Abstract
Hypertension is a major risk factor for cardiovascular and cerebrovascular diseases. To effectively prevent end‐organ damage, maintain vascular integrity and reduce morbidity and mortality, it is essential to decrease and adequately control blood pressure (BP) throughout each 24‐hour period. Exaggerated early morning BP surge (EMBS) is one component of BP variability (BPV), and has been associated with an increased risk of stroke and cardiovascular events, independently of 24‐hour average BP. BPV includes circadian, short‐term and long‐term components, and can best be documented using out‐of‐office techniques such as ambulatory and/or home BP monitoring. There is a large body of evidence linking both BPV and EMBS with increased rates of adverse cardio‐ and cerebrovascular events, and end‐organ damage. Differences in hypertension and related cardiovascular disease rates have been reported between Western and Asian populations, including a higher rate of stroke, higher prevalence of metabolic syndrome, greater salt sensitivity and more common high morning and nocturnal BP readings in Asians. This highlights a need for BP management strategies that take into account ethnic differences. In general, long‐acting antihypertensives that control BP throughout the 24‐hour period are preferred; amlodipine and telmisartan have been shown to control EMBS more effectively than valsartan. Home and ambulatory BP monitoring should form an essential part of hypertension management, with individualized pharmacotherapy to achieve optimal 24‐hour BP control particularly the EMBS and provide the best cardio‐ and cerebrovascular protection. Future research should facilitate better understanding of BPV, allowing optimization of strategies for the detection and treatment of hypertension to reduce adverse outcomes.
Keywords: 24‐hour blood pressure, Asia, blood pressure variability, cardiovascular risk, hypertension, morning blood pressure surge
1. INTRODUCTION
Chronic elevation of blood pressure (BP), a complex disorder is a major global health issue. Hypertension is a major risk factor for cardiovascular and cerebrovascular disease,1 and accounts for approximately 45% of cardiovascular disease mortality and morbidity worldwide.2 The goal of hypertension management is not only to reduce BP but also to prevent end‐organ damage and maintain vascular integrity. The importance of decreasing and adequately controlling BP throughout each 24‐hour period for reducing mortality and morbidity cannot be overemphasized. BP shows circadian variation, being higher during the day (or awake periods) and lower at night (or during sleep). In addition, cardiovascular events are more likely to occur in the morning.3, 4, 5, 6, 7, 8 It has been suggested that extreme BP variability (BPV) results in large dynamic surges triggering adverse cardiovascular events (the resonance hypothesis) and that cardiovascular risk is particularly exaggerated in high‐risk patients with vascular disease.9 One important phenotype of BPV is the early morning blood pressure surge (EMBS), which probably contributes to the high rate of cardiovascular events occurring at that time of day.
The systemic hemodynamic atherothrombotic syndrome (SHATS), a concept proposed by Kario and colleagues, is a vicious cycle of hemodynamic stress and vascular disease that advances organ damage and triggers cardiovascular disease.10 Clinical phenotypes of SHATS include large‐artery atherothrombotic diseases (such as stroke, coronary artery disease, and aortic and peripheral artery disease), heart failure, small‐artery disease, and microcirculation‐related disease (such as chronic kidney disease and retinopathy). Appropriate detection of BP and BPV, and the early detection and management of SHATS, should facilitate more effective individualized cardiovascular protection.
2. MORNING BLOOD PRESSURE SURGE
Morning BP surge is a normal physiological phenomenon of circadian or diurnal BPV. It has been defined as the difference between morning BP (measured 2 hours after awakening) and the lowest night‐time BP (Figure 1).6 High morning surge may lead to target organ damage such as left ventricular hypertrophy and left ventricular mass index (LVMI),11 arterial stiffness and carotid atherosclerosis,12, 13, 14, 15 and renal albuminuria.16 In addition, exaggerated EMBS surge is associated with other adverse cardiovascular5, 6 and cerebrovascular events, especially hemorrhagic stroke.6, 8, 17
Figure 1.
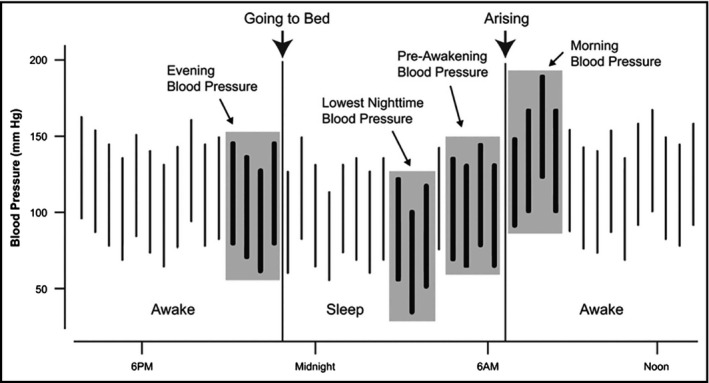
Derivation of the morning blood pressure surges from 24‐hour ambulatory blood pressure recordings. The sleep‐trough morning surge is the difference between the morning pressure and the lowest night‐time blood pressure. The pre‐awakening morning surge is the difference between the morning blood pressure and the pre‐awakening blood pressure (From: Ref. 1; reproduced with permission)
3. BLOOD PRESSURE VARIABILITY
Variability is an intrinsic property of BP, and is essential for physiological adaptability. BPV can be spontaneous and is especially striking during physical activity, anxiety and emotional episodes. The concept of BPV is not new and was recognized in the early 1970s, with an editorial by James Conway noting that “The diagnosis of hypertension has always been bedeviled by variability in blood pressure.”18
In a simple sense, BPV can be defined as the average variation of BP throughout the day, quantified as the standard deviation (SD) of ambulatory blood pressure monitoring (ABPM) recordings. There are circadian, short‐term and long‐term components to BPV. Diurnal BPV refers to day‐to‐night changes in BP. There is also temporal variability in BP with age due to arteriosclerosis and variability due to differences between central and peripheral arteries. As a result, BPV may need to be confirmed using a variety of measures, including beat‐to‐beat assessment, multiple manual or digital recordings, and home and/or ambulatory blood pressure monitoring. Table 1 summarizes the different indices used to assess BPV.
