Abstract
Little is known on the effect of sodium intake on BP of children with clinical conditions. Our objective was therefore to review systematically studies that have assessed the association between sodium intake and BP in children with various clinical conditions. A systematic search of several databases was conducted and supplemented by a manual search of bibliographies and unpublished studies. Experimental and observational studies assessing the association between sodium intake and BP and involving children or adolescents between 0 and 18 years of age with any clinical condition were included. Out of the 6861 records identified, 51 full texts were reviewed, and 16 studies (10 experimental and 6 observational), involving overall 2902 children and adolescents, were included. Ten studies were conducted in children with elevated BP without identifiable cause, two in children with familial hypertension, one in children with at least one cardiovascular risk factor, one in children with chronic renal insufficiency, one in children with urolithiasis, and one in premature infants. A positive association between sodium intake and BP was found in all studies, except one. The meta‐analysis of six studies among children with elevated BP without identifiable cause revealed a difference of 6.3 mm Hg (95% CI 2.9‐9.6) and 3.5 mm Hg (95% CI 1.2‐5.7) in systolic and diastolic BP, respectively, for every additional gram of sodium intake per day. In conclusion, our results indicate that the BP response to salt is greater in children with clinical conditions, mainly hypertension, than in those without associated clinical conditions.
Keywords: blood pressure, cardiovascular risk factors, children, elevated blood pressure, familial hypertension, prematurity, renal insufficiency, salt, sodium, urolithiasis
1. INTRODUCTION
Hypertension is a major risk factor for cardiovascular disease and an important cause of morbidity and mortality worldwide.1, 2, 3 Because high sodium intake is a modifiable cause of elevated blood pressure (BP) in adults,4, 5, 6 the World Health Organization recommends sodium intake reduction in the population as a key strategy to reduce the burden of cardiovascular diseases.7 However, there is a debate around this recommendation, as the sensitivity of BP to sodium intake has been shown to vary between individuals, suggesting that sodium intake reduction should be targeted at individuals with certain clinical conditions, instead of the whole population. For instance, it has been shown that older adults with elevated BP, diabetes, or chronic kidney disease have a BP that is more sensitive to a reduction in sodium intake than adults without these conditions.8, 9, 10
Much less is known about the association of sodium intake and BP among children, and especially among those with clinical conditions. To this date, only three systematic reviews have been conducted on the association between sodium intake and BP in children.11, 12, 13 Two of these reviews included experimental studies and showed that sodium reduction can significantly reduce BP in children. Of note, these reviews did not separate studies between children with or without clinical conditions.11, 12 Hence, these reviews could not demonstrate if children with a clinical condition have a different response to sodium intake compared to children without a clinical condition. More recently, we have conducted a systematic review of both experimental and observational studies assessing the association between salt intake and BP in children without clinical conditions.13 Eighteen studies of high quality showed that systolic and diastolic BP was reduced by approximately 1 mm Hg for a sodium reduction of 1 g.13
To our knowledge, no systematic review examined the association between sodium intake and BP in children with clinical conditions. Our objective was therefore to assess this association through a systematic review of both experimental and observational studies.
2. METHODS
Details of the research methods are given in the protocol of this systematic review registered in PROSPERO (registration number CRD42016038245) and published.14 The first review included studies with children without clinical conditions was recently published.13 The second review, which is the subject of the current report, included only studies with children with a clinical condition. Both reviews were conducted following the PRISMA guidelines.15
2.1. Eligibility criteria
Both reviews followed the same eligibility criteria, apart from the presence and absence of clinical conditions respectively. We included randomized and non‐randomized controlled and non‐controlled trials, quasi‐experimental studies, case‐control, cohort, and cross‐sectional studies, which had assessed the association between sodium intake and BP. Studies involving children or adolescents between 0 and 18 years of age with any clinical condition were included. Experimental studies assessing the acute or short‐term effect of sodium intake (ie, duration of 7 days or less) on blood pressure were excluded.
2.2. Search strategy
A systematic search of the MEDLINE, EMBASE, CINAHL, and CENTRAL databases was conducted in March 2017 and supplemented by a manual search of the bibliographies of the included studies, Google Scholar, Web of Science, and unpublished studies in trial registries.14
2.3. Data collection process
Study selection, quality assessment, and data extraction were performed in duplicate by two independent reviewers. Disagreements were resolved by discussion and, if necessary, by a third reviewer. The study selection was conducted using Covidence.16 The quality of experimental studies was evaluated with the Cochrane's risk of bias tool17 and the observational studies with the Newcastle‐Ottawa Scale.18 Sodium intake measurement was considered of high quality when assessed by 24‐hour urine collection controlled for completeness or using duplicates of foods measured for their sodium content. BP measurement was considered of high quality when measured multiple times, by trained professional, using standardized procedures, and, for the oscillometric method, using a clinically validated device. Data extraction was performed in Excel.
