Abstract
The relationships between orthostatic hypotension (OH) and some kinds of cardiovascular disease are inconsistent among studies. This updated meta‐analysis was conducted in hopes of producing progress on this topic. A systematic database search was performed in electronic databases, including the Chinese Biomedical Database (CBM), PubMed, Web of Science, and the Cochrane Library. Summary hazard ratio (HR) estimates with 95% confidence intervals (CIs) were calculated by a random‐effects model. Statistical heterogeneity was assessed with Cochran's Q test and the I 2 statistic. From 1462 potentially eligible records, 15 studies met the inclusion criteria. Subjects with OH had a high risk of heart failure (HF) and atrial fibrillation (AF) (pooled HR 1.34, 95% CI 1.17‐1.52, P < 0.001 and pooled HR 1.51, 95% CI 1.28‐1.79, P < 0.001, respectively). This meta‐analysis also showed significant associations between OH and the risks of developing coronary heart disease (CHD) (pooled HR 1.44, 95% CI 1.18‐1.75, P < 0.001) and myocardial infarction (MI) (pooled HR 1.52, 95% CI 1.12‐2.06, P = 0.008). Our study suggests that OH is positively associated with high risks of HF and AF. Moreover, it may be related to high risks of CHD and MI.
Keywords: atrial fibrillation, coronary heart disease, heart failure, myocardial infarction, orthostatic hypotension
1. INTRODUCTION
Orthostatic hypotension (OH) has been related to increased morbidity, leading to increased hospitalizations.1 It is traditionally characterized by a sustained reduction of at least 20 mm Hg in systolic blood pressure (SBP) and/or a decrease in diastolic blood pressure (DBP) of at least 10 mm Hg within 3 minutes of standing or tilting the head up to at least 60° on a table.2, 3, 4 OH is prevalent among the elderly population, with a pooled prevalence of 22.2%, but is often assessed and treated inadequately.5 Studies have reported effects of OH on cardiovascular diseases (CVD), cerebrovascular events, and mortality.6, 7, 8, 9, 10, 11, 12, 13 However, OH as an independent risk factor for CVD has been controversial. Therefore, we systematically reviewed the literature from 1996 to update the association between OH and some kinds of cardiovascular diseases, including coronary heart disease (CHD), myocardial infarction (MI), heart failure (HF), and atrial fibrillation (AF).
2. METHODS
2.1. Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses statement. A comprehensive literature search was conducted in online databases. The Chinese Biomedical Database, PubMed, Web of Science, and the Cochrane Library were searched from January 1996 (when the consensus definition of OH was established) through January 2019. The search terms were as follows: (orthostatic hypotension OR postural hypotension) combined with the following keywords: (“cardiovascular diseases” OR “coronary heart disease” OR CHD OR “myocardial infarction” OR MI OR “heart failure” OR HF OR “atrial fibrillation” OR AF). The references of relevant articles were hand searched. Our search was restricted to English and Chinese literature. Two authors (Min Min and Tingting Shi) reviewed the titles and abstracts to identify potential studies and subsequently obtained the full texts (Figure 1).
Figure 1.
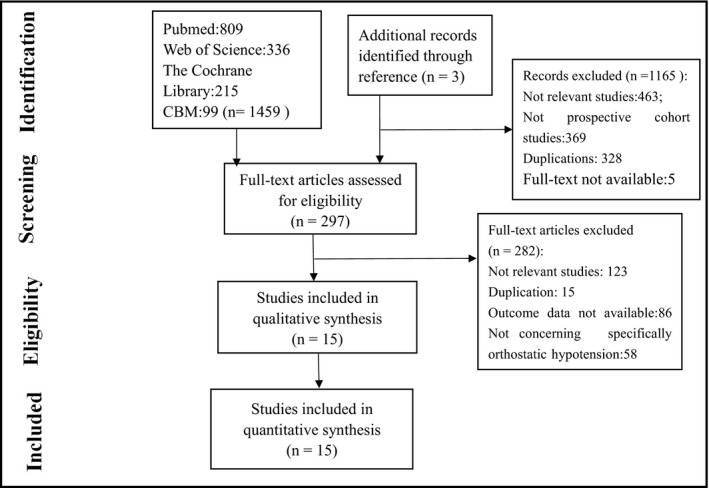
Flow diagram of the detailed process of selection of articles
2.2. Study selection
To be eligible for inclusion in our systematic review, studies had to meet the following criteria: (a) use of comparable definitions and measurements of OH based on the 1996 consensus; (b) availability of the full text in English or Chinese in a peer‐reviewed journal; (c) prospective cohort design; and (d) inclusion of an adult population (≥18 years). The exclusion criteria were (a) retrospective studies, case‐control studies, case reports, reviews, commentaries, and letters, as well as experimental studies; (b) studies with no clear definition of OH; and (c) studies concerning hypotension generally and not OH specifically.
