In their study, Padwal and coworkers tested whether long‐term (20 years) home blood pressure telemonitoring (BPT) plus pharmacist case management was more cost‐effective than usual care in a cohort of high‐risk Canadian patients with a recent non‐disabling cerebrovascular disease.1 Authors ran a cost‐utility analysis using a Markov decision model applied to a previously published BPT study in a large population of adults with uncontrolled BP followed in a primary care setting under a pharmacist‐physician collaborative practice agreement and modified it in order to fit the post‐stroke and Canadian context.2 The baseline characteristics of the cohort were based on a randomized controlled study performed in patients with a recent minor cerebrovascular event.3 Achieved BP, risk of future cardiovascular (CV) events, attendant consequences on quality‐adjusted life years (QALY), and Canadian dollar ($) costs were modeled. BPT was assumed to occur intensively for 3 months, then quarterly. In the base case analysis, the BPT intervention resulted in total costs per patient of 21 640$ and 8.83 QALY, whereas corresponding values for usual care were 23 020$ and 8.00. Home BPT and pharmacist case management resulted in an incremental 0.83 QALY and cost savings of 1929$ compared to usual care. Some sensitivity analyses were run in order to evaluate different scenarios, confirming the intervention dominance even in case the systolic blood pressure (SBP) efficacy was reduced or BPT costs were increased. The authors concluded that the intervention was dominant, achieving improved health at a reduced cost.
In the past, few randomized controlled studies based on BPT in hypertensive patients have concluded that the use of technologies may only modestly increase healthcare costs compared to usual care and that the BPT is a cost‐effective strategy. Unfortunately, most of these studies suffered from methodological flaws in the economic analysis, were based on a relatively small sample of subjects, short observation periods, and were often performed in mixed populations, including both low‐ and high‐risk patients for which the cost‐benefit of the intervention may substantially vary. Evidence on long‐term economic benefits of BPT is substantially lacking. In a systematic review of randomized controlled studies that we performed a few years ago,4 including 6 studies (8 comparisons), the use of BPT was associated with lower medical costs, a finding confirmed in more recent studies5, 6, 7 supporting the cost‐effectiveness of this intervention. However, medical costs were offset by those of the equipment and technologies, which contributed to the increase in the overall healthcare costs. The total expenditure was approximately 660 Euros larger in the BPT group than in the usual care group, with a substantial heterogeneity across the studies and a rather broad oscillation of costs (from 640 to 1035 Euros) (Figure1). The incremental cost‐effectiveness ratio for the healthcare expenditure averaged to nearly 400 Euros for SBP and to nearly 800 Euros for diastolic blood pressure (DBP) over a median follow‐up period of 4 years, which means 100‐200 Euros per person per year per 1 mmHg of BP reduction. However, when only medical costs were considered, the mean incremental cost‐effectiveness ratio dropped to approximately 30 Euros for SBP and 25 Euros for DBP, namely an economically worthwhile intervention.
Figure 1.
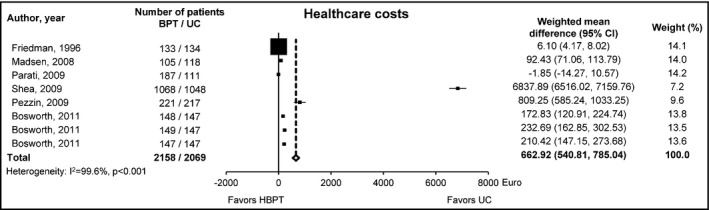
Weighted mean difference (95% confidence interval) in the healthcare costs (in Euros) between the group randomized to home blood pressure telemonitoring and the group under usual care. BPT, Blood pressure telemonitoring; UC, usual care; CI, confidence interval. [Redrawn from 4]
Few of the economic studies published so far specifically evaluated the cost‐benefit of pharmacist case management plus BPT.8, 9, 10 These randomized studies were set in outpatient primary care clinics staffed with clinical pharmacy specialists and evaluated direct costs of the intervention on a relatively short time interval (6‐12 months). The remote monitoring intervention was not homogeneous so that in one study8 patients had to enter their BP readings and information on BP control on a website, no automatic BP transmission being available. In one study,9 patients had two options: to input their BP readings on a free web application or to automatically upload into a PC data stored on home BP machines equipped with a USB port. In the most recent study,10 whose efficacy data served as a base for the economic simulation of Padwal and coworkers, home BPT was performed more appropriately with devices able to store and automatically transmit BP data to a secure website via modem. In all studies, home BP was measured at least 2‐3 times per week and uploaded weekly. Pharmacist care management was delivered through web, e‐mail, or telephone contacts together with remote BPT and education (review of the medication regimen, counseling on lifestyle changes, and antihypertension medications adjustment) under a collaborative practice agreement with the clinics’ primary care team. The studies enrolled uncontrolled adult hypertensive patients at relatively low risk, most of them treated with antihypertensive medications. Patients were excluded in case of major comorbidities such as recent cardiovascular disease (including stroke or ischemic heart disease) or advanced or end‐stage renal disease: the overall proportion of patients with diabetes or chronic kidney disease was small (39.5%) as well as that with a previous history of CV disease (9.6% in the Hyperlink study). As shown in Table1, in all studies, the intervention resulted in a better BP control than usual care and was cost‐effective.
Table 1.