2.4. Data analysis
The reported effect sizes were transformed to unstandardized regression coefficients, that is, the change or difference in BP for every additional gram in sodium intake. If standard errors (SE) were not available, they were calculated from standard deviations, confidence intervals (CI), P‐values, t‐values, approximated using the Taylor expansion or imputed as the weighted mean of all standard errors of all studies included in the systematic review.14, 17
Random effects meta‐analyses were performed when there was a sufficient number of studies involving the same condition. If a study reported separately several groups, they were first pooled into one estimate using a fixed effect meta‐analysis, before being pooled with other studies. Heterogeneity was assessed with the I2 statistic.17 The findings of the studies which could not be meta‐analyzed were summarized narratively. The statistical analyses were performed with R (version 3.3.1) and R Analytic Flow (version 3.0.6).
3. RESULTS
Figure 1 shows the study selection process. After screening and reviewing, 16 studies (10 experimental studies and 6 observational studies, from 18 articles) involving overall 2902 children and adolescents with various clinical conditions were included.
Figure 1.
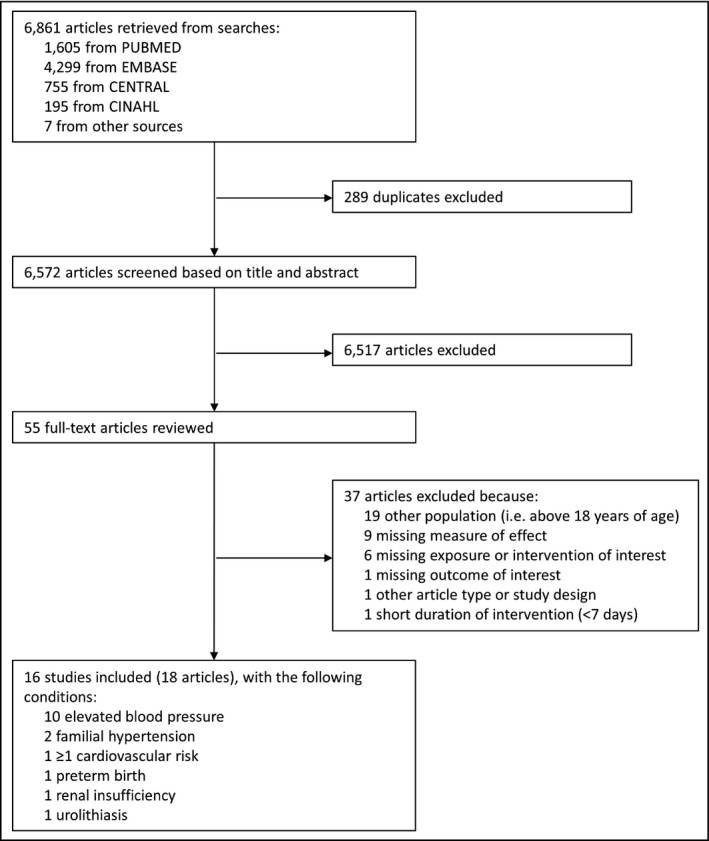
Flowchart of the study selection
The characteristics of each study are shown in Table 1. The majority of the studies (n = 10) were conducted in children with elevated BP without identifiable cause.19, 20 Only one of these studies specifically excluded children under anti‐hypertensive treatment.20 Two studies were conducted among children with familial hypertension,30, 31 one among children with cardiovascular risk factors,32, 33 one among children with chronic renal insufficiency,34 one among children with urolithiasis,35 and one among premature infants.36, 37
Table 1.