2.3. Data extraction and study quality assessment
We extracted adjusted odds ratios (ORs) or hazard ratios (HRs) and 95% confidence intervals (CIs) directly from original studies. When the study populations of included studies partially overlapped, we extracted data from the study with the largest sample size or with a longer follow‐up. To assess the quality of selected studies, we used the Newcastle‐Ottawa instrument, which is recommended for cohort and case‐control observational studies. It contains eight questions in three parts: study selection, comparability and verification of exposure, and outcome investigation. The quality of selected studies can be classified into three groups: (a) low quality—when receiving up to 3 stars, (b) moderate quality—when getting 4‐6 stars, and (c) high quality—from 7 to 9 stars.14
2.4. Statistical analysis
All statistical analyses were performed using STATA software (version 14.0; Stata Corp). The pooled effects for the outcomes and 95% confidence intervals were calculated using the Mantel‐Haenszel method, adopting a random‐effect model. Cochrane's Q (χ 2) statistic and the I 2 statistic were used to assess the heterogeneity across studies. When the I 2 statistic was <25%, heterogeneity between studies was considered low; a value of between 25% and 50% suggested moderate heterogeneity; and a value of more than 50% indicated high heterogeneity. For the Cochran Q test, a P value of <0.05 is considered a possibility of significant heterogeneity.15 To detect potential publication bias, Begg's test and Egger's test were used, and a P value < 0.05 is considered evidence of publication bias.16, 17 Sensitivity analysis was performed to evaluate the robustness of the results and the impact of each single study on the summary estimate of effect. Moreover, subgroup analysis and meta‐regression were performed to examine the source of heterogeneity.
3. RESULTS
3.1. The basic characteristics of participants
In total, 1462 articles were retrieved from the literature, of which four articles18, 19, 20, 21 had the same population. We chose the article written by Juraschek et al21 for its long follow‐up. Finally, 15 met the inclusion criteria and were included in the meta‐analysis.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 The majority of studies were performed in Europe,22, 23, 24, 25, 26, 27, 28 five were conducted in North America,21, 29, 30, 31, 32 and three were in Asia.33, 34, 35 Most of the cohorts included the general population,21, 22, 23, 24, 25, 26, 28, 29, 30, 32, 33, 34, 35 and one included hypertensive patients.27 The other one included patients with both hypertension and diabetes mellitus.31 The sample size of the cohorts ranged from 190 to 32 797. The mean age of the participants varied from 45.6 to 83.4 years. The mean length of follow‐up ranged from 1.0 to 26.0 years. The quality assessment of the 15 studies scored 8.2 stars on average. Detailed characteristics of the included studies are displayed in Table 1. Devices utilized for blood pressure measurement and diagnosis criteria of OH are listed in Table S1.
Table 1.