Table 1.
Blood pressure variability indices, measures and methodology
| Index/Parameter of BPV | Proposed by | Methodology | Comments/Results |
|---|---|---|---|
| Standard deviation (SD) | Clement et al68 | BPV assessment using SD and CoV was performed in 70 untreated hypertensive patients |
When assessing BPV, SD depended more on BP level than CoV. Sympathetic activity also correlated with BP and the SD of BP but not with CoV |
| Coefficient of variation (CoV) | Mancia et al69 | Continuous intra‐arterial pressure recordings | SD, but not CoV, was highly dependent on the BP level |
| Weighted SD | Bilo et al70 | Weighted SD is the difference between MMD (maximum minus minimum BP) and ARV (average real variability). These were measured by ABPM and represent the mean of day and night SD values weighted for the number of hours covered by these two periods. ARV is the average of the absolute differences between consecutive readings, weighted for the between‐reading time intervals and accounting for the order of the BP recordings | Weighted SD also depends on the BP level |
| Variability independent of the mean (VIM) | Rothwell et al34 | VIM is the within‐subject SD derived from the within‐subject mean BP to the power of x and multiplied by the population mean BP to the power x (the power x is obtained by fitting a curve through a plot of SD against mean BP level, using a model where SD = a × meanx, where x is derived by non‐linear regression) | VIM correlated best with indices and outcomes associated with BPV, rather than BP |
ABPM, ambulatory blood pressure monitoring; BP, blood pressure; BPV, blood pressure variability.
3.1. Factors affecting blood pressure variability
Available data indicate that ethnicity, age, sex, body mass index, socioeconomic status, a sedentary lifestyle, diabetes mellitus, history of hypertension, cardiovascular disease, renal disease, and use of drugs such as beta‐blockers are some of the important factors associated with BPV (Table 2).19, 20, 21, 22 Even environmental pollution can have an effect on BPV according to a recent study.23
Table 2.
Factors associated with blood pressure variability and morning blood pressure surge
| Factor | |
|---|---|
| Risk factors | Aging |
| Hypertension | |
| Glucose abnormality | |
| Metabolic syndrome | |
| Behaviors | Alcohol drinking |
| Smoking (Tobacco intake) | |
| Emotional state | |
| Diet | Salt intake |
| Psychological stress | |
| Excessive physical activity in the morning | |
| Sleep conditions | Poor sleep quality |
| Nocturnal hypoxia | |
| Clocks | Monday |
| Winter season | |
| Central and peripheral clock genes |
3.2. Clinical manifestations of blood pressure variability
The intrinsic variability in BP can make accurate diagnosis of hypertension difficult, particularly when based on individual BP readings. Use of 24‐hour ABPM along with routine clinic or hospital recordings can help to accurately and reliably diagnose true hypertension. Using this approach, patients can be categorized into four groups (Figure 2):
True normotension: normal BP readings by both clinic and out‐of‐office BP measurement;
True/sustained hypertension: elevated BP readings by both methods;
White coat hypertension: elevated clinic BP, normal BP on ABPM;
Masked hypertension: normal clinic BP, elevated BP on ABPM.
Figure 2.

The clinical spectrum of hypertension based on clinic and ambulatory blood pressure monitoring (ABPM) recordings. SBP, systolic blood pressure
In the context of BPV, a new way of looking at white coat hypertension could be described as increased BPV with normal out‐of‐office BP, and masked hypertension could be described as increased BPV and elevated out‐of‐office BP.24 From a therapeutic perspective, white coat hypertension is considered to be benign, but the detection of masked hypertension is essential because it is associated with significantly increased cardiovascular risk if undetected.25 However, data from the Spanish ABPM registry suggest that white coat hypertension may not in fact be a benign condition.26
4. BLOOD PRESSURE VARIABILITY AND MORNING BP SURGE
4.1. The synergistic resonance hypothesis
The circadian rhythm of normal BP is controlled by various neurohumoral factors. Activation of the sympathetic autonomic nervous system and suppression of vagal tone elevates BP on awakening.27, 28, 29 At the same time, other hemodynamic parameters, including heart rate, vasomotor tone, and blood viscosity, are increased.29, 30 Significantly elevated plasma epinephrine levels, posture‐related sympathetic neural reflexes,27 and early morning release of renin and angiotensin II,31 may all contribute to morning surges. Daytime physical activity is the main factor responsible for elevating BP during periods of wakefulness. Other factors affecting BPV are summarized in Table 3.
Table 3.
Factors that affect blood pressure variability
| √Genetic influences |
| √Mechanical forces generated during ventilation |
| √Local vasomotor phenomenon |
| √Neurohumoral factors |
| √Arterial wall thickness |
| √Baroreflex mechanisms |
| √Seasonal influences on body temperature |
As per the synergistic resonance hypothesis proposed by Kario et al,9 the EMBS can be potentiated by synergistic resonance of a variety of other BPV components that contribute to increased cardiovascular risk.32, 33, 34 These include beat‐by‐beat, visit‐to‐visit, orthostatic, diurnal, seasonal, annual, and physical or psychological stress‐induced BPV.
