Abstract
In a multicenter, randomized trial, we investigated whether the long half‐time dihydropyridine calcium channel blocker amlodipine was more efficacious than the gastrointestinal therapeutic system (GITS) formulation of nifedipine in lowering ambulatory blood pressure (BP) in sustained hypertension (clinic systolic/diastolic BP 140‐179/90‐109 mm Hg and 24‐hour systolic/diastolic BP ≥ 130/80 mm Hg). Eligible patients were randomly assigned to amlodipine 5‐10 mg/day or nifedipine‐GITS 30‐60 mg/day. Ambulatory BP monitoring was performed for 24 hours at baseline and 4‐week treatment and for 48 hours at 8‐week treatment with a dose of medication missed on the second day. After 8‐week treatment, BP was similarly reduced in the amlodipine (n = 257) and nifedipine‐GITS groups (n = 248) for both clinic and ambulatory (24‐hour systolic/diastolic BP 10.3/6.5 vs 10.9/6.3 mm Hg, P ≥ 0.24) measurements. However, after missing a dose of medication, ambulatory BP reductions were greater in the amlodipine than nifedipine‐GITS group, with a significant (P ≤ 0.04) between‐group difference in 24‐hour (–1.2 mm Hg) and daytime diastolic BP (–1.5 mm Hg). In conclusion, amlodipine and nifedipine‐GITS were efficacious in reducing 24‐hour BP. When a dose of medication was missed, amlodipine became more efficacious than nifedipine‐GITS.
Keywords: ambulatory blood pressure, amlodipine, dihydropyridine calcium channel blocker, hypertension, nifedipine‐GITS
1. INTRODUCTION
Current guidelines recommend the use of long‐acting antihypertensive drugs for the chronic management of hypertension.1, 2 Long‐acting compounds are available for all five classes of guideline‐recommended antihypertensive drugs. Nonetheless, various slow‐ or controlled‐release formulations of short‐acting compounds are commonly used in many countries, such as China.3 These slow‐ or controlled‐release formulations are successful in preventing adverse events by reducing the maximum plasma concentration.4 However, these special formulations are often not so successful in elongating the duration of blood pressure–lowering action because of the interindividual variability in the bioavailability and the time of drug ingestion.5 For instance, if a controlled‐release drug is excreted too early or if a dose of medication is delayed or missed, the duration of action would not be sufficiently long to cover 24 hours. If administered on monotherapy, these drugs would be less efficacious in blood pressure lowering and increase blood pressure variability6 and hence may be less protective against cardiovascular events.7
In the class of dihydropyridine calcium channel blockers, amlodipine is a long‐acting compound because of its more than 50 hours of long half‐life time,8, 9 and nifedipine gastrointestinal therapeutic system (GITS) uses a controlled‐release technique.10, 11 Several previous studies compared the 24‐hour blood pressure–lowering effect of these two drugs, but produced inconsistent results.12, 13 One13 but not the other studies demonstrated that amlodipine was more efficacious than nifedipine‐GITS in blood pressure lowering, especially during the trough hours in the morning or after having missed one or two doses of medication. These previous studies had relatively small sample size (<120 randomized patients per group) and hence probably inadequate power. More importantly, these studies were conducted in clinic instead of ambulatory hypertension. Ambulatory blood pressure, compared with clinic blood pressure, substantially improves diagnostic accuracy of hypertension and risk prediction of cardiovascular events.15
The present randomized controlled trial was designed to compare 8‐week open‐label treatment of amlodipine and nifedipine‐GITS monotherapy in controlling ambulatory blood pressure in patients with sustained hypertension, before and after having missed a dose of medication.
2. METHODS
2.1. Study design
The present randomized, actively controlled, parallel‐group trial (ClinicalTrials.gov identifier, NCT01030081) was conducted from November 2009 to February 2013 in five hospitals across China. The study protocol was approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, and, as necessary, also by the ethics committees of the participating hospitals. All patients gave written informed consent.
The study consisted of a 4‐week run‐in washout period and a subsequent 8‐week randomized treatment period (Figure S1). If at a screening visit, previously untreated patients had a systolic/diastolic blood pressure of 140‐179/90‐109 mm Hg, and previously treated patients on monotherapy had a systolic/diastolic blood pressure of 140‐159/90‐99 mm Hg and were willing to discontinue previous antihypertensive therapy, they entered the run‐in period without any treatment. If eligible according to the average of six blood pressure readings obtained at two clinic visits and the 24‐hour ambulatory blood pressure at the end of the run‐in period, patients were randomized into the 8‐week study treatment period with amlodipine or nifedipine‐GITS after stratification for center. The patients were followed up at 4 and 8 weeks of treatment. The study medication could be stopped in the presence of symptomatic hypotension or any other serious adverse events related to the study medication. Patients were instructed to take the study medication at 06:00‐08:00 every morning before breakfast except on the day of clinic visit, when medication was administered after blood pressure was measured and ambulatory blood pressure monitoring was started. The study medication was supplied free of charge for the whole study period.
Clinic blood pressure was measured at each of the clinic visits. Ambulatory blood pressure monitoring was performed for 24 hours at the end of the run‐in period and 4 weeks of treatment and for 48 hours at 8 weeks of treatment. During the 48‐hour ambulatory monitoring, the study medication was taken on the first but not the second day.
2.2. Study population
Men and women of 40‐70 years old were eligible for the trial, if the following inclusion and exclusion criteria were fulfilled. Inclusion criteria were as follows. Blood pressure was in the range of 140‐179 mm Hg systolic or 90‐109 mm Hg diastolic for the average of the six clinic readings and was at least 130 mm Hg systolic or 80 mm Hg diastolic for the 24‐hour ambulatory measurement, regardless of no treatment or treatment with a single drug prior to the entry into the run‐in period. In addition, the patient should be able to attend the clinic visit on his/her own.