Characteristics of the included studies
| Author and year | Study type | Study design | Duration of follow‐up | Country | Sex | Age range | Sample size | Condition | Quality of sodium intake measurement | Quality of BP measurement |
|---|---|---|---|---|---|---|---|---|---|---|
| Berenson et al (1979) | Observational | Cross‐sectional study | Not applicable | United States | Boys and girls | 7‐15 y | 278 | Elevated BP | High | High |
| Couch et al (2014) | Experimental | Randomized controlled trial | 6 mo | United States | Boys and girls | Not reported | 159 | Elevated BP | Low | Unclear |
| Gillum et al (1981) | Experimental | Randomized controlled trial | 1 y | United States | Boys and girls | 6‐9 y | 80 | Elevated BP | Low | Unclear |
| Howe et al (1985), Howe et al (1986) | Experimental | Non‐randomized cross‐over trial | 3 wk | Australia | Boys and girls | 11‐14 y | 21 | Elevated BP | Low | Low |
| Howe et al (1991) | Experimental | Randomized cross‐over trial | 4 wk | Australia | Boys and girls | 11‐14 y | 100 | Elevated BP | Low | Low |
| Johnson et al (1991) | Experimental | Non‐randomized controlled trial | 12 wk | United States | Boys and girls | 9‐13 y | 19 | Elevated BP | High | Unclear |
| Maiorano et al (1987) | Observational | Cross‐sectional study | Not applicable | Italy | Boys and girls | 11‐14 y | 120 | Elevated BP | High | High |
| Sinaiko et al (1993) | Experimental | Randomized controlled trial | 3 y | United States | Boys and girls | 10‐14 y | 210 | Elevated BP | Unclear | High |
| Tochikubo et al (1986a) | Experimental | Non‐randomized non‐controlled trial | 10 wk | Japan | Boys | 15‐18 y | 41 | Elevated BP | High | Unclear |
| Tochikubo et al (1986b) | Experimental | Non‐randomized non‐controlled trial | 6 mo | Japan | Boys | 15‐18 y | 111 | Elevated BP | High | Unclear |
| Siervogel et al (1980) | Observational | Cross‐sectional study | Not applicable | United States | Boys and girls | 8‐18 y | 154 | Familial hypertension | High | High |
| ten Berge‐van der Schaaf & May (1990) | Observational | Cross‐sectional study | Not applicable | Netherlands | Boys and girls | 10‐13 y | 750 | Familial hypertension | Unclear | Low |
| Kokanovic et al (2011), Kokanovic et al (2014) | Experimental | Non‐randomized non‐controlled trial | 2 mo | Croatia | Boys and girls | Not reported | 17 | One or more cardiovascular risk | Low | Unclear |
| Trachtman et al (1995) | Observational | Cross‐sectional study | Not applicable | United States | Boys and girls | 1.5‐10 y | 118 | Chronic renal insufficiency | Low | Unclear |
| Nikolis et al (2017) | Observational | Case‐control study | Not applicable | United States | Boys and girls | 3‐17 y | 124 | Urolithiasis | High | Low |
| Lucas et al (1988), Singhal et al (2011) | Experimental | Randomized controlled trial | 18 mo, 13‐16 y | England | Boys and girls | 0‐18 mo, 13‐16 y | 347 | Prematurity | High | Unclear |
BP, blood pressure.
Assessment of study quality is reported in Figure 2 and Figures S1 and S2. Overall, the overall quality of the studies was relatively low. Eight studies (50%) had a measurement of sodium intake of high quality, four (25%) had a BP measurement of high quality, and three (19%) had measurements of both sodium intake and BP of high quality.
Figure 2.
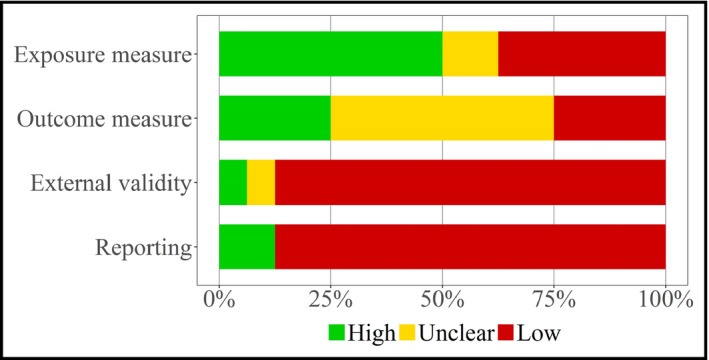
Quality assessment of all studies (n = 16)
3.1. Elevated blood pressure
The association between sodium intake and BP in children with elevated BP without identifiable cause was assessed in 10 studies. Eight of these studies were experimental and two were observational. Data from six studies, including 381 children, could be pooled together.20, 23, 25, 26, 29 The meta‐analysis of these studies showed a positive and substantial association between sodium intake and systolic and diastolic BP (Figure 3), that is, a difference of 6.3 mm Hg (95% CI: 2.9‐9.6) of systolic BP and of 3.5 mm Hg (95% CI: 1.2‐5.7) of diastolic BP for a difference of 1 g of sodium intake. There were no statistically significant differences in effect estimates between the experimental and observational studies (P = 0.26 for systolic BP and P = 0.77 for diastolic BP).