High‐risk patients with advanced vascular disease are at increased risk of adverse cardiac and stroke events. BPV and related hemodynamic stress in the setting of diseased macro‐ and microvasculature contributes to a vicious cycle resulting in end‐organ damage and SHATS.32, 33, 35, 36 Vascular aging and remodeling, increased vascular resistance combined with increased sympathetic vascular tone and reduced baroreceptor sensitivity are the most important factors underlying the pathophysiology of EMBS and SHATS.35, 36 In addition, time (morning), day (Monday), and season (winter) are also contributing factors. Daily circadian variation in BP hemodynamics is influenced by electrolytes and neurohormonal factors.37
Exaggerated or abnormal BPV is also associated with more rapid progression of left ventricular mass index (LVMI) and increased carotid intima‐medial thickness.14 This was found to be an independent predictor of cerebrovascular events34 and was a stronger predictor than mean BP and day‐night variability in BP.38 Hemodynamic factors associated with BPV based on ABPM were recently investigated.39 The hemodynamic variable that showed the most significant independent association with diastolic BP average real variability (ARV) on ABPM was arterial pulse wave reflection.39 Elevation of inflammatory markers such as C‐reactive protein (CRP), interleukin (IL)‐6, and IL‐18 might contribute to target organ damage at the molecular level associated with the EMBS.12
4.2. Cardiovascular risk
There is significant evidence for a relationship between both BPV and EMBS and increased rates of adverse cardiovascular events, renal decline, and end‐organ damage.40, 41 It appears that BPV and cardiovascular risk may have a U‐shaped curve relationship, which is even more marked among patients with hypertension than those with normal BP.32 Thus, both lack of BPV and exaggerated BPV appear to be pathological.38
A number of epidemiological studies have documented a strong association between the timing of cardiovascular event onset and the circadian variation in BP.3, 4, 5, 6, 7, 8 It has also been shown that abnormal circadian BPV with non‐dipping pattern increases the risk of having a stroke event.42 In elderly hypertensive patients, Kario et al were first to demonstrate that exaggerated EMBS measured using ABPM was independently associated with stroke risk.6 Specifically, the risk of future stroke was 2.7 times higher in patients who were in the top decile of morning BP surge (≥55 mm Hg) compared with those whose morning surge was <55 mm Hg.6 Pierdominico and colleagues found that in addition to coronary events, stroke was also associated with exaggerated EMBS, independent of 24‐hour BP.17 There was a 24% increase in stroke risk for each 10 mm Hg increase in EMBS (P = 0.004).17 Data from meta‐analyses showed that EMBS was associated with higher relative risks for stroke (49%), myocardial infarction (40%), and sudden cardiac death (29%).43, 44 The International Database in Ambulatory BP monitoring in relation to Cardiovascular Outcomes (IDACO) study found that both daytime and nocturnal blood pressure monitoring provided valuable prognostic information, stating that even isolated nocturnal rise of BP in normotensives and normal daytime BP recordings predict cardiovascular outcomes.45 Table 4 summarizes six important studies using ABPM to investigate associations between the EMBS and cardiovascular events.
Table 4.
Studies demonstrating the association between early morning blood pressure surge (based on ambulatory blood pressure monitoring) and cardiovascular events
| Author | Morning surge definition (ABPM) | Patients | Mean follow‐up | CV endpoint | Main findings |
|---|---|---|---|---|---|
| Kario et al6 (JMS‐ABPM study) | Sleep‐trough surge, prewaking surge | 519 unmedicated elderly Japanese pts with hypertension (mean 72 y) | 3.4 y | Stroke | Those with sleep‐trough surge >55 mm Hg (highest decile) had a higher stroke incidence than those with a surge <55 mm Hg (19.0% vs 7.3%; P = 0.004). After matching for age and 24‐h BP, the RR in the surge vs nonsurge group was 2.7 (P = 0.04) |
| Gosse et al5 (Bordeaux cohort) | Rising surge | 507 untreated pts with hypertension (mean 49 y) | 7.7 y | CV events | The rate of CV events increased in parallel with increasing BP surge quartile (Q1: 4.0%; Q2: 2.3%; Q3: 7.1%; Q4: 11.0%; P = 0.02). In multivariate analysis, the association between rising BP surge and CV events remained a significant independent predictor. Independent of age and 24‐h BP (P = 0.009) |
| Metoki et al8 (Ohasama study) | Prewaking surge, sleep‐trough surge | 1430 community‐dwelling subjects aged ≥40 y (mean 61 y) | 10.4 y | Stroke | Those with a prewaking surge >25 mm Hg (highest quintile: Q5) had a higher hemorrhagic stroke risk vs Q2 (surge 3.0‐11.0 mm Hg) (HR 4.0; P = 0.04). Those with a sleep‐trough surge >40 mm Hg (Q5) had a higher hemorrhagic stroke risk vs Q2 (16.0‐23.0 mm Hg surge) (HR 8.9; P < 0.05) |
| Dolan et al4 (Dublin Outcome Study) | Prewaking surge | 11 291 referred hypertensive patients off‐medication (mean 55 y) | 5.3 y | CV mortality, stroke mortality, and cardiac mortality | HR values for total CV, stroke, and cardiac mortality associated with a 10‐mm Hg increase in morning surge were 1.38 (95% CI 1.31‐1.45), 1.37 (1.23‐1.51), and 1.39 (1.30‐1.49), respectively. These remained statistically significant after adjusting for covariates including age and 24‐h BP |
| Amici et al3 | Sleep‐trough surge | 10 normotensive and 32 well‐controlled hypertensive elderly outputs (mean 66 y) with mean 24‐hour BP <135/85 mm Hg | 5.0 y | CV events | Those with a sleep‐trough surge ≥34 mm Hg (highest tertile: T3) had higher CV risk than those with a surge <34 mm Hg (T1 and T2; 5 events vs 0 events; P = 0.001) |
| Li et al7 (IDACO) | Sleep‐trough surge, prewaking surge | 5645 subjects from eight populations (mean 53 y) | 11.4 y | CV, cardiac, coronary, and cerebrovascular events | For all CV, cardiac, coronary and cerebrovascular events, and all‐cause mortality, the HR values for the top decile of the systolic sleep‐trough surge (>37 mm Hg) compared with the average risk in the whole study population were 1.30 (P = 0.01), 1.52 (P = 0.004), 1.45 (P = 0.03), 0.95 (P = 0.74), and 1.32 (P = 0.004), respectively |