Exclusion criteria included the presence of any life‐threatening disease, history of myocardial infarction or stroke within two years, the presence of any contraindication to dihydropyridine calcium channel blockers, and current participation in another trial or trials.
Our study did not exclude patients with diabetes mellitus or chronic kidney disease. We defined diabetes mellitus as a plasma glucose of at least 7.0 mmol/L fasting or as the use of antidiabetic agents and chronic kidney disease as the presence of microalbuminuria or an estimated glomerular filtration rate (eGFR) < 60 mL/min × 1.73 m2. eGFR was calculated using the modified MDRD (Modification of Diet in Renal Disease) equations in Chinese.16
2.3. Antihypertensive treatment and follow‐up
Patients were randomly assigned to amlodipine 5 mg once daily or to nifedipine‐GITS 30 mg once daily using a computer‐based system. At 4 weeks of follow‐up, the study medication could be up‐titrated to amlodipine 10 mg once daily or nifedipine‐GITS 60 mg once daily to control clinic systolic/diastolic blood pressure to a level below 140/90 mm Hg.
At 4 and 8 weeks of treatment, patients were followed up in normal working hours in the morning. The precise follow‐up time was recorded. At each follow‐up visit, in addition to the clinic and ambulatory blood pressure measurements, the responsible physician measured pulse rate and collected information on the use of medications, adverse events, and serious adverse events. Patients’ compliance to drug treatment was evaluated by the pill count approach at 4 and 8 weeks of follow‐up.
2.4. Clinic and ambulatory blood pressure measurements
Clinic blood pressure was measured three times consecutively with a 30‐ to 60‐second interval after at least five‐minute rest in the sitting position using a validated automated blood pressure monitor (HEM 705, Omron Healthcare, Kyoto, Japan). These three blood pressure readings were averaged for the clinical decision at 4 weeks of follow‐up and for the present analysis.
Ambulatory blood pressure monitoring was performed using the monitors routinely used at each of the participating hospitals. The ambulatory blood pressure monitors included SpaceLabs 90207 and 90217 (SpaceLabs, Redmond, WA, USA), Mobil‐O‐Graph (IEM. GmbH, Stolberg, Germany), A&D TM2430 (A&D CO., LTD., Tokyo, Japan), CB (BIOX Instruments CO., LTD., Wuxi, Jiangsu Province, China), and MGY‐ABP1 (Meigaoyi, Beijing, China). We programmed these oscillometric ambulatory blood pressure monitors to obtain ambulatory blood pressure readings at 20‐minute intervals in the day (06:00‐22:00) and at 30‐minute intervals at night (22:00‐06:00). Daytime, nighttime, and morning were defined as the clock time intervals from 08:00 to 18:00, from 23:00 to 05:00, and from 05:00 to 08:00, respectively. Valid recordings covered more than 20 hours and included at least 10 in the daytime and five readings at night. The 24‐hour mean values of blood pressure and pulse rate were weighted for the time interval between consecutive readings. For the 48‐hour monitoring, mean values were calculated for the first and second 24‐hour periods separately.
For both clinic and ambulatory blood pressure measurements, a standard cuff was used when the arm circumference was 32 cm or smaller. Otherwise, a large cuff was used. To avoid the influence of inter‐arm blood pressure difference on blood pressure evaluations,17 both clinic and ambulatory measurements were performed on the left arm during the whole trial.
2.5. Efficacy and safety evaluations
The primary efficacy variable was the change from baseline in the mean systolic blood pressure during the morning hours (05:00‐08:00) of the first 24‐hour ambulatory blood pressure recording at 8 weeks after randomization.
The secondary efficacy variables included the change from baseline in the mean systolic blood pressure during the morning hours (05:00‐08:00) of the 24‐hour ambulatory blood pressure recording at 4 weeks of treatment and the change in the mean systolic blood pressure at night (23:00‐5:00) at 4 and 8 weeks of treatment. Other efficacy variables included the changes from baseline in the mean diastolic blood pressure during the morning hours and at night and in the mean systolic and diastolic blood pressure over 24 hours and daytime. The changes from baseline in ambulatory blood pressure after missing a dose of medication at 8 weeks of treatment were also pre‐specified efficacy variables.
All adverse events were documented for information on symptoms, severity, treatment, and outcome. The routine and biochemical tests of blood and urine were performed for clinical laboratory safety evaluations. Any clinically significant changes in physical examinations and laboratory findings were also recorded as adverse events.
2.6. Statistical analysis
Sample size estimation was based on a 12 mm Hg standard deviation of morning systolic blood pressure and a 4 mm Hg difference of morning systolic blood pressure (05:00‐08:00) between amlodipine and nifedipine‐GITS at 8 weeks of follow‐up in favor of amlodipine. If assuming an α = 0.05 and a power = 90%, the study would require a sample size of 190 hypertensive patients per group to detect the projected 4 mm Hg difference of morning systolic blood pressure. After accounting for 5% of dropout rate and 20% of patients on add‐on therapy, the sample size for each group was 250. Thus, the number of required subject for the whole trial was 500.