Figure 3.
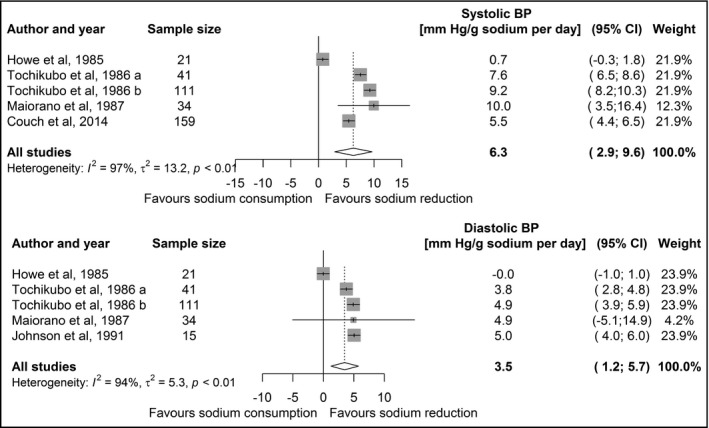
Forest plot of the association between sodium intake and systolic (upper panel) and diastolic (lower panel) BP among studies including children with elevated BP. BP, blood pressure; CI, confidence interval
The four other studies are summarized narratively here. Two studies (n = 80, n = 210) aimed at reducing sodium intake in children with elevated BP through educational interventions over long periods, that is, 1 and 3 years.21, 27 However, the difference in sodium intake at the end of the intervention between the control and intervention groups was negligible, that is, −0.17 g sodium/d and −0.03 g sodium/d, respectively, and the effect of sodium on BP could therefore not be further investigated. In another study (n = 100) conducted in a group of children with normal and elevated BP, sodium intake was reduced through an intervention combining education and provision of low sodium bread and was not associated with a change in BP.22 The change in sodium intake in children with elevated BP was not reported and therefore could not be included in our meta‐analysis. Finally, an observational study (n = 278) among children with different levels of BP found “a general tendency […] toward positive association in the high BP stratum” between sodium intake and BP, but did not report any figures.19
3.2. Familial hypertension
In a population‐based study conducted in the 1970s in the United States,30 over 20 000 men aged 35‐58 years of age were screened for essential hypertension. The children (n = 154) of the men diagnosed with essential hypertension were invited to have an assessment of BP and sodium excretion. The association between sodium excretion and BP was positive with a difference of 0.9 mm Hg (SE: 0.3) systolic BP and 0.4 mm Hg (SE: 0.2) diastolic BP for 1 g difference in sodium intake, respectively. However, upon adjustment for age and body anthropometry, the associations were reduced for both systolic BP and diastolic BP, and no longer reached statistical significance.
In a sub‐analysis of a study conducted in 1980s in the Netherlands in children (n = 750) of mothers with a diastolic BP equal or above 90 mm Hg,31 a significant positive association was found between BP and sodium intake with a difference of 1.5 mm Hg (SE: 0.3) systolic BP and of 0.7 mm Hg (SE: 0.3) diastolic BP for a 1 g difference in sodium intake.
3.3. Children with at least one cardiovascular risk factor
In a non‐controlled trial conducted in the 2000s in Croatia,32, 33 17 adolescents with at least one cardiovascular risk factor (ie, family history of cardiovascular disease, elevated BP, or obesity) were given a 2‐hour individual education session with dietary recommendations, notably to reduce sodium intake. The effect of the session on sodium intake and BP was assessed after 2 months. For a reduction of 1 gram of sodium, the decrease in systolic BP and diastolic BP was 8.9 mm Hg (SE: 0.5) and 2.7 mm Hg (SE: 0.5), respectively. Data were not analyzed separately for family history of cardiovascular disease, elevated BP, or obesity.
3.4. Chronic renal insufficiency
In a cross‐sectional study including 118 children with chronic renal insufficiency between 18 months and 10 years of age, nutritional intake was assessed in detail.34 Sixteen children were hypertensive and six took anti‐hypertensive medication. In multi‐variate analysis, sodium intake was associated with systolic BP with a difference of 1.0 mm Hg (SE: 1.7, P = 0.571) systolic BP and of 0.9 mm Hg (SE: 1.8, P = 0.608) diastolic BP for a 1 g difference in sodium intake.