Characteristics of the included studies
| First author | Year | Country | Sample | Follow‐up (y) | Mean age (y) | Men (%) | Diabetes (%) | Hypertension (%) | BMI (kg/m2) | OH prevalence (%) | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Juraschek21 | 2018 | USA | 9139 | 26 | 54.2 | 43 | 8.8 | 28.9 | 27.2 | 3 | 9 |
| Fedorowski22 HF | 2010 | Sweden | 32 669 | 24 | 45.6 | 68.2 | 4.7 | 40.2 | 24.6 | 6.1 | 9 |
| Fedorowski23 AF | 2010 | Sweden | 32 797 | 22.7 | 45.6 | 68.2 | 4.8 | 40.3 | 24.6 | 6.6 | 9 |
| Fedorowski24 MPP | 2010 | Sweden | 32 797 | 22.7 | 45.6 | 68.2 | 4.8 | 40.3 | 24.6 | 6.6 | 9 |
| Verwoert25 | 2008 | Netherlands | 5064 | 6.8 | 68.1 | 38.4 | 8.9 | 26.9 | 26.2 | 17.8 | 9 |
| Casiglia26 | 2013 | Italy | 1016 | 9.3 | 71.7 | 41.2 | 26.8 | — | 27.3 | 16.5 | 8 |
| Fedorowski27 CPP | 2013 | Sweden | 8788 | 6 | 52.3 | 52.2 | 6.2 | 100 | 27.9 | 12.1 | 9 |
| Yasa28 | 2018 | Sweden | 30 528 | 15 | 58 | 40 | 3.4 | 61 | 26 | 1.6 | 9 |
| Agarwal29 | 2013 | USA | 12 071 | 18 | 54.1 | 45 | 9 | 34 | 27.5 | 5 | 9 |
| Ko30 | 2018 | USA | 1736 | 13.5 | 71.7 | 39.8 | 6.6 | 44.6 | 26.5 | 14.8 | 9 |
| Fleg31 | 2016 | USA and Canada | 4266 | 3.9 | 62.1 | 53.4 | 100 | 100 | 32.1 | 20 | 7 |
| Alagiakrishnan32 | 2013 | USA | 3510 | 13 | 74 | 42 | 16.6 | 59.2 | 25.9 | 25.2 | 8 |
| Lin33 | 2011 | China | 1174 | 1.1 | 81.1 | 95.6 | 41.2 | 72.3 | 25.1 | 25.6 | 7 |
| Chou34 | 2015 | China | 13 486 | 4.5 | 54.8 | 47 | 24.8 | 42.4 | — | 0.09 | 8 |
| Li35 | 2018 | China | 190 | 1 | 82.6 | 67.9 | 54.2 | 73.1 | 25.1 | 34.7 | 5 |
3.2. Meta‐analysis for the association between OH and HF
Eight studies reported data about the relationship between OH and heart failure.21, 22, 25, 26, 28, 31, 32, 35 The pooled result demonstrated a positive relationship between OH and HF (summary HR 1.34, 95% CI 1.17‐1.52, P < 0.001), with moderate heterogeneity (I 2 = 48.7%). The forest plot is shown in Figure 2.
Figure 2.
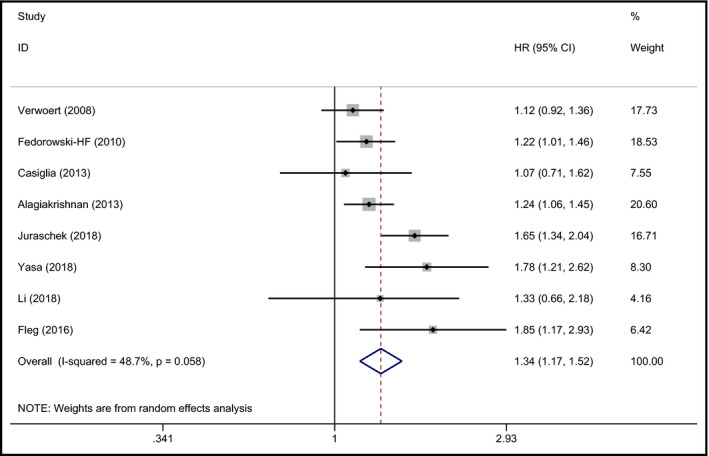
Forest plot of the association between OH and HF
3.3. Meta‐analysis for the association between OH and AF
Four studies comprising 77 132 participants assessed the prospective association between OH and AF.23, 28, 29, 30 Fedorowski et al observed a high risk of developing AF among individuals with OH.23 However, the ARIC study found a positive association among those with and without hypertension.29 The study conducted by Ko et al also suggested a significant association between OH and AF after adjusting for hypertension and other cardiovascular risk factors.30 Another population‐based cohort study indicated that a history of OH hospitalization predicted atrial fibrillation,28 where the symptoms of OH were more severe than in the other three studies. The strength of the association between OH and AF was expressed by hazard ratios (HRs) ranging from 1.3 to 1.89 in individual studies. When pooled together, the summary HR was 1.51 (95% CI 1.28‐1.79, P < 0.001) with moderate heterogeneity (I 2 = 48.5%). The pooled HR of the association between OH and AF was 1.51 (95% CI 1.28‐1.77, P < 0.001, I 2 = 0) for the hypertensive population and 1.33 (95% CI 0.84‐2.09, P = 0.224, I 2 = 0) for the diabetic population. The forest plot is shown in Figure 3.