We performed intention‐to‐treat analyses in all patients who entered the study treatment period and per‐protocol analyses in the patients who completed the 8‐week study on study medication. Safety analyses were performed in all patients who had ever started the study medication. Continuous and categorical variables were compared using the Student t test and chi‐square test, respectively. Blood pressure and pulse rate changes from baseline were calculated by subtracting values at baseline from those during follow‐up. Analysis of covariance was performed to calculate the least square mean changes with standard error and the mean between‐group difference with 95% confidence intervals, with baseline values as covariate and treatment group as a factor.
3. RESULTS
3.1. Baseline characteristics of patients
Of the 529 screened patients, 505 (95.5%) were randomly assigned to receive amlodipine 5 mg per day (n = 257) or nifedipine‐GITS 30 mg per day (n = 248, Figure 1). Table 1 shows the baseline characteristics of the 505 randomized patients, of whom, 459 and 420 completed the 4 and 8 weeks of follow‐up, respectively. The corresponding number of patients with valid 24‐hour ambulatory recordings was 417 and 387, respectively. A valid 48‐hour ambulatory recording was obtained at 8 weeks or the end of follow‐up in 363 patients.
Figure 1.
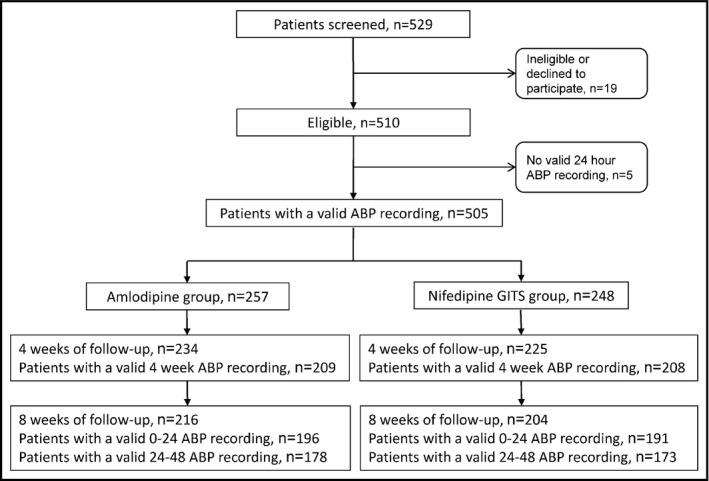
Flow of patients
Table 1.
Baseline characteristics of the study subjects by randomization group
| Characteristics |
Amlodipine (n = 257) |
Nifedipine‐GITS (n = 248) |
P value |
|---|---|---|---|
| Men, n (%) | 141 (54.9) | 131 (52.8) | 0.65 |
| Age, years | 53.7 ± 8.3 | 53.9 ± 7.3 | 0.87 |
| Body mass index, kg/m2 | 25.2 ± 3.2 | 25.3 ± 3.0 | 0.76 |
| Current smoking, n (%) | 76 (29.6) | 65 (26.2) | 0.40 |
| Alcohol intake, n (%) | 77 (30.0) | 62 (25.0) | 0.21 |
| Clinic blood pressure, mm Hg | |||
| Systolic | 151.4 ± 10.2 | 151.2 ± 10.2 | 0.85 |
| Diastolic | 92.5 ± 7.6 | 91.8 ± 8.3 | 0.34 |
| Clinic pulse rate, beats/minute | 75.8 ± 8.8 | 75.7 ± 7.8 | 0.83 |
| Ambulatory blood pressure, mm Hg | |||
| 24‐h systolic | 140.3 ± 10.6 | 139.7 ± 11.2 | 0.59 |
| 24‐h diastolic | 89.1 ± 7.2 | 91.8 ± 8.3 | 0.67 |
| Daytime systolic | 146.4 ± 11.6 | 145.2 ± 11.8 | 0.27 |
| Daytime diastolic | 93.3 ± 8.2 | 92.7 ± 8.1 | 0.37 |
| Nighttime systolic | 128.3 ± 12.9 | 128.4 ± 13.8 | 0.91 |
| Nighttime diastolic | 81.0 ± 8.5 | 81.3 ± 9.5 | 0.70 |
| Morning systolic | 136.3 ± 14.0 | 136.3 ± 13.8 | 0.99 |
| Morning diastolic | 87.6 ± 9.2 | 87.7 ± 8.6 | 0.84 |
| 24‐h ambulatory pulse rate, beats/minute | 73.6 ± 7.8 | 73.4 ± 6.9 | 0.76 |
| Diabetes mellitus, n (%) | 22 (8.6) | 23 (9.3) | 0.78 |
| Chronic kidney disease, n (%) | 45 (17.5) | 37 (14.9) | 0.43 |
Daytime, nighttime, and morning were defined as 08:00‐18:00, 23:00‐05:00, and 05:00‐08:00, respectively. Diabetes mellitus was defined as a plasma glucose of at least 7.0 mmol/L fasting or as the use of antidiabetic agents and chronic kidney disease as the presence of microalbuminuria or as an estimated glomerular filtration rate < 60 mL/min·1.73 m2.
During the study treatment period, amlodipine remained at 5 mg per day in 224 (87.2%) patients and nifedipine‐GITS at 30 mg per day in 213 (85.9%) patients. The corresponding percentages of patients who up‐titrated the study medication were 12.8% (n = 33) and 14.1% (n = 35), respectively. The use of other antihypertensive agents or drugs potentially active in blood pressure lowering was reported in 1 patient enrolled in the nifedipine‐GITS group.
3.2. Efficacy of treatment in the whole trial
In the intention‐to‐treat analysis, systolic blood pressure in the morning (05:00‐08:00) of the first 24‐hour ambulatory monitoring at 8 weeks of follow‐up, the primary efficacy variable, was reduced by 10.2 ± 14.6 and 12.1 ± 14.4 mm Hg in the amlodipine and nifedipine‐GITS groups, respectively (P = 0.14 for the between‐group comparison, Table 2). The corresponding changes in diastolic blood pressure were –6.8 ± 9.6 and −7.3 ± 9.1 mm Hg, respectively (P = 0.55).