| Israel et al71 | Difference between average BP in last hour before waking and first hour after waking | 2627 pts with hypertension (mean | 22 353 person‐y (median 6.5 y) | All‐cause mortality | After adjustment for age, sex, hypertension and diabetes treatments, and 24‐h SBP, morning surge above vs below the median (12 mm Hg) was associated with significantly lower mortality (HR 0.61, 95% CI 0.47‐0.79; P < 0.001). This association was significant in dipping (HR 0.49, 95% CI 0.34‐0.73;P < 0.001), but not non‐dipping (HR 0.90, 95% CI 0.60‐1.34) pts |
| Verdecchia et al48 | Sleep‐trough morning surge | 3792 pts with initially untreated hypertension (mean 51 y) | 8.4 y | Major CV events | BP surge of ≤9.5 mm Hg was associated with increased risk of CV events (adjusted HR 1.66, 95% CI 1.14‐2.42; P = 0.009). Excessive BP surge did not increase the risk of CV events |
| Pierdomenico et al17 | Prewaking surge | 1191 pts with treated hypertension (mean 69 y) | 9.1 y | Stroke | Based on tertiles of morning surge in SBP (≤2.5 mm Hg, >2.5 and ≤11.5 mm Hg, and >11.5 mm Hg), the number of stroke events was 51, 39 and 49, respectively (NS). In dippers, morning surge in SBP of >23 mm was associated with a significantly higher risk of stroke (HR 2.08, 95% CI 1.03‐4.23; P = 0.04) |
| Pierdomenico et al72 | Prewaking surge | 1191 pts with treated hypertension (mean 69 y) | 9.1 y | Coronary events | Dippers in the third tertile of morning surge SBP (>23 mm Hg) and nondippers were at higher coronary event risk than dippers with morning SBP surge<23 mm Hg (HR 1.912, 95% CI 1.048‐3.488; P = 0.03 and HR 1.739, 95% CI 1.074‐2.815;P = 0.02) |
| Abdel‐Khalik et al73 | Sleep‐trough morning surge | 81 pts with hypertension (mean 56 y) | 3 y | CV events | Pts with a CV event had higher morning surge (P < 0.0001) and a nondipper pattern (P = 0.0171). A morning surge cut‐off of 41 mm Hg has sensitivity/specificity of 100%/80% to predict cardiovascular events, and a cut‐off of 33 mm Hg had sensitivity/specificity of 91%/79% to predict acute coronary syndrome (both P < 0.001). |
| Cheng et al74 | Sleep‐trough morning surge | 2020 pts with hypertension (mean 55 y) | Median 19.7 y | All‐cause and CV mortality | The rate of increase (but not the magnitude of the increase) in morning BP was significantly associated with all‐cause (HR 1.7, 95% CI 1.2‐2.3) and CV mortality (HR 2.6, 95% CI 1.6‐4.4). |
| Pierdomenico et al75 | Prewaking surge | 391 elderly pts with treated hypertension (mean 69 y) | 9.3 y | Composite of stroke, coronary events, heart failure and peripheral revascularization | Compared to dippers with normal morning surgein adjusted multivariate analysis, dippers with high morning surge (HR 2.52, 95% CI 1.29‐4.93; P = 0.007) and nondippers (HR 2.09, 95% CI 1.19‐3.68; P = 0.01) had significantly higher CV risk |
BP, blood pressure; CI, confidence interval; CV, cardiovascular; h, hour; HR, hazard ratio; NS, not statistically significant; pts, patients; Q, quartile or quintile; RR, relative risk; SBP, systolic BP; T, tertile; y, years.
4.3. Controversies
There is a reasonable body of evidence for the association between abnormal BPV and EMBS and higher rates of adverse cardiac and cerebrovascular outcomes, including mortality. However, there is still some disagreement about the prognostic significance of BPV and EMBS.32, 46 In the large International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) study, exaggerated EMBS was independently associated with cardiovascular, but not non‐cardiovascular, death.7 This may be because the patient population in IDACO was younger than that enrolled in some other studies.6, 17 A study from Denmark that included 100 patients with newly detected diabetes used ABPM to study EMBS and the systolic night‐day ratio (SND).47 In hypertensive patients, these parameters have been associated with a higher rate of cardiovascular events. However, the Danish study did not show any association between EMBS and SND and subclinical markers of early vascular target organ damage, including pulse wave velocity (PWV), white matter lesions (WML) on brain magnetic resonance imaging (MRI), and urine‐albumin creatinine ratio (UAE). In a cohort of 3012 patients with untreated hypertension, Verdecchia et al reported that although a blunted sleep‐trough and pre‐awakening BP surge were associated with an excess risk of major cardiovascular events, there was no corresponding increase in mortality risk.48 The results of a Systolic Hypertension in Europe (Syst‐Eur) trial substudy suggested that BP‐lowering therapy reduced cardiovascular complications by decreasing systolic BP rather than BPV and that it is the absolute value of systolic BP that predicts risk than variability indices.49 Thus, published data indicate that BPV definitions and terminology are inconsistent between studies, and the fact that BPV values have poor reproducibility might contribute to conclusions that BPV is only a weak predictor of cardiovascular risk.5, 7, 8, 47, 50 The validity of the recordings, the predictability of variability indices, and the confounding effects of timing of antihypertensive medication intake all need to be considered when evaluating available evidence. Some authors suggest that EMBS could be an epiphenomenon of BPV and that it may represent only a part of 24‐hour BPV.50
5. EVIDENCE FROM ASIA
Geographical location, race, and ethnicity are well‐recognized factors influencing the prevalence of hypertension. Differences in the rates of hypertension and related cardiovascular diseases have been reported between Western and Asian populations. Specific differences include the following:
Stroke (especially hemorrhagic stroke) is more common than myocardial infarction in Asians;
Asians show a steeper association between BP and cardiovascular disease;
Asians have higher salt intake than Westerners, leading to higher salt sensitivity;
There is a high prevalence of obesity and metabolic syndrome in Asia;
High morning and nocturnal BP readings are more common in Asians.