Figure 3.
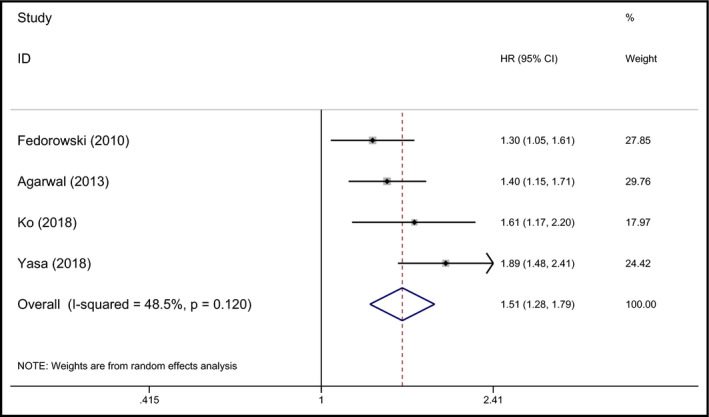
Forest plot of the association between OH and AF
3.4. Meta‐analysis for the association between OH and CHD
Coronary heart disease was defined on the basis of the International Classification of Diseases 9th and 10th Revisions or fatal and non‐fatal myocardial infarction. Ten studies explored the relationship between OH and CHD.21, 24, 25, 26, 27, 31, 32, 33, 34, 35 Five out of the ten studies suggested that baseline OH had no significant association with CHD,26, 27, 31, 32, 34 and the other 5 studies showed contrary results.21, 24, 25, 33, 35 The meta‐analysis results indicated that baseline OH had an independent association with high risk for CHD (pooled HR 1.44, 95% CI 1.18‐1.75, P < 0.001), with significant heterogeneity (I 2 = 75.4%). The forest plot is shown in Figure 4.
Figure 4.
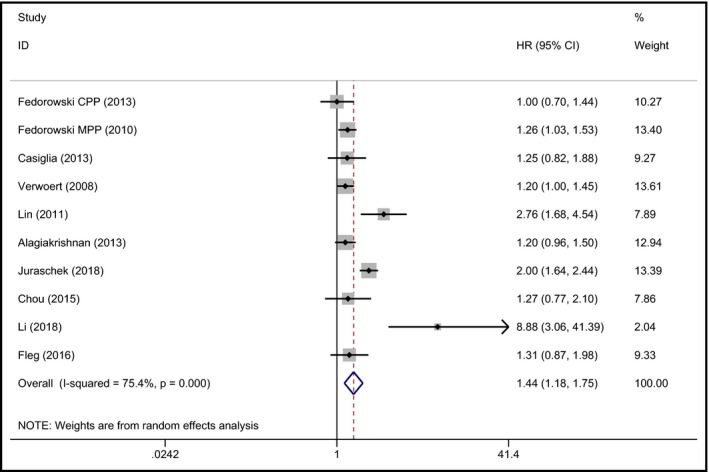
Forest plot of the association between OH and CHD
3.5. Meta‐analysis for the association between OH and MI
Eight studies reported data about MI.21, 24, 27, 31, 32, 33, 34, 35 The 8 studies included 73 350 participants. The pooled result demonstrated that OH was related to a high risk of developing MI (pooled HR 1.52, 95% CI 1.12‐2.06, P = 0.008), but the heterogeneity was large (I 2 = 77.2%). The forest plot is shown in Figure 5. Summary effects for the association between OH and HF, AF, CHD, and MI are shown in Table 2.