Table 2.
Blood pressure and pulse rate changes from baseline after 4 or 8 wk of treatment
| Variables |
Amlodipine (n = 257) |
Nifedipine‐GITS (n = 248) |
Between‐group difference (95% CI) |
P value |
|---|---|---|---|---|
| After 4 wk of treatment | ||||
| Clinic blood pressure, mm Hg | ||||
| Systolic | −14.1 ± 0.8 | −14.7 ± 0.8 | 0.6 (−1.6, 2.8) | 0.59 |
| Diastolic | −8.0 ± 0.4 | −7.8 ± 0.5 | −0.2 (−1.5, 1.1) | 0.78 |
| Clinic pulse rate, beats/minute | 1.9 ± 0.5 | 1.9 ± 0.5 | 0.0 (−1.4, 1.4) | 0.99 |
| Ambulatory blood pressure, mm Hga | ||||
| 24‐h systolic | −10.0 ± 0.6 | −10.4 ± 0.6 | 0.5 (−1.3, 2.2) | 0.61 |
| 24‐h diastolic | −6.0 ± 0.4 | −5.8 ± 0.4 | −0.2 (−1.3, 0.9) | 0.72 |
| Daytime systolic | −10.5 ± 0.8 | −9.5 ± 0.7 | −1.0 (−3.1, 1.0) | 0.32 |
| Daytime diastolic | −6.3 ± 0.5 | −5.1 ± 0.4 | −1.1 (−2.3, 0.1) | 0.07 |
| Nighttime systolic | −8.9 ± 0.8 | −10.7 ± 0.8 | 1.8 (−0.4, 3.9) | 0.10 |
| Nighttime diastolic | −5.5 ± 0.5 | −6.1 ± 0.5 | 0.7 (−0.7, 2.1) | 0.35 |
| Morning (5‐8 am) systolic | −9.4 ± 0.8 | −11.0 ± 0.9 | 1.5 (−0.8, 3.9) | 0.21 |
| Morning (5‐8 am) diastolic | −6.1 ± 0.6 | −6.8 ± 0.5 | 0.8 (−0.8, 2.3) | 0.34 |
| 24‐h ambulatory pulse rate, beats/minute | 1.2 ± 0.4 | 1.6 ± 0.4 | −0.4 (−1.4, 0.6) | 0.44 |
| After 8 wk of treatment | ||||
| Clinic blood pressure, mm Hg | ||||
| Systolic | −14.5 ± 0.8 | −14.4 ± 0.8 | −0.1 (−2.4, 2.2) | 0.91 |
| Diastolic | −7.5 ± 0.5 | −6.7 ± 0.5 | −0.8 (−2.1, 0.5) | 0.24 |
| Clinic pulse rate, beats/minute | −1.1 ± 0.5 | −1.3 ± 0.5 | 0.2 (−1.2, 1.6) | 0.78 |
| Ambulatory blood pressure, mm Hga | ||||
| 24‐h systolic | −10.3 ± 0.7 | −10.9 ± 0.7 | 0.6 (−1.3, 2.5) | 0.56 |
| 24‐h diastolic | −6.5 ± 0.4 | −6.3 ± 0.4 | −0.1 (−1.3, 1.0) | 0.83 |
| Daytime systolic | −10.7 ± 0.8 | −10.5 ± 0.8 | −0.2 (−2.4, 2.0) | 0.85 |
| Daytime diastolic | −6.6 ± 0.5 | −6.2 ± 0.5 | −0.5 (−1.8, 0.9) | 0.49 |
| Nighttime systolic | −9.0 ± 0.8 | −10.7 ± 0.8 | 1.7 (−0.5, 3.9) | 0.12 |
| Nighttime diastolic | −5.7 ± 0.5 | −6.6 ± 0.5 | 0.9 (−0.6, 2.4) | 0.22 |
| Morning (5‐8 am) systolic | −10.2 ± 0.9 | −12.1 ± 0.9 | 1.9 (−0.6, 4.5) | 0.14 |
| Morning (5‐8 am) diastolic | −6.8 ± 0.6 | −7.3 ± 0.6 | 0.5 (−1.1, 2.1) | 0.55 |
| 24‐h ambulatory pulse rate, beats/minute | 1.0 ± 0.4 | 1.1 ± 0.3 | −0.1 (−1.0, 0.8) | 0.84 |
Values per group are least square mean change from baseline ± standard error. The between‐group difference was calculated by subtracting the blood pressure or pulse rate changes from baseline in the nifedipine‐GITS group from those in the amlodipine group. Negative values of the between‐group difference indicate greater blood pressure reduction in the amlodipine than nifedipine‐GITS group. For further explanations, see Methods.
On the first day of the 48‐h ambulatory blood pressure recording.
The ambulatory blood pressure changes from baseline did not significantly differ between the two treatment groups for systolic and diastolic blood pressure over 24 hours, in the daytime, and at night at either 4 or 8 weeks of follow‐up (Table 2). After having missed a dose of study medication at 8 weeks of follow‐up, ambulatory blood pressure changes were greater in the amlodipine than nifedipine‐GITS group (Figure 2), with a significant between‐group difference in 24‐hour (–1.2 mm Hg, 95% confidence interval −1.8 to −0.6, P = 0.04) and daytime diastolic blood pressure (−1.5 mm Hg, 95% confidence interval −2.2 to 0.8 mm Hg, P = 0.02, Table 3, Figure 3).