5.1. China
Qin et al used ABPM retrospectively to investigate BPV, EMBS, and associated factors in 513 elderly (≥65 years) and 188 younger (<65 years) Chinese patients with hypertension.51 The EMBS was lower in younger versus elderly patients, and was highest in those aged 75 to 80 years (29.0 ± 13.4 mm Hg). Compared with other groups, patients aged ≥80 years showed significantly greater BPV (P < 0.05). Multiple linear regression analysis showed that body mass index (BMI; P < 0.01), smoking history of up to 50 years (P ≤ 0.03), and circadian BPV (P < 0.01) were associated with EMBS.51 In a study of 340 patients from China, the proportion of subjects with EMBS was higher in patients with hypertension compared with normotensive participants.52 In addition, daytime SBP was the best predictor of the rate of morning surge in SBP, and EMBS was associated with cardiovascular and stroke events.52
5.2. Japan
The Jichi Medical University—Ambulatory Blood Pressure Monitoring (JMU‐ABPM) study investigated the association between the time of onset of events and morning BP surge. The results showed that the incidence of stroke events in the morning hours was higher in subjects with exaggerated morning BP surge than in those without exaggerated morning BP surge (Table 4).6 Hoshide et al showed that ethnic differences exist between European and Japanese patients with hypertension, including a higher sleep‐trough morning surge in those from Japan versus Europe.53 For patients with resistant hypertension, Japanese have significantly higher morning SBP levels, moving peak morning SBP, morning dynamic surge and night dynamic surge compared with Black and White Americans, again highlighting the presence of ethnic differences.54
5.3. India
The incidence of hypertension, diabetes, and cardiovascular disease burden in India is rapidly increasing. However, there are few large‐scale studies on BPV and EMBS in the Indian population. One study in 122 consecutive patients with diabetes showed that poor glycemic control and insulin resistance predicted the occurrence of morning BP surge.55 Sultana et al studied the role of genetic factors in BPV among the Kumba community from the state of Chattisgarh, India.56 The study compared members of two families consisting of three to four generations which showed significant differences in 24‐hour mean BP values, BPV, and nocturnal dipping in systolic BP.56
5.4. Taiwan
The prognostic value of long‐term BPV was studied in a Taiwanese community‐based population (n = 1257) using ABPM.57 The population included normotensives and those with untreated hypertension, and looked at the relationship between baseline BPV and 20‐year all‐cause and cardiovascular mortality. BPV was assessed using the read‐to‐read average real variability of 24‐hour systolic and diastolic BP values (ARVs and ARVd, respectively). In a Cox proportional hazards analysis, ARVd predicted cardiovascular mortality independently of office systolic BP. In patients with hypertension, a high versus low ARVd predicted cardiovascular mortality, even after adjustment for conventional risk factors, and office systolic BP or 24‐hour systolic BP. Associations for ARVs were similar, but not as strong. However, no such associations were seen in the normotensive group.57
6. MANAGEMENT STRATEGIES
Given the role of BPV and EMBS in contributing to an increased risk of cardiovascular events, effective detection and management of these important manifestations of hypertension is critical. Several international guidelines advocate the use of out‐of‐office BP measurements for the diagnosis and monitoring of hypertension.58, 59, 60 These approaches maximize the chances of detecting BPV and EMBS, facilitating appropriate interventions to reduce cardiovascular risk.
In general, long‐acting antihypertensive drugs that control BP for 24 hours are preferred to short‐ or intermediate‐acting drugs. However, even long‐acting drugs vary in their ability to effectively control 24‐hour BP, including the EMBS. In the VALUE (Valsartan Antihypertensive Long‐term Use‐Evaluation) trial, amlodipine was more effective at controlling the morning BP surge compared with valsartan.61 Among angiotensin receptor blockers (ARBs), an 80 mg dose of telmisartan was better at controlling EMBS than valsartan.62 Current guidelines recommend the use of full doses or maximum tolerable doses of antihypertensive drugs, either alone or as combination therapy.58, 63, 64, 65, 66 Combination therapy with two agents (one of which should ideally be a diuretic, plus an angiotensin‐converting enzyme inhibitor or ARB) are preferred over higher doses of monotherapy. If short‐ or intermediate‐acting drugs or split drug dosing is used, bedtime intake may improve morning BP control.
Based on the pathophysiological basis that morning BP surge may be related to activation of the sympathetic nervous system and renin‐angiotensin‐aldosterone system (RAAS), it would be logical to use drugs that have mechanisms of action involving these pathways. However, the best clinical evidence comes from the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA), which included ambulatory BP recordings in 1905 high‐risk hypertensive patients.67 The results showed that amlodipine was more effective than atenolol for controlling nocturnal BP due to its longer duration of action. Available evidence suggests that it is appropriate to consider home and ambulatory BP monitoring as an essential part of hypertension assessment, and to tailor pharmacotherapy to achieve optimal 24‐hour BP control and therefore maximize the benefits of treatment with respect to mitigation of cardio‐ and cerebrovascular disease risk.