Figure 5.
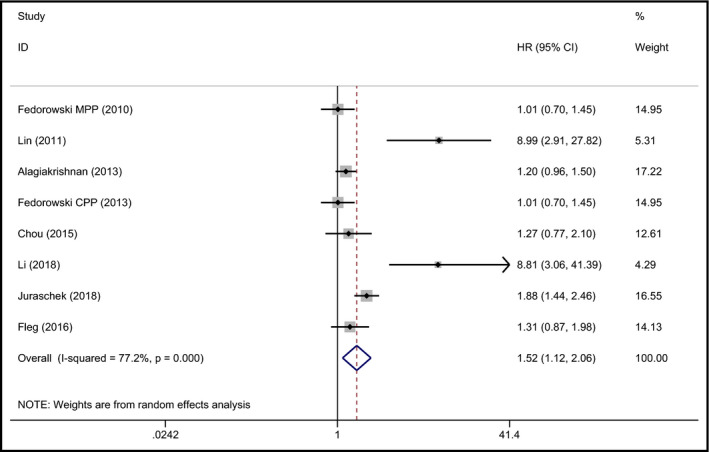
Forest plot of the association between OH and MI
Table 2.
Summary of effect measure
| Group | No. of studies | Test of association | Test of heterogeneity | Publication bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | Z | P | χ 2 | P | I 2 (%) | Model | Egger's test | Begger's test | ||
| AF | 4 | 1.51 (1.28‐1.79) | 4.91 | <0.001 | 5.83 | 0.120 | 48.5 | R | 0.139 | 0.470 |
| CHD | 10 | 1.44 (1.18‐1.75) | 3.55 | <0.001 | 36.66 | <0.001 | 75.4 | R | 0.309 | 0.074 |
| HF | 8 | 1.34 (1.17‐1.52) | 4.31 | <0.001 | 13.65 | 0.058 | 48.7 | R | 0.165 | 0.386 |
| MI | 8 | 1.52 (1.12‐2.06) | 2.67 | 0.008 | 30.71 | <0.001 | 77.2 | R | 0.418 | 0.458 |
Abbreviations: AF, atrial fibrillation; CHD, coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; MI, myocardial infarction.
3.6. Subgroup analysis
The subgroup analysis was conducted based on stratification by variables such as the mean age, gender, mean BMI, proportions of participants with hypertension and diabetes mellitus, prevalence of OH, follow‐up duration, and study quality. For the association between OH and CHD, a meta‐regression was conducted. The results of subgroup analysis and meta‐regression are presented in Tables S2 and S3, which suggested that these variables did not seem to affect the prospective association between OH and cardiovascular diseases. Three studies reported the association between OH and CHD among the Chinese population.33, 34, 35 After pooling, the pooled HR was 2.63 (95% CI 1.13‐6.15, P = 0.025, I 2 = 79.5%), indicating a stronger correlation than that among the total population. However, it was not significant for the association between OH and MI among the Chinese population (the pooled HR was 4.29, 95% CI 0.95 to 19.40, P = 0.059, I 2 = 86.6%).
3.7. Publication bias
Begg's funnel plot was made to assess publication bias through visual assessments (Figures [Link], [Link], [Link], [Link]). Furthermore, Egger's test and Begg's test were performed to quantitatively assess the publication bias. For AF, HF, CHD, and MI, both Egger's test and Begg's test showed no publication bias (Table 2).