3.5. Urolithiasis
One case‐control study35 compared children (n = 124) below 18 years of age with urolithiasis (so‐called stone formers, n = 71) and children with non‐glomerular hematuria (non‐stone formers, n = 53). The stone formers had higher systolic and diastolic BP (systolic BP: 109.4 vs 103.0 mm Hg, P = 0.01 and diastolic BP: 65.0 vs 61.4 mm Hg, P = 0.02) and higher 24‐hour sodium excretion (2.8 vs 2.4 g). In stone formers, systolic BP was associated positively with 24‐hour urine sodium with a difference of 3.6 mm Hg (SE: 1.1, P = 0001) for a 1 g difference in sodium intake. Upon adjustment for age, sex, and BMI z‐score, the association remained statistically significant. The association with diastolic BP was not reported.
3.6. Prematurity
One randomized controlled trial was conducted among premature children born between 1982 and 1985 in the United Kingdom.36, 37 Some 347 premature infants without any major congenital anomalies and with a low birthweight (<1850 g) were assigned to preterm formula (high sodium, 0.5 g sodium/L), standard term formula (low sodium, 0.2 g sodium/L), or banked breast milk (low sodium, 0.2 g sodium/L). Further, in each group, the feeds could be complemented with expressed breast milk (0.3 g sodium/L). Infants received the assigned diets until they reached 2000 g or were discharged. The infants were followed up at 18 months and at 15 years of age. This study found no differences in BP between groups at 18 months of age. However, differences in BP were found at 15 years of age between breast milk and the preterm and term formulas (BP breastmilk − BP preterm formula: 2.7 mm Hg for systolic BP (P = 0.075) and 3.1 mm Hg for diastolic BP (P = 0.016); BP breastmilk − BP term formula: 4.2 mm Hg for systolic BP [P‐value not reported] and 2.9 mm Hg for diastolic BP [P‐value not reported]). This study suggests that, compared to formula, breast milk during infancy in premature infants could lower BP later in life, possibly due to factors other than the amount of sodium provided. However, a relatively low‐sodium (standard term) formula was not associated with a lower BP compared to relatively high‐sodium (preterm) formula.
4. DISCUSSION
In this systematic review, 16 studies, involving overall 2902 children and adolescents, with various clinical conditions were identified. Ten studies were conducted in children with elevated BP without identifiable cause, two studies in children with familial hypertension, one study in children with one or more cardiovascular risk factors, one study in children with renal insufficiency, one study in children with urolithiasis, and one study in premature infants. A positive association between sodium intake and BP was found in all studies (n = 16), except one.22 Sodium intake was strongly associated with BP in children and adolescents with elevated BP without identifiable cause. A positive association was found in children with other selected clinical conditions, however, with a lower level of evidence. Since BP tracks across the life course, our findings support the reduction of sodium intake among children with elevated BP to lower BP and, ultimately, to prevent the development of hypertension later in life.
The meta‐analyses of studies with children with elevated BP showed a stronger association between sodium intake and BP than in another systematic review and meta‐analysis with only healthy children (see Table 2).13 Similarly, in adults, the association between sodium intake and BP has been shown to be higher in hypertensives, than in normotensives.10, 12, 38 The greater sensitivity of BP to sodium intake in children and adults with elevated BP suggests that reducing sodium intake at all ages can have a substantial effect on the level of BP. It may also indicate that salt sensitivity could track from childhood to adulthood, similarly to the tracking on BP.39, 40
Table 2.
Comparison of the association between sodium intake and systolic and diastolic blood pressure (BP) in adults and children with normal and elevated BP
| Population group | Systolic BP (95% CI) (mm Hg/g sodium per day) | Diastolic BP (95% CI) (mm Hg/g sodium per day) |
|---|---|---|
| Adults with normal BP4 | 1.4 (0.7, 2.1) | 0.6 (0.1, 1.1) |
| Adults with hypertension4 | 3.1 (2.4, 3.8) | 1.6 (1.2, 2.1) |
| Children without any clinical conditions13 | 0.8 (0.4, 1.3) | 0.7 (0.0, 1.4) |
| Children with elevated BP (this review) | 6.3 (2.9, 9.6) | 3.5 (1.2, 5.7) |
The number indicates the difference in systolic or diastolic BP that is expected for a reduction in one gram of sodium intake per day. CI, confidence interval.