Figure 2.
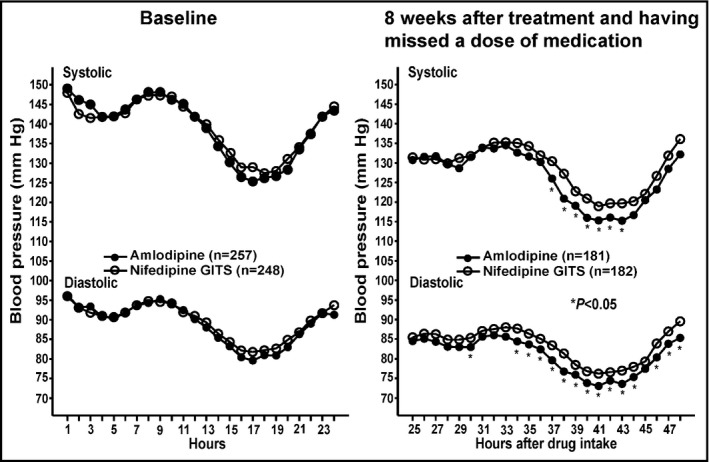
Ambulatory systolic and diastolic blood pressure at baseline (left) and after 8 wk of treatment and having missed a dose of medication of amlodipine (dot) and nifedipine‐GITS (circle) according to hours after drug intake (right). Asterisks denote P value ≤ 0.05 for the difference between the two groups
Table 3.
Ambulatory blood pressure and pulse rate change from baseline after 8 wk of treatment and having missed a dose of medication
| Variables |
Amlodipine (n = 257) |
Nifedipine‐GITS (n = 248) |
Between‐group difference (95% CI) |
P value |
|---|---|---|---|---|
| Ambulatory blood pressure, mm Hg | ||||
| 24‐h systolic | −8.7 ± 0.6 | −7.4 ± 0.7 | −1.3 (−3.1, 0.6) | 0.19 |
| 24‐h diastolic | −5.5 ± 0.4 | −4.3 ± 0.4 | −1.2 (−2.3, −0.1) | 0.04 |
| Daytime SBP | −9.5 ± 0.7 | −8.3 ± 0.8 | −1.2 (−3.3, 0.9) | 0.27 |
| Daytime DBP | −6.0 ± 0.5 | −4.5 ± 0.5 | −1.5 (−2.8, −0.2) | 0.02 |
| Nighttime SBP | −7.3 ± 0.7 | −6.3 ± 0.7 | −1.0 (−3.0, 1.1) | 0.34 |
| Nighttime DBP | −4.7 ± 0.5 | −4.3 ± 0.5 | −0.4 (−1.6, 1.0) | 0.63 |
| Morning (5‐8 am) SBP | −8.8 ± 0.8 | −6.7 ± 0.9 | −2.1 (−4.4, 0.2) | 0.08 |
| Morning (5‐8 am) DBP | −5.6 ± 0.6 | −4.0 ± 0.6 | −1.6 (−3.2, 0.1) | 0.06 |
| 24‐h ambulatory pulse rate, beats/minute | 0.9 ± 0.3 | 0.1 ± 0.3 | 0.8 (−0.1, 1.7) | 0.09 |
Values per group are least square mean changes from baseline ± standard error. The between‐group difference was calculated by subtracting the blood pressure or pulse rate changes from baseline in the nifedipine‐GITS group from those in the amlodipine group. Negative values of the between‐group difference indicate greater blood pressure reduction in the amlodipine than nifedipine‐GITS group. For further explanations, see Methods.
Figure 3.

Twenty‐four‐hour, daytime, nighttime, and morning blood pressure changes from baseline after 8 wk of treatment and having missed a dose of medication. Black and gray bars denote mean change from baseline in the amlodipine and nifedipine‐GITS groups, respectively. Vertical lines denote standard error. The P value is given for the difference between two groups
Clinic systolic/diastolic blood pressures were significantly (P < 0.0001 for the changes from baseline) and similarly (P ≥ 0.24 for the between‐group comparisons) reduced by a mean of 14.5/7.5 and 14.4/6.7 mm Hg in the amlodipine and nifedipine‐GITS groups, respectively, at 8 weeks of treatment (Table 2). The corresponding percentages of patients who attained the goal clinic systolic/diastolic blood pressure (<140/90 mm Hg) were 50.2% and 52.0%, respectively (P = 0.68).
Pulse rate was significantly (P ≤ 0.03) reduced in both the amlodipine (–1.1 ± 7.9 beat/min) and nifedipine‐GITS groups (–1.3 ± 7.7 beat/min) on clinic measurement. But it significantly (P ≤ 0.008) increased on ambulatory measurement (Table 2).
In the per‐protocol analysis, similar findings were observed for ambulatory and clinic blood pressures and pulse rate (data not shown).
3.3. Subgroup analyses
We performed subgroup analyses on blood pressure control in 83 (16.4%) patients with chronic kidney disease at baseline and in 78 (15.4%) patients who up‐titrated the study medication during follow‐up.
In patients with chronic kidney disease at baseline, the blood pressure changes from baseline tended to be slightly smaller in the amlodipine (n = 45) than nifedipine‐GITS group (n = 38) for both clinic and ambulatory measurements at 4 and 8 weeks of treatment (Table S1). Statistical significance was achieved for the nighttime systolic blood pressure with a between‐group mean difference of 5.6 mm Hg (95% confidence interval 0.3‐10.9, P = 0.04) in favor of nifedipine‐GITS. Accordingly, the percentage of patients who attained the goal clinic systolic/diastolic blood pressure was slightly lower in the amlodipine than nifedipine‐GITS group (35.6% vs 47.4%, P = 0.23). After having missed a dose, the blood pressure changes from baseline were not statistically different between the two groups (P ≥ 0.30, Table S1).