3.8. Sensitivity analysis
By omitting the included studies sequentially, sensitivity analysis was conducted. The results are shown in Table S4. The removal of each study only made limited differences to the results, and the direction of the pooled effect remained the same. No individual study significantly affected the pooled effect when omitted, which indicated reasonably robust results were obtained.
4. DISCUSSION
In this updated meta‐analysis, we found that OH was strongly associated with future risks of CHD, HF, AF, and MI. Subgroup analysis showed that among the Chinese population, the pooled HR of the association between OH and CHD was 2.63 (95% CI 1.13‐6.15, P = 0.025, I 2 = 79.5%), indicating a stronger correlation than that among the total population. The results of this updated meta‐analysis corroborated findings from a previous systematic review.11, 12, 13 In a meta‐analysis of four studies, individuals with OH predicted a 30% higher risk for developing HF in the future than did those without OH.12 Another meta‐analysis including 13 prospective cohort studies demonstrated that OH was an independent predictor of increased mortality and HF and CHD.11 However, a recent trial on blood pressure reduction in a population with diabetes mellitus demonstrated that OH was not associated with CVD events.31
There are several explanations linking OH and cardiovascular diseases, including AF, HF, CHD, and MI. First, OH may increase the risk of cardiovascular diseases by affecting heart structure, such as left ventricular hypertrophy (LVH). OH may provoke intermittent ischemic bouts and increase afterload, finally leading to LVH.6, 36 Moreover, LVH has been regarded as a risk factor for AF37 and HF.38 One study demonstrated that OH was associated with high risks of future HF, MI, and CHD, as well as concurrent subclinical markers of atherosclerosis and intermediate‐term elevations in biomarkers reflecting myocardial injury and strain,21 which suggests pathways relating OH and the pathogenesis of HF, MI, and CHD. In addition, sustained and enhanced pro‐inflammatory activity might be one of the mechanisms underlying the longitudinal association between OH and cardiovascular morbidity.39 One study demonstrated that patients with OH have elevated plasma levels of inflammatory biomarkers, particularly immunoglobulin‐like transcript 3 (ILT‐3), midkine (MK), and regenerating islet‐derived protein 4 (REG‐4), independently of age, sex, prevalent cardiovascular disease, and risk factors.40 These elevated plasma levels of inflammatory biomarkers may suggest a complex interplay among inflammation, autonomic dysfunction, and atherothrombosis, which could be potentially related to the pathophysiological pathway underlying the link between classical OH and CHD, HF and MI.41 Furthermore, autonomic dysfunction underlying OH may contribute to the relationship between OH and AF.42, 43 Artery stiffness44 and baroreflex dysfunction,45, 46 which failed to maintain cardiovascular homeostasis, also have been proposed to explain the relationship between OH and a series of cardiovascular diseases. CHD, AF, HF, and MI could affect each other, and they shared some common risk factors. Further studies are warranted to explore the specific mechanism underlying the association of OH and AF, HF, MI, and CHD.
Some strengths of our study should be mentioned: It included a large number of studies, including the newest relevant studies, with large sample sizes or long follow‐up. Furthermore, compared with previous systematic reviews, our meta‐analysis analyzed the relationship between OH and two other cardiovascular events (AF and MI) and included more studies from the Asian population. Thus, our study may provide more robust scientific evidence on this topic than previous ones. Meanwhile, several limitations also need to be addressed. The diagnostic criteria for cardiovascular diseases were unclear in some included studies. Second, the operational protocols for measuring OH varied among individual studies. Finally, some traditional CVD risk factors, such as aging, smoking, diabetes, hypertension, obesity, dyslipidemia, and previous cardiovascular disease, may confound the relationship between OH and CVD, even though these factors have been partly adjusted for in individual studies.
5. CONCLUSIONS
Our study further confirms that OH may predict a high risk of developing a series of cardiovascular diseases, including AF, HF, CHD, and MI.