Our review indicates that evidence linking sodium intake and BP is very limited among children with conditions other than elevated BP, notably familial hypertension, prematurity, and chronic renal failure. Of note, we could not identify studies conducted among children with diabetes, while diabetes in adults has been shown to increase BP sensitivity to sodium intake.41
High sodium intakes are one of the causes of progression of renal insufficiency.42 Patients with renal insufficiency should therefore reduce sodium intake. Several studies in adults with chronic renal disease have shown the high sensitivity of BP to sodium intake.42 In the study by Trachtman et al,34 a positive yet non‐statistically significant association was found between sodium intake and BP in children with chronic renal insufficiency. Some of the children included in this study took anti‐hypertensive medication, which could have partly masked the association between sodium intake and BP. Nevertheless, because of the equivocal and limited evidence in children with chronic kidney disease, further studies are needed to estimate the effect of sodium reduction on BP in this population.
A rise in the frequency of urinary stones has been reported worldwide and could be due to the increases in sodium intake.43, 44 High sodium intake can increase urinary calcium excretion, which can in turn increase the formation of calcium stones.45 Patients with urinary stone diseases are therefore encouraged to limit sodium intake.43, 44 The study by Nikolis et al35 suggests that the association between sodium intake and systolic BP could be higher in children with urolithiasis than in healthy children.13 This finding supports the reduction of sodium intake in children with urolithiasis not only to prevent the formation of stones but also to reduce BP during childhood and prevent hypertension later in life. Of note, urinary stones are uncommon in children and many children with stones have either an underlying abnormality of the urinary tract or a metabolic disease and often a reduced renal function.
Numerous studies have shown that children born prematurely or with low birth weight have relatively high BP later in life.46 A study, in which the duration of the intervention was of only 7 days and which was therefore excluded from this review, compared the salt sensitivity of children born preterm and at term.47 This study found that children with low birth weight and small‐for‐gestational age were more likely to be salt sensitive. Salt sensitivity was inversely correlated with kidney length, which was smaller in low birth weight children. The Barker and the Brenner hypotheses both support that fetal and perinatal exposures can have long‐term effects on the development of hypertension and coronary heart diseases.48, 49 The study by Lucas et al showed that breast milk could reduce BP later in life among preterm infants compared to formula, irrespective of sodium content. This study suggests not only that the first months of life may be a key time window for the development of high BP later in life36, 37 but also that nutrients other than sodium present in breast milk could have an effect on BP.
To our knowledge, this is the first systematic review on the association between sodium intake and BP in children with clinical conditions. The major strengths of this study are the comprehensive and systematic review of the literature and the assessment of the study quality. The limitations of this review are that too few studies were identified to determine with confidence the association for each condition and that the overall quality of the studies was low. We were only able to pool results of some studies in children with elevated BP. There was nevertheless high heterogeneity between these studies, limiting our confidence in the pooled estimates. Moreover, due to the limited number of studies, we were not able to assess the presence of publication bias through an analysis of funnel plots or Egger's test. We can however suspect that studies showing no association between sodium intake and BP may not have been published. As a result, we may have overestimated the association between sodium intake and BP. Moreover, due to the limited number of studies for which data was poolable, we were not able to assess whether the association between sodium intake and BP differs by age, sex, or weight. To overcome this limitation, we would need to conduct individual data analyses. Interestingly, in our previous review of studies including children without a clinical condition, we identified a large number of studies and we could show that the association was stronger among overweight children and children having a low potassium intake; no substantial difference was observed with age or sex.13
Our findings suggest that targeting salt reduction in children with elevated BP, with a condition associated with elevated BP or at risk for elevated BP in the future (such as with familial history), could be efficient as these children seem to be more salt sensitive than children with normal BP. For example, clinicians who identify children with elevated BP could provide dietary counseling to reduce salt intake in those children. This targeted high‐risk approach could complement a population‐based approach to, for example, reduce sodium content of foods consumed by children.13
5. CONCLUSIONS
In conclusion, our systematic review suggests that sodium intake is positively associated with BP in children and adolescents with clinical conditions, in particularly in those with elevated BP. Consistent with findings in adults, the association between sodium intake and BP is stronger in children with elevated BP than in healthy children. Our findings support therefore the reduction of sodium intake in children with elevated BP to ultimately prevent the development of hypertension later in life.
CONFLICT OF INTEREST
The authors report no conflict of interest to disclose.
AUTHORS’ CONTRIBUTIONS
ArC and MRL designed the research protocol. MRL conducted the databases and manual searches, statistical analyses, and wrote the manuscript. MRL, CB, and AnC selected the studies, extracted the data, and assessed the quality, and ArC resolved conflicts. ArC provided guidance for the statistical analyses. ArC, CB, PB, AnC, GP, MiB, MuB, RT, and VS provided inputs to the manuscript. ArC had primary responsibility for final content. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Chantal Petoud Dei Rossi for her help in finding the full texts of the selected articles. This manuscript has not been published and is not being considered for publication elsewhere.