7. CONCLUSION
Systemic hypertension is a major modifiable cardiovascular risk factor. BPV and EMBS are important hypertension biomarkers and significant, independent predictors of adverse cardiovascular events and SHATS. Single clinic or office BP readings may underestimate both the prevalence and severity of hypertension and associated risks. Ideally, beat‐to‐beat continuous monitoring is the best way to assess hypertension and BPV, and the EMBS is a simple and predictable measure of BPV. Systemic hypertension and associated risks have a multifactorial etiology, including intrinsic neurohumoral factors (eg, the RAAS) and external behavioral and environmental factors. Therefore, a holistic approach to primary and secondary prevention is essential for avoiding end‐organ damage. Although there are inconsistencies in current definitions and parameters of BPV, ongoing and future research should facilitate better scientific understanding, which in turn will help to optimize strategies for the detection and treatment of arterial hypertension to reduce adverse outcomes. Existing available technology and future advances in digital non‐invasive miniaturized sensors could help early detection of hypertension and its variability for planning appropriate therapeutic strategies.
DISCLOSURES
K Kario received research grant from Teijin Pharma Limited; Omron Healthcare Co., Ltd.; Fukuda Denshi Co., Ltd.; Bayer Yakuhin Ltd.; A &D Co., Ltd.; Daiichi Sankyo Company, Limited; Mochida Pharmaceutical Co., Ltd.; EA pharma; Boehringer Ingelheim Japan Inc; Tanabe Mitsubishi Pharma Corporation; Shionogi & Co., Ltd.; MSD KK; Sanwa Kagaku Kenkyusho Co., Ltd.; Bristol‐Myers Squibb KK; Pfizer Japan Inc; Otsuka Holdings Co., Ltd. and honoraria from Takeda Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; Omron Healthcare Co., Ltd.; Terumo Corporation. S Park has received research grants and honoraria from Pfizer. S Siddique has received honoraria from Bayer, GlaxoSmithKline, Pfizer, ICI, Novartis and Servier; and travel, accommodation and conference registration support from Atco Pharmaceutical, Werrick Pharma, Highnoon Laboratories, Horizon Pharma, ICI, OBS and Pfizer. YC Chia has received speaker honoraria and sponsorship to attend conferences and CME seminars from Abbott, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Merck Sharp & Dohme, Novartis, Orient Europharma, Pfizer, and Sanofi; and a research grant from Pfizer. CH Chen has received honoraria as a member of a speaker's bureau for Pfizer. R Divinagracia has received honoraria as a member of speakers’ bureaus for Bayer, Novartis, and Pfizer. J Sison has received honoraria from Pfizer, AstraZeneca, Boehringer Ingelheim and Novartis. GP Sogunuru has received a research grant related to hypertension monitoring and treatment from Pfizer. JC Tay has received advisory board and consultant honoraria from Pfizer. JG Wang has received research grants from Bayer, Pfizer, and Phillips; and lecture and consulting fees from Bayer, Daiichi‐Sankyo, Merck Sharp & Dohme, Pfizer, Sanofi, and Servier. L Wong has received honoraria from Bristol‐Myers Squibb and Pfizer. Y Zhang has received research grants from Bayer, Novartis, and Shuanghe; and lecture fees from Bayer, Daiichi Sankyo, Novartis, Pfizer, Sanofi, Servier, and Takeda. All other authors report no potential conflicts of interest in relation to this article.
ACKNOWLEDGMENTS
Editing assistance was provided by Nicola Ryan, independent medical writer, funded by Pfizer.
Sogunuru GP, Kario K, Shin J, et al. Morning surge in blood pressure and blood pressure variability in Asia: Evidence and statement from the HOPE Asia Network. J Clin Hypertens. 2019;21:324–334. 10.1111/jch.13451
Contributor Information
Guru P. Sogunuru, Email: drcardio73@gmail.com.
Kazuomi Kario, Email: kkario@jichi.ac.jp.
REFERENCES
- 1. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765‐773. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . A global brief on hypertension: silent killer, global public health crisis. http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf?ua=1. Accessed March 6, 2018.