6. AUTHOR CONTRIBUTIONS
All authors take full responsibility for the work as a whole, including the study design, and the decision to submit and publish the manuscript. Min Min was involved in research conception, article writing, and revision; Min Min, Tingting Shi, Mingming Liang, and Yun Zhang participated in literature search, and data extraction and interpretation; and Chenyu Sun, Guangbo Qu, and Yehuan Sun were involved in article revision. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors report no conflicts of interest to disclose.
Supporting information
Min M, Shi T, Sun C, et al. Orthostatic hypotension and the risk of atrial fibrillation and other cardiovascular diseases: An updated meta‐analysis of prospective cohort studies. J Clin Hypertens. 2019;21:1221–1227. 10.1111/jch.13613
REFERENCES
- 1. Ricci F, Manzoli L, Sutton R, et al. Hospital admissions for orthostatic hypotension and syncope in later life: insights from the Malmo Preventive Project. J Hypertens. 2017;35:776‐783. [DOI] [PubMed] [Google Scholar]
- 2. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Clin Auton Res. 1996;6:125‐126. [DOI] [PubMed] [Google Scholar]
- 3. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69‐72. [DOI] [PubMed] [Google Scholar]
- 4. Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567‐1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saedon NI, Tan MP, Frith J. The prevalence of orthostatic hypotension: a systematic review and meta‐analysis. J Gerontol A Biol Sci Med Sci. (Epub ahead of print] 29 August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shibao C, Biaggioni I. Orthostatic hypotension and cardiovascular risk. Hypertension. 2010;56:1042‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mol A, Reijnierse EM, Bui Hoang P, van Wezel R, Meskers C, Maier AB. Orthostatic hypotension and physical functioning in older adults: a systematic review and meta‐analysis. Ageing Res Rev. 2018;48:122‐144. [DOI] [PubMed] [Google Scholar]
- 8. Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. ARIC Study Investigators. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velilla‐Zancada SM, Escobar‐Cervantes C, Manzano‐Espinosa L, Prieto‐Diaz MA, Ramalle‐Gomara E, Vara‐Gonzalez LA. Impact of variations in blood pressure with orthostatism on mortality: the HOMO study. Blood Press Monit. 2017;22:184‐190. [DOI] [PubMed] [Google Scholar]
- 10. Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta‐analysis. J Hypertens. 2014;32:1562‐1571. [DOI] [PubMed] [Google Scholar]
- 11. Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta‐analysis of prospective observational studies. Eur Heart J. 2015;36:1609‐1617. [DOI] [PubMed] [Google Scholar]
- 12. Xin W, Lin Z, Li X. Orthostatic hypotension and the risk of congestive heart failure: a meta‐analysis of prospective cohort studies. PLoS ONE. 2013;8:e63169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xin W, Mi S, Lin Z, Wang H, Wei W. Orthostatic hypotension and the risk of incidental cardiovascular diseases: a meta‐analysis of prospective cohort studies. Prev Med. 2016;85:90‐97. [DOI] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Ottawa, ON, Canada: The Ottawa Hospital; 2014. [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088‐1101. [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000;31:2307‐2313. [DOI] [PubMed] [Google Scholar]
- 19. Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13:571‐578. [DOI] [PubMed] [Google Scholar]
- 20. Jones CD, Loehr L, Franceschini N, et al. Orthostatic hypotension as a risk factor for incident heart failure: the atherosclerosis risk in communities study. Hypertension. 2012;59:913‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juraschek SP, Daya N, Appel LJ, et al. Hypotension and risk of clinical and subclinical cardiovascular disease in middle‐aged adults. J Am Heart Assoc. 2018;7(10):e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fedorowski A, Engström G, Hedblad BO, Melander O. Orthostatic hypotension predicts incidence of heart failure: the Malmö preventive project. Am J Hypertens. 2010;23:1209‐1215. [DOI] [PubMed] [Google Scholar]