In patients who up‐titrated the study medication during follow‐up, the clinic and ambulatory blood pressure changes from baseline were similar between the amlodipine (n = 33) and nifedipine‐GITS groups (n = 35, P ≥ 0.08, Table S2). The percentage of patients who attained the goal clinic systolic/diastolic blood pressure was also similar in the amlodipine and nifedipine‐GITS groups (33.3% vs 37.1%, P = 0.74).
We also performed subgroup analyses according to the use of various ambulatory blood pressure monitors and did not find any significant difference in the ambulatory blood pressure reductions from baseline (P ≥ 0.08, Table S3).
3.4. Safety
During the whole study treatment period, no serious adverse event was reported. Adverse event was reported in 40 (15.6%) and 42 (16.9%) patients in the amlodipine and nifedipine‐GITS groups, respectively (P = 0.68, Table 4). The incidence of headache tended to be lower in the amlodipine than nifedipine‐GITS group (1.6% vs 4.0%, P = 0.09).
Table 4.
Incidence rate of adverse events
| Adverse events |
Amlodipine (n = 257) |
Nifedipine‐GITS (n = 248) |
P value |
|---|---|---|---|
| Number of patients (%) | Number of patients (%) | ||
| Elevation of alanine or aspartate transaminase | 10 (3.9) | 8 (3.2) | 0.69 |
| Dizziness | 7 (2.7) | 6 (2.4) | 0.83 |
| Ankle edema | 7 (2.7) | 4 (1.6) | 0.39 |
| Erubescence | 5 (1.9) | 5 (2.0) | 0.79 |
| Headache | 4 (1.6) | 10 (4.0) | 0.09 |
| Joint pain | 3 (1.2) | 0 | 0.26 |
| Palpitation | 2 (0.8) | 4 (1.6) | 0.65 |
| Cough | 2 (0.8) | 2 (0.8) | 0.64 |
| Constipation | 0 | 3 (1.2) | 0.23 |
| Total | 40 (15.6) | 42 (16.9) | 0.68 |
Adverse events are listed in the descending order of the incidence rate in the amlodipine group and then in the nifedipine‐GITS group.
In 410 (81.2%) of the 505 randomized patients, serum creatinine and urinary albumin excretion were re‐measured at the end of follow‐up. In 350 (85.4%) patients without chronic kidney disease at baseline, 51 (12.4%) developed incident chronic kidney disease during follow‐up, with a similar rate in the amlodipine (n = 28, 15.6%) and nifedipine‐GITS groups (n = 23, 13.5%; P = 0.59). In 60 (14.6%) patients with chronic kidney disease at baseline, 22 (36.7%) had normal eGFR and urinary albumin excretion at the end of follow‐up, with a slightly but non‐significantly (P = 0.28) higher rate in the amlodipine (n = 13, 43.3%) than nifedipine‐GITS group (n = 9, 30.0%).
4. DISCUSSION
Our study in sustained hypertension showed that long‐acting dihydropyridine calcium channel blockers amlodipine and nifedipine‐GITS had similar blood pressure–lowering effects on clinic and ambulatory measurements. Only when a dose of medication was missed, amlodipine was more efficacious in lowering ambulatory diastolic blood pressure.
Our study did not show significant difference in the primary efficacy variable, systolic blood pressure in the morning. The difference became significant beyond 24 hours of drug ingestion. These findings are in keeping with the results of three previous studies of similar design in clinic hypertension.12, 14, 18
In a randomized double‐blind crossover study, 42 patients were randomly allocated to amlodipine (5‐10 mg) or nifedipine‐GITS (30‐60 mg) once daily for 12 weeks.14 At 8, 10, and 12 weeks of the study treatment period, patients missed zero, one, or two doses of the study medication. Amlodipine and nifedipine‐GITS had similar blood pressure–lowering effect at 0‐24 hours post‐dose. After one or two doses of medication were missed, amlodipine, compared with nifedipine‐GITS, had a greater diastolic blood pressure–lowering effect at 24‐48 hours post‐dose (3.3 mm Hg, P = 0.005) and at 48‐72 hours post‐dose (5.5 mm Hg, P < 0.0001). The study also demonstrated that at 48 hours post‐dose, the plasma concentration of nifedipine was only 30% of the 24‐hour post‐dose concentration (from 115.0 ± 63.0 to 34.3 ± 29.6 ng/mL), while the plasma concentration of amlodipine remained at 79% of the 24‐hour post‐dose value (from 28.0 ± 16.0 to 22.1 ± 13.3 ng/mL). At 72 hours post‐dose, the corresponding percentages were <25% and 61%, respectively.
Two double‐blind, double‐dummy, parallel‐group comparison trials also compared blood pressure–lowering efficacy after having missed one or two doses of medication. In an earlier study in 58 patients, amlodipine maintained blood pressure–lowering effect up to 72 hours post‐dose at 58% and 60% of the peak effect on systolic and diastolic pressure, respectively.18 The corresponding values for nifedipine‐GITS were 14% and 16%, respectively. In a later and larger study in Asians (n = 222), similar results were observed.12 The mean ambulatory blood pressure rise (±standard deviation) during the last 9 hours on day two after having missed a dose was significantly less with amlodipine than with nifedipine‐GITS (4.4 ± 7.0 vs 11.2 ± 11.3 mm Hg for systolic blood pressure and 2.4 ± 6.3 mm Hg vs 6.0 ± 6.0 mm Hg for diastolic blood pressure, P ≤ 0.0002).