- 3. Amici A, Cicconetti P, Sagrafoli C, et al. Exaggerated morning blood pressure surge and cardiovascular events. A 5‐year longitudinal study in normotensive and well‐controlled hypertensive elderly. Arch Gerontol Geriatr. 2009;49:e105–e109. [DOI] [PubMed] [Google Scholar]
- 4. Dolan E, McCormack P, Staessen JA, O’Brien E. The morning surge in systolic blood pressure predicts cardiovascular mortality: Dublin Outcomes Study. J Hypertens. 2008;26:S30. [Google Scholar]
- 5. Gosse P, Lasserre R, Minifie C, Lemetayer P, Clementy J. Blood pressure surge on rising. J Hypertens. 2004;22:1113‐1118. [DOI] [PubMed] [Google Scholar]
- 6. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401‐1406. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Thijs L, Hansen TW, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040‐1048. [DOI] [PubMed] [Google Scholar]
- 8. Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149‐154. [DOI] [PubMed] [Google Scholar]
- 9. Kario K. New insight of morning blood pressure surge into the triggers of cardiovascular disease‐synergistic resonance of blood pressure variability. Am J Hypertens. 2016;29:14‐16. [DOI] [PubMed] [Google Scholar]
- 10. Kario K. Systemic hemodynamic atherothrombotic syndrome and resonance hypothesis of blood pressure variability: triggering cardiovascular events. Korean Circ J. 2016;46:456‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwajima I, Mitani K, Miyao M, Suzuki Y, Kuramoto K, Ozawa T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: relation to arising time. Am J Hypertens. 1995;8:29‐33. [DOI] [PubMed] [Google Scholar]
- 12. Marfella R, Siniscalchi M, Nappo F, et al. Regression of carotid atherosclerosis by control of morning blood pressure peak in newly diagnosed hypertensive patients. Am J Hypertens. 2005;18:308‐318. [DOI] [PubMed] [Google Scholar]
- 13. Marfella R, Siniscalchi M, Portoghese M, et al. Morning blood pressure surge as a destabilizing factor of atherosclerotic plaque: role of ubiquitin‐proteasome activity. Hypertension. 2007;49:784‐791. [DOI] [PubMed] [Google Scholar]
- 14. Yano Y, Hoshide S, Inokuchi T, Kanemaru Y, Shimada K, Kario K. Association between morning blood pressure surge and cardiovascular remodeling in treated elderly hypertensive subjects. Am J Hypertens. 2009;22:1177‐1182. [DOI] [PubMed] [Google Scholar]
- 15. Zakopoulos NA, Tsivgoulis G, Barlas G, et al. Time rate of blood pressure variation is associated with increased common carotid artery intima‐media thickness. Hypertension. 2005;45:505‐512. [DOI] [PubMed] [Google Scholar]
- 16. Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797‐805. [DOI] [PubMed] [Google Scholar]
- 17. Pierdomenico SD, Pierdomenico AM, Cuccurullo F. Morning blood pressure surge, dipping, and risk of ischemic stroke in elderly patients treated for hypertension. Am J Hypertens. 2014;27:564‐570. [DOI] [PubMed] [Google Scholar]
- 18. Conway J. Hemodynamic aspects of hypertension. Labile hypertension: the problem. Circ Res. 1970;27:43‐47. [PubMed] [Google Scholar]
- 19. de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466‐472. [DOI] [PubMed] [Google Scholar]
- 20. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160‐166. [DOI] [PubMed] [Google Scholar]
- 21. Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469‐480. [DOI] [PubMed] [Google Scholar]
- 22. Schutte R, Thijs L, Liu YP, et al. Within‐subject blood pressure level–not variability–predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60:1138‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quinn AK, Ae‐Ngibise KA, Kinney PL, et al. Ambulatory monitoring demonstrates an acute association between cookstove‐related carbon monoxide and blood pressure in a Ghanaian cohort. Environ Health. 2017;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang IS, Pyun WB, Shin J, et al. Higher blood pressure variability in white coat hypertension; from the Korean Ambulatory Blood Pressure Monitoring Registry. Korean Circ J. 2016;46:365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28:521‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood‐pressure measurements and mortality. N Engl J Med. 2018;378:1509‐1520. [DOI] [PubMed] [Google Scholar]
- 27. Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71‐76. [DOI] [PubMed] [Google Scholar]
- 28. Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210‐1215. [DOI] [PubMed] [Google Scholar]
- 29. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha‐sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986‐990. [DOI] [PubMed] [Google Scholar]
- 30. Jovicic A, Mandic S. Circadian variations of platelet aggregability and fibrinolytic activity in healthy subjects. Thromb Res. 1991;62:65‐74. [DOI] [PubMed] [Google Scholar]
- 31. Portaluppi F, Bagni B, Uberti E, Montanari L, Cavallini R, Trasforini G. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. J Hypertens. 1990;8:85‐95. [DOI] [PubMed] [Google Scholar]
- 32. Kario K. Prognosis in relation to blood pressure variability: pro side of the argument. Hypertension. 2015;65:1163‐1169. [DOI] [PubMed] [Google Scholar]
- 33. Kario K. Essential Manual of 24‐Hour Blood Pressure Management from Morning to Nocturnal Hypertension. London, UK: Wiley‐Blackwell; 2015. [Google Scholar]
- 34. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895‐905. [DOI] [PubMed] [Google Scholar]
- 35. Kario K. Orthostatic hypertension – a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726‐738. [DOI] [PubMed] [Google Scholar]
- 36. Kario K, Tomitani N, Kanegae H, et al. Development of a new ICT‐based multisensor blood pressure monitoring system for use in hemodynamic biomarker‐initiated anticipation medicine for cardiovascular disease: the National IMPACT Program Project. Prog Cardiovasc Dis. 2017;60:435‐449. [DOI] [PubMed] [Google Scholar]
- 37. White WB. Relevance of blood pressure variation in the circadian onset of cardiovascular events. J Hypertens Suppl. 2003;21:S9‐S15. [DOI] [PubMed] [Google Scholar]
- 38. Mancia G, Ferrari A, Gregorini L, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96‐104. [DOI] [PubMed] [Google Scholar]
- 39. Hsu PF, Cheng HM, Sung SH, et al. Hemodynamic determinants of the short‐term blood pressure variability: differential roles of arterial stiffness and wave reflection. Am J Hypertens. 2017;30:256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chia YC, Ching SM, Lim HM. Visit‐to‐visit SBP variability and cardiovascular disease in a multiethnic primary care setting: 10‐year retrospective cohort study. J Hypertens. 2017;35(suppl 1):S50‐S56. [DOI] [PubMed] [Google Scholar]