- 23. Fedorowski A, Hedblad B, Engström G, Gustav Smith J, Melander O. Orthostatic hypotension and long‐term incidence of atrial fibrillation: the Malmö Preventive Project. J Intern Med. 2010;268:383‐389. [DOI] [PubMed] [Google Scholar]
- 24. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all‐cause mortality and coronary events in middle‐aged individuals (The Malmö Preventive Project). Eur Heart J. 2010;31:85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verwoert GC, Mattace‐Raso F, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56:1816‐1820. [DOI] [PubMed] [Google Scholar]
- 26. Casiglia E, Tikhonoff V, Caffi S, et al. Orthostatic hypotension does not increase cardiovascular risk in the elderly at a population level. Am J Hypertens. 2014;27:81‐88. [DOI] [PubMed] [Google Scholar]
- 27. Fedorowski A, Wahlstrand B, Hedner T, Melander O. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens. 2014;32:75‐81. [DOI] [PubMed] [Google Scholar]
- 28. Yasa E, Ricci F, Magnusson M, et al. Cardiovascular risk after hospitalisation for unexplained syncope and orthostatic hypotension. Heart. 2018;104:487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agarwal SK, Alonso A, Whelton SP, et al. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi‐ethnic population based study. PLoS ONE. 2013;8:e79030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko D, Preis SR, Lubitz SA, et al. Relation of orthostatic hypotension with new‐onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2018;121:596‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016;68:888‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alagiakrishnan K, Patel K, Desai RV, et al. Orthostatic hypotension and incident heart failure in community‐dwelling older adults. J Gerontol A Biol Sci Med Sci. 2014;69:223‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin ZQ, Xie ZQ, Wu ZQ, et al. [Correlation between orthostatic hypotension and cardiovascular risks in elderly population]. Zhonghua Yi Xue Za Zhi. 2011;91:2530‐2533. [PubMed] [Google Scholar]
- 34. Chou R‐H, Liu C‐J, Chao T‐F, et al. Association between orthostatic hypotension, mortality, and cardiovascular disease in Asians. Int J Cardiol. 2015;195:40‐44. [DOI] [PubMed] [Google Scholar]
- 35. Li LZ, Chang Y. Correlation between orthostatic hypotension and cardiovascular risks in elderly population. Chong Qing Yi Xue. 2018;47:2507‐2509. [PubMed] [Google Scholar]
- 36. Maule S, Milan A, Grosso T, Veglio F. Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens. 2006;19:1049‐1054. [DOI] [PubMed] [Google Scholar]
- 37. Magnusson M, Holm H, Bachus E, et al. Orthostatic hypotension and cardiac changes after long‐term follow‐up. Am J Hypertens. 2016;29:847‐852. [DOI] [PubMed] [Google Scholar]
- 38. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724‐730. [DOI] [PubMed] [Google Scholar]
- 39. Fedorowski A, Östling G, Persson M, et al. Orthostatic blood pressure response, carotid intima‐media thickness, and plasma fibrinogen in older nondiabetic adults. J Hypertens. 2012;30(3):522‐529. [DOI] [PubMed] [Google Scholar]
- 40. Johansson M, Ricci F, Aung N, Sutton R, Melander O, Fedorowski A. Inflammatory biomarker profiling in classical orthostatic hypotension: insights from the SYSTEMA cohort. Int J Cardiol. 2018;259:192‐197. [DOI] [PubMed] [Google Scholar]
- 41. Ibrahim NE, Januzzi JL, Magaret CA, et al. A clinical and biomarker scoring system to predict the presence of obstructive coronary artery disease. J Am Coll Cardiol. 2017;69:1147‐1156. [DOI] [PubMed] [Google Scholar]
- 42. Shen MJ, Choi E‐K, Tan AY, et al. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;9:30‐39. [DOI] [PubMed] [Google Scholar]
- 43. Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mattace‐Raso F, van der Cammen T, Knetsch AM, et al. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam study. J Hypertens. 2006;24:339‐344. [DOI] [PubMed] [Google Scholar]
- 45. Sabbah HN. Baroreflex activation for the treatment of heart failure. Curr Cardiol Rep. 2012;14:326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl. 1):2‐7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