Taken the results of these previous studies12, 14, 18 and our present study together, amlodipine but not nifedipine‐GITS may maintain its blood pressure–lowering effect after having missed one or two doses of medication. This pharmacologic difference may have clinical implications when a patient's drug adherence is low. Indeed, patient compliance in the chronic management of hypertension is often not more than 50%.19, 20 The clinical significance of these findings may also be extrapolated to the delay of medication dosing. When a dose is delayed for hours on monotherapy, blood pressure–lowering efficacy of nifedipine‐GITS may shrink substantially.
Our observation in the subgroup of patients with chronic kidney disease at baseline is intriguing, but remains incompletely understood. A possible explanation is that the elimination time increases in the presence of chronic kidney disease for nifedipine‐GITS but not amlodipine. In this case, nifedipine‐GITS would have a prolonged and probably also increased blood pressure–lowering effect. There is no direct pharmacokinetic comparison study between these two drugs. However, two previous studies provided useful evidence by comparing bedtime with morning dosing of nifedipine‐GITS.22, 23 Bedtime, compared with morning, dosing significantly increased the blood pressure–lowering efficacy of nifedipine‐GITS over 24 hours and in the morning. The time‐to‐peak concentration was longer in bedtime than morning dosing of nifedipine‐GITS. It would be therefore clinically relevant to investigate whether the blood pressure–lowering effect of amlodipine and nifedipine‐GITS be dependent on renal function in future data analysis or randomized controlled trials.
Our study should be interpreted in the context of its strengths and limitations. All of our randomized patients had sustained hypertension. Repeated ambulatory blood pressure recordings were performed during follow‐up. However, a relatively large proportion of patients did not complete ambulatory recordings, especially at 8 weeks of follow‐up. The incomplete follow‐up of ambulatory blood pressure monitoring may to some extent influence our study results. However, the results in the per‐protocol analysis, restricting to patients with complete follow‐up, were not different from those in the intention‐to‐treat analysis in all study participants. Despite that only about half of the patients reached the goal clinic blood pressure in both groups, few patients up‐titrated their study medication. Underdose of medication may decrease the power of our study in the detection of a possible significant difference between the two treatment groups. Nonetheless, the magnitude of the blood pressure reductions was similar to that of several previous studies on monotherapy of calcium channel blockers.24, 25 Finally, the open‐label design of our study may also have affected the results, for example, via changes in lifestyle.
In conclusion, both amlodipine and nifedipine‐GITS are efficacious in reducing clinic and ambulatory blood pressure. However, when a dose of medication is missed or delayed, amlodipine was more efficacious than nifedipine‐GITS in lowering blood pressure.
DISCLOSURES
Dr Wang reports having received consulting and lecture fees from Pfizer China (Shanghai). The other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
This appendix formed part of the original submission and has been peer‐reviewed. Author contributions for: A randomized controlled trial on the blood pressure‐lowering effect of amlodipine and nifedipine‐GITS in sustained hypertension. Ji‐Guang Wang involved in conceptualization, funding acquisition, project administration, resources, and validation. Qi‐Fang Huang, Chang‐Sheng Sheng, Yan Li, Yu Dou, Mei‐Sheng Zheng, and Zhi‐Ming Zhu involved in data curation. Qi‐Fang Huang, Chang‐Sheng Sheng, Yan Li, and Ji‐Guang Wang participated in the formal analysis. Qi‐Fang Huang, Yan Li, Ji‐Guang Wang involved in methodology. Qi‐Fang Huang involved in software programming. Yan Li, Yu Dou, Mei‐Sheng Zheng, Zhi‐Ming Zhu, and Ji‐Guang Wang involved in supervision. Ji‐Guang Wang involved in validation. Qi‐Fang Huang and Chang‐Sheng Sheng involved in visualization. Qi‐Fang Huang, Ji‐Guang Wang wrote the original draft. Qi‐Fang Huang, Chang‐Sheng Sheng, Yan Li, Yu Dou, Mei‐Sheng Zheng, Zhi‐Ming Zhu, Ji‐Guang Wang investigated, wrote, reviewed and edited the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors gratefully acknowledge the participation of the patients and the contribution of the investigators from 5 hospitals. The participating hospitals are listed in the order of the number of randomized patients, with the principal investigator and the number of randomized patients in the parenthesis. Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai (Yan Li, n = 340), Jiangsu Province Hospital for Governmental Employees, Nanjing, Jiangsu Province (Yu Dou, n = 83), Wuhu Chinese Medicine Hospital, Wuhu, Anhui Province (Mei‐Sheng Zheng, n = 40), Daping Hospital, The Third Military Medical University, Chongqing (Zhi‐Ming Zhu, n = 30) and Yancheng City No. 1 People's Hospital, Yancheng, Jiangsu Province (Yu‐Lin Wang, n = 12).
Huang Q‐F, Sheng C‐S, Li Y, et al. on behalf of the Amlodipine Morning Blood Pressure Surge Study (ARMORS) Investigators . A randomized controlled trial on the blood pressure–lowering effect of amlodipine and nifedipine‐GITS in sustained hypertension. J Clin Hypertens. 2019;21:648–657. 10.1111/jch.13543
Funding information
The study was partially funded by Pfizer. The authors were also financially supported by grants from the National Natural Science Foundation of China (grants 81170245, 81400312, 91639203, 81721001 and 81770455), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 15XD1503200) and Health (grant 15GWZK0802 and a special grant for “Leading Academics”), Shanghai, China.