Rios‐Leyvraz M, Bloetzer C, Chatelan A, et al. Sodium intake and blood pressure in children with clinical conditions: A systematic review with meta‐analysis. J Clin Hypertens. 2019;21:118–126. 10.1111/jch.13436
Funding information
This work was funded by the Swiss Federal Food Safety and Veterinary Office (FSVO) (funding reference number 5.15.03).
REFERENCES
- 1. Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension . Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371(9623):1513‐1518. [DOI] [PubMed] [Google Scholar]
- 2. Bochud M, Marques‐Vidal P, Burnier M, Paccaud F. Dietary salt intake and cardiovascular disease: summarizing the evidence. Public Health Rev. 2012;33(2):530‐552. [Google Scholar]
- 3. Global Burden of Disease Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 5. Graudal NA, Hubeck‐Graudal T, Jürgens G. Effects of low‐sodium diet vs. high‐sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens. 2012;25(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 6. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85(2):679‐715. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization . Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 8. Mente A, O'Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388(10043):465‐475. [DOI] [PubMed] [Google Scholar]
- 9. Chiolero A, Wurzner G, Burnier M. Renal determinants of the salt sensitivity of blood pressure. Nephrol Dial Transplant. 2001;16(3):452‐458. [DOI] [PubMed] [Google Scholar]
- 10. Elijovich F, Weinberger MH, Anderson CA, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68(3):e7–e46. [DOI] [PubMed] [Google Scholar]
- 11. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta‐analysis of controlled trials. Hypertension. 2006;48(5):861‐869. [DOI] [PubMed] [Google Scholar]
- 12. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leyvraz M, Chatelan A, da Costa BR, et al. Sodium intake and blood pressure in children and adolescents: a systematic review and meta‐analysis of experimental and observational studies. Int J Epidemiol. 2018;(dyy121):1–15. [DOI] [PubMed] [Google Scholar]
- 14. Leyvraz M, Taffe P, Chatelan A, et al. Sodium intake and blood pressure in children and adolescents: protocol for a systematic review and meta‐analysis. BMJ Open. 2016;6(9):e012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Covidence [computer program]. Prahran, VIC: Alfred Health; 2013. [Google Scholar]
- 17. Higgins J, Cochrane GS. Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration.2011.
- 18. Wells GA, Shea B, O'Connell D, et al.The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2014.
- 19. Berenson GS, Voors AW, Dalferes ER, Webber LS, Shuler SE. Creatinine clearance, electrolytes, and plasma renin activity related to the blood pressure of white and black children – the Bogalusa Heart Study. J Lab Clin Med. 1979;93(4):535–548. [PubMed] [Google Scholar]
- 20. Couch SE, Saelens BE, Hinn K, et al. Effects of a clinic‐initiated behavioral nutrition intervention emphasizing the dash diet on blood pressure control in adolescents with elevated blood pressure. J Am Soc Hypertens. 2014;8(4S):e116. [Google Scholar]
- 21. Gillum RF, Elmer PJ,Prineas RJ. Changing sodium intake in children. The Minneapolis Children's Blood Pressure Study. Hypertension. 1981;3(6):698–703. [DOI] [PubMed] [Google Scholar]
- 22. Howe PR, Cobiac L, Smith RM. Lack of effect of short‐term changes in sodium intake on blood pressure in adolescent schoolchildren. J Hypertens. 1991;9(2):181–186. [DOI] [PubMed] [Google Scholar]
- 23. Howe P, Jureidini KF, Smith RM. Sodium and blood pressure in children – a short‐term dietary intervention study. Proc Nutr Soc Aust. 1985;10:121–124. [Google Scholar]
- 24. Howe PR, Rogers PF, Smith RM, Jureidini KF. Effects of short‐term modification of dietary sodium intake on plasma catecholamines and blood pressure in prehypertensive children. Clin Exp Pharmacol Physiol. 1986;13(4):305–309. [DOI] [PubMed] [Google Scholar]
- 25. Johnson CC, Nicklas TA, Arbeit ML, et al. Cardiovascular intervention for high‐risk families: the Heart Smart Program. South Med J. 1991;84(11):1305–1312. [DOI] [PubMed] [Google Scholar]