- 41. Chia YC, Lim HM, Ching SM. Long‐term visit‐to‐visit blood pressure variability and renal function decline in patients with hypertension over 15 years. J Am Heart Assoc. 2016;5(11):e003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet. 1988;2:397. [DOI] [PubMed] [Google Scholar]
- 43. Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta‐analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512‐1516. [DOI] [PubMed] [Google Scholar]
- 44. Elliott WJ. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke. 1998;29:992‐996. [DOI] [PubMed] [Google Scholar]
- 45. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 46. Asayama K, Wei FF, Hara A, Hansen TW, Li Y, Staessen JA. Prognosis in relation to blood pressure variability. Hypertension. 2015;65(6):1170‐1179. [DOI] [PubMed] [Google Scholar]
- 47. Lyhne JM, Laugesen E, Hoyem P, et al. Morning blood pressure surge and target organ damage in newly diagnosed type 2 diabetic patients: a cross sectional study. BMC Endocr Disord. 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verdecchia P, Angeli F, Mazzotta G, et al. Day‐night dip and early‐morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60:34‐42. [DOI] [PubMed] [Google Scholar]
- 49. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350:757‐764. [DOI] [PubMed] [Google Scholar]
- 50. Bombelli M, Fodri D, Toso E, et al. Relationship among morning blood pressure surge, 24‐hour blood pressure variability, and cardiovascular outcomes in a white population. Hypertension. 2014;64:943‐950. [DOI] [PubMed] [Google Scholar]
- 51. Qin X, Zhang Q, Yang S, Sun Z, Chen X, Huang H. Blood pressure variability and morning blood pressure surge in elderly Chinese hypertensive patients. J Clin Hypertens. 2014;16:511‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo Y, Wang YL, Wu YB, et al. Association between the rate of the morning surge in blood pressure and cardiovascular events and stroke. Chin Med J. 2013;126:510‐514. [PubMed] [Google Scholar]
- 53. Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750‐756. [DOI] [PubMed] [Google Scholar]
- 54. Kario K, Bhatt DL, Brar S, Bakris GL. Differences in dynamic diurnal blood pressure variability between Japanese and American treatment‐resistant hypertensive populations. Circ J. 2017;81:1337‐1345. [DOI] [PubMed] [Google Scholar]
- 55. Nuthalapati RK, Indukuri BR. Association between glycemic control and morning blood surge with vascular endothelial dysfunction in type 2 diabetes mellitus patients. Indian J Endocrinol Metab. 2016;20:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sultana R, Pati AK. Blood pressure variability and pedigree analysis of nocturnal SBP dipping in Kumbas from rural Chhattisgarh, India. Indian J Exp Biol. 2014;52:542‐548. [PubMed] [Google Scholar]
- 57. Hsu PF, Cheng HM, Wu CH, et al. High short‐term blood pressure variability predicts long‐term cardiovascular mortality in untreated hypertensives but not in normotensives. Am J Hypertens. 2016;29:806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 59. National Institute for Health and Care Excellence . Hypertension: clinical management of primary hypertension in adults (update). Clinical guideline 127 (2011). https://www.nice.org.uk/guidance/cg127/chapter/1-guidance. Accessed December 2016.
- 60. Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 61. Pedersen OL, Mancia G, Pickering T, et al. Ambulatory blood pressure monitoring after 1 year on valsartan or amlodipine‐based treatment: a VALUE substudy. J Hypertens. 2007;25:707‐712. [DOI] [PubMed] [Google Scholar]
- 62. White WB, Lacourciere Y, Davidai G. Effects of the angiotensin II receptor blockers telmisartan versus valsartan on the circadian variation of blood pressure: impact on the early morning period. Am J Hypertens. 2004;17:347‐353. [DOI] [PubMed] [Google Scholar]
- 63. Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1‐47. [DOI] [PubMed] [Google Scholar]
- 64. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Assoc. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 65. Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579‐615. [PubMed] [Google Scholar]
- 66. Shin J, Park JB, Kim KI, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension: part I‐epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dolan E, Stanton AV, Thom S, et al. Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients – an Anglo‐Scandinavian cardiac outcomes trial substudy. J Hypertens. 2009;27:876‐885. [DOI] [PubMed] [Google Scholar]
- 68. Clement DL, Mussche MM, Vanhoutte G, Pannier R. Is blood pressure variability related to activity of the sympathetic system? Clin Sci. 1979;57:217s–219s. [DOI] [PubMed] [Google Scholar]
- 69. Mancia G, Ferrari A, Gregorini L, et al. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci. 1980;59:401s–404s. [DOI] [PubMed] [Google Scholar]
- 70. Bilo G, Giglio A, Styczkiewicz K, et al. A new method for assessing 24‐h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058‐2066. [DOI] [PubMed] [Google Scholar]
- 71. Israel S, Israel A, Ben‐Dov IZ, Bursztyn M. The morning blood pressure surge and all‐cause mortality in patients referred for ambulatory blood pressure monitoring. Am J Hypertens. 2011;24:796‐801. [DOI] [PubMed] [Google Scholar]
- 72. Pierdomenico SD, Pierdomenico AM, Di Tommaso R, et al. Morning blood pressure surge, dipping, and risk of coronary events in elderly treated hypertensive patients. Am J Hypertens. 2016;29:39‐45. [DOI] [PubMed] [Google Scholar]
- 73. Abdel‐Khalik MY, Mahrous SA, Shanab AA, Alshehri AM, Rashed MH, Azoz AM. Morning blood pressure surge as a predictor of outcome in patients with essential hypertension. Saudi J Med Med Sci. 2017;5:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheng HM, Wu CL, Sung SH, et al. Prognostic utility of morning blood pressure surge for 20‐year all‐cause and cardiovascular mortalities: results of a community‐based study. J Am Heart Assoc. 2017;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pierdomenico SD, Pierdomenico AM, Coccina F, Lapenna D, Porreca E. Prognostic value of nondipping and morning surge in elderly treated hypertensive patients with controlled ambulatory blood pressure. Am J Hypertens. 2017;30:159–165. [DOI] [PubMed] [Google Scholar]