REFERENCES
- 1. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 2. Liu LS, Writing Group of 2010 Chinese Guidelines for the Management of Hypretension . 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579‐615. [PubMed] [Google Scholar]
- 3. Yang T, Jiang Y, Hao Y, et al. Comparison of bisoprolol to a metoprolol CR/ZOK tablet for control of heart rate and blood pressure in mild‐to‐moderate hypertensive patients: the CREATIVE study. Hypertens Res. 2017;40:79‐86. [DOI] [PubMed] [Google Scholar]
- 4. Parker RB, Soberman JE. Effects of paroxetine on the pharmacokinetics and pharmacodynamics of immediate‐release and extended‐release metoprolol. Pharmacotherapy. 2011;31:630‐641. [DOI] [PubMed] [Google Scholar]
- 5. Deroubaix X, Lins RL, Lens S, et al. Comparative bioavailability of a metoprolol controlled release formulation and a bisoprolol normal release tablet after single oral dose administration in healthy volunteers. Int J Clin Pharmacol Ther. 1996;34:61‐70. [PubMed] [Google Scholar]
- 6. Fogari R, Preti P, Zoppi A, et al. Effect of telmisartan/hydrochlorothiazide combination versus nifedipine GITS on ambulatory blood pressure and sympathetic activation. Am J Hypertens. 2005;18:577‐583. [DOI] [PubMed] [Google Scholar]
- 7. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. Br Med J. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 1992;80:31‐36. [DOI] [PubMed] [Google Scholar]
- 9. Kim JR, Kim S, Huh W, et al. No pharmacokinetic interactions between candesartan and amlodipine following multiple oral administrations in healthy subjects. Drug Des Devel Ther. 2018;12:2475‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grundy JS, Foster RT. The nifedipine gastrointestinal therapeutic system (GITS) Evaluation of pharmaceutical, pharmacokinetic and pharmacological properties. Clin Pharmacokinet. 1996;30:28‐51. [DOI] [PubMed] [Google Scholar]
- 11. Toal CB, Meredith PA, Elliott HL. Once daily nifedipine: the formulation dictates the pharmacokinetic characteristics and the therapeutic responses. Int J Clin Pharmacol Ther. 2012;50:202‐217. [DOI] [PubMed] [Google Scholar]
- 12. Ongtengco I, Morales D, Sanderson J, et al. Persistence of the antihypertensive efficacy of amlodipine and nifedipine GITS after two 'missed doses': a randomised, double‐blind comparative trial in Asian patients. J Hum Hypertens. 2002;16:805‐813. [DOI] [PubMed] [Google Scholar]
- 13. Nussinovitch N, Rosenberg G, Peleg E, Rosenthal T. A comparative crossover evaluation of amlodipine and nifedipine GITS before and after a missed dose: 48‐h blood pressure profiles. Am J Hypertens. 2002;15:580‐582. [DOI] [PubMed] [Google Scholar]
- 14. Elliott HL, Elawad M, Wilkinson R, et al. Persistence of antihypertensive efficacy after missed dises: comparison of amlodipine and nifedipine gastrointestinal therapeutic system. J Hypertens. 2002;20:333‐338. [DOI] [PubMed] [Google Scholar]
- 15. Balietti P, Spannella F, Giulietti F, et al. Ten‐year changes in ambulatory blood pressure: the prognostic value of ambulatory pulse pressure. J Clin Hypertens. 2018;20:1230‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma Y‐C, Zuo LI, Chen J‐H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937‐2944. [DOI] [PubMed] [Google Scholar]
- 17. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta‐analysis. Lancet. 2012;379:905‐914. [DOI] [PubMed] [Google Scholar]
- 18. Hernández Hernández R, Armas‐Hernández MJ, Chourio JAC, et al. Comparative effects of amlodipine and nifedipine GITS during treatment and after missing two doses. Blood Press Monit. 2001;6:47‐57. [DOI] [PubMed] [Google Scholar]
- 19. Morrissey EC, Durand H, Nieuwlaat R, et al. Effectiveness and content analysis of interventions to enhance medication adherence in hypertension: a systematic review and meta‐analysis protocol. Systematic Rev. 2016;5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. Br Med J. 2008;336:1114‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elliot WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens. 2008;10:20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermida RC, Ayala DE, Mojon A, Fernandez JR. Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens. 2008;21:948‐954. [DOI] [PubMed] [Google Scholar]
- 23. Hermida RC, Ayala DE, Mojón A, Alonso I, Fernández JR. Reduction of morning blood pressure surge after treatment with nifedipine GITS at bedtime, but not upon awakening, in essential hypertension. Blood Press Monit. 2009;14:152‐159. [DOI] [PubMed] [Google Scholar]
- 24. Toal CB, Meredith PA, Elliott HL. Long‐acting dihydropyridine calcium‐channel blockers and sympathetic nervous system activity in hypertension: a literature review comparing amlodipine and nifedipine GITS. Blood Press. 2012;21:3‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen QI, Huang Q‐F, Kang Y‐Y, et al. Efficacy and tolerability of initial high vs low doses of S‐(‐)‐amlodipine in hypertension. J Clin Hypertens. 2017;19:973‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu S‐K, Huang Q‐F, Zeng W‐F, Sheng C‐S, Li Y, Wang J‐G. A randomized multicenter study on ambulatory blood pressure and arterial stiffness in patients treated with valsartan/amlodipine or nifedipine GITS. J Clin Hypertens. 2019;21:252‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