- 26. Maiorano G, Contursi V, Petrelli G, et al. Anthropometric data, urinary electrolytes excretion, and blood pressure in adolescents. J Clin Hypertens. 1987;3(2):164–172. [PubMed] [Google Scholar]
- 27. Sinaiko AR, Gomez‐Marin O, Prineas RJ. Effect of low sodium diet or potassium supplementation on adolescent blood pressure. Hypertension. 1993;21(6 Pt 2):989–994. [DOI] [PubMed] [Google Scholar]
- 28. Tochikubo O, Sasaki O, Umemura S, Goto E, Fujishima S, Kaneko Y. Cation imbalance in erythrocytes, serum and 24‐hour urine from patients with essential hypertension and adolescents with high blood pressure. Jpn Circ J. 1982;46(5):512–522. [DOI] [PubMed] [Google Scholar]
- 29. Tochikubo O, Sasaki O, Umemura S, Kaneko Y. Management of hypertension in high school students by using new salt titrator tape. Hypertension. 1986;8(12):1164–1171. [DOI] [PubMed] [Google Scholar]
- 30. Siervogel RM, Frey MA, Kezdi P, Roche AF, Stanley EL. Blood pressure, electrolytes, and body size: their relationships in young relatives of men with essential hypertension. Hypertension. 1980;2(4 Pt 2):83–92. [PubMed] [Google Scholar]
- 31. ten Berge‐van der Schaaf J, May JF. Self‐screening of blood pressure and sodium in a 24‐hour urine sample as part of a school health programme. J Hum Hypertens. 1990;4(4):337–338. [PubMed] [Google Scholar]
- 32. Kokanovic A, Mandic ML, Banjari I. Does individual dietary intervention have any impact on adolescents with cardiovascular health risks? Med Glas (Zenica). 2014;11(1):234–237. [PubMed] [Google Scholar]
- 33. Kokanovic A, Mandic M, Banjari I. Impact of dietary intervention on cardiovascular risks in adolescents. Ann Nutr Metab. 2011;58(suppl 3):285–286. [Google Scholar]
- 34. Trachtman H, Chan JC, Boyle R, et al. The relationship between calcium, phosphorus, and sodium intake, race, and blood pressure in children with renal insufficiency: a report of the Growth Failure in Children with Renal Diseases (GFRD) Study. J Am Soc Nephrol. 1995;6(1):126–131. [DOI] [PubMed] [Google Scholar]
- 35. Nikolis L, Seideman C, Palmer LS, et al. Blood pressure and urolithiasis in children. J Pediatr Urol. 2017;13(1):54.e1–54.e6. [DOI] [PubMed] [Google Scholar]
- 36. Lucas A, Morley R, Hudson GJ, et al. Early sodium intake and later blood pressure in preterm infants. Arch Dis Child. 1988;63(6):656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357(9254):413–419. [DOI] [PubMed] [Google Scholar]
- 38. Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–611. [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta‐regression analysis. Circulation. 2008;117(25):3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leyvraz M, Wahlen R, Bloetzer C, Paradis G, Bovet P, Chiolero A. Persistence of elevated blood pressure during childhood and adolescence: a school‐based multiple cohorts study. J Hypertens. 2018;36(6):1306–1310. [DOI] [PubMed] [Google Scholar]
- 41. Suckling RJ, He FJ, Macgregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev. 2010(12):CD006763. [DOI] [PubMed] [Google Scholar]
- 42. Smyth A, O'Donnell MJ, Yusuf S, et al. Sodium intake and renal outcomes: a systematic review. Am J Hypertens. 2014;27(10):1277–1284. [DOI] [PubMed] [Google Scholar]
- 43. Afsar B, Kiremit MC, Sag AA, et al. The role of sodium intake in nephrolithiasis: epidemiology, pathogenesis, and future directions. Eur J Intern Med. 2016;35:16–19. [DOI] [PubMed] [Google Scholar]
- 44. Prezioso D, Strazzullo P, Lotti T, et al. Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch Ital Urol Androl. 2015;87(2):105–120. [DOI] [PubMed] [Google Scholar]
- 45. Ticinesi A, Nouvenne A, Maalouf NM, Borghi L, Meschi T. Salt and nephrolithiasis. Nephrol Dial Transplant. 2016;31(1):39–45. [DOI] [PubMed] [Google Scholar]
- 46. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59(2):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG. Salt sensitivity of children with low birth weight. Hypertension. 2008;52(4):625–630. [DOI] [PubMed] [Google Scholar]
- 48. Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25(7):457–463. [DOI] [PubMed] [Google Scholar]
- 49. Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure: less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


