8. [Google Scholar]
- 46. Sarfo FS, Mobula LM, Sarfo‐Kantanka O, et al. Estimated glomerular filtration rate predicts incident stroke among Ghanaians with diabetes and hypertension. J Neurol Sci. 2019;396:140‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akinyemi R, Tiwari HK, Arnett DK, et al. APOL1, CDKN2A/CDKN2B, and HDAC9 polymorphisms and small vessel ischemic stroke. Acta Neurol Scand. 2018;137(1):133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parsa A, Kao WL, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sarfo F, Treiber F, Gebregziabher M, et al. PINGS (Phone‐Based Intervention Under Nurse Guidance After Stroke) interim results of a pilot randomized controlled trial. Stroke. 2018;49(1):236‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sarfo FS, Treiber F, Jenkins C, et al. Phone‐based Intervention under Nurse Guidance after Stroke (PINGS): study protocol for a randomized controlled trial. Trials. 2016;17(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]