Cost‐benefit studies of blood pressure telemonitoring studies with pharmacist case management. Data are shown as mean and 95% confidence interval. P‐value refers to the statistical significance of the comparison between the intervention and the usual care group
| Study | Follow‐up duration (mo) | n | BP <140/90 (%) | ΔSBP (mmHg) | ΔDBP (mmHg) | Healthcare costs ($) | ICER for achievement of BP control ($) | ICER for mmHg of SBP reduction ($) | ICER for mmHg of DBP control ($) | Cost per additional life‐year gained ($) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| e‐BP study8 | 12 | BPT | 247 | 56.0 (49.0, 62.0) | −14.2 (−16.0, −12.4) | −7.0 (−8.0, −6.0) | 400.36 (263.41, 565.77) | 16.65 (15.37, 17.94)a | 65.29 (59.91, 70.67) | 114.82 (111.90, 117.74) | 1035 (1690.42, 2379.58) |
| UC | 237 | 31.0 (25.0, 37.0) | −5.3 (−7.1, −3.5) | −3.5 (−4.5, −2.5) | 10.56 (8.48, 12.64) | – | – | – | – | ||
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | – | – | – | – | |||
| Heart360‐based study9 | 6 | BPT | 175 | 54.1 (61.5, 46.8) | −20.7 (−17.9, −23.5) | −10.5 (−12.0, −9.0) | 1590.00 (934.00, 2841.00) | 1331.00b | 20.50 | N.A. | 3330.00 |
| UC | 173 | 35.4 (28.1, 42.8) | −8.2 (−4.4, −12.0) | −4.8 (−6.9, −2.7) | 1283.00 (681.00, 2383.00) | – | – | – | – | ||
| P‐value | <0.001 | <0.001 | <0.001 | 0.008 | – | – | – | – | |||
| Hyperlink study10 | 12 | BPT | 150 | 71.2 (62.0, 78.9) | −22.5 (−25.1, −19.9) | −9.3 (−11.0, −7.7) | 4500.00 | 7337.00 (2278.00, 26 329.00)b | 139.00 (46.00, 347.00) | 265.00 (83.00, 743.00) | – |
| UC | 148 | 52.8 (45.4, 60.2) | −12.9 (−15.5, −10.2) | −4.3 (−5.9, −2.7) | 4453.00 | – | – | – | – | ||
| P‐value | 0.005 | <0.001 | <0.001 | N.S. | – | – | – | – | |||
BP, blood pressure; BPT, blood pressure telemonitoring; DBP, diastolic blood pressure; e‐BP, electronic communications and home blood pressure monitoring; Hyperlink, home blood pressure telemonitoring and case management to control hypertension; ICER, incremental cost‐effectiveness ratio; N.A., not available; N.S., not significant; SBP, systolic blood pressure; UC, usual care.
ICER for 1% improvement in the number of patients with controlled BP.
ICER per person achieving BP control.
The study of Padwal and coworkers adds further evidence to the one provided by the studies mentioned above, particularly because it is the only one so far evaluating cost‐benefit of pharmacist intervention in high‐risk post‐stroke patients. Although based on simulation of data taken from randomized controlled trials and on data extrapolated from a recent meta‐analysis, the strength of this study lies in the attempt to evaluate cost‐effectiveness over a long‐period of time, incorporating the long‐term benefits of BP control on cardiovascular risk and quality of life.
Although the first of its kind, the study by Padwal and coworkers is not free of important critical issues. First of all, the study results and conclusions are based on a simulation, and thus, they may not fully reflect what it may be observed in a real‐life study. Additionally, the simulation took as reference a Canadian study in stroke patients: results may be not fully interchangeable with situations occurring in other countries and different settings.
There are also a couple of methodological assumptions which may be not completely appropriate.
Padwal and coworkers set the reference difference in SBP changes between usual care and intervention to 9.7 mmHg over 4 years. Although sensitivity analysis included reducing the SBP efficacy to 4.9 mmHg, this might not match with actual effects of prolonged BPT. As a matter of fact, a recent meta‐analysis 11 suggests that BP control/reduction in patients with stroke performing self‐BP monitoring (with some studies including BPT) may be consistently less than in patients without stroke. In addition, it is well known that the impact of BPT in terms of BP reduction is time‐dependent. A progressive attenuation in the effect of the intervention may be observed over months or years, for instance due to a reduction in patient's motivation and adherence to the BPT program, or because of pharmacist's inertia. This might require adaptation or changes in strategies in order to ensure sustainability and cost‐efficacy of the intervention in the long‐term, particularly when prevention of future cardiovascular events is considered as the main target of the intervention.
Although Padwal and coworkers performed a sensitivity analysis using a reference of 4.5 mmHg and assumed a BPT program occurring initially for 3 months and then quarterly, this might be not yet appropriate leading to over‐estimation of cost‐effectiveness of the intervention, as it may be inferred from two analyses of the e‐BP and Hyperlink studies.12, 13 These evaluated the benefit of the pharmacist's intervention months after its withdrawal. In the e‐BP Study 1 year after the completion of the intervention, the BPT group still had significantly larger SBP difference than the usual care group, but the differential effect narrowed (−3.6 mmHg vs −8.9 at the end of the 1‐year follow‐up).12 In a recent update of the Hyperlink study, the differential SBP and DBP reductions from baseline between the BPT and usual care group at 54 months were very much attenuated compared to the ones observed at 12 months (−2.5/−1.0 vs −9.7/−5.1 mmHg).13
Whether the cost‐benefit of a pharmacist case management of the hypertensive patients may be extended on the cost related to the disease still needs to be investigated in economic studies. A potential positive effect is suggested by the simulation of Padwal and coworkers and by the recently published economic evaluation of the Hyperlink study. In this trial, an almost 500$ reduction in cardiovascular disease‐related hospital costs (P = 0.112) was observed over the 12 months of the observation, suggesting that over a longer period of time, significant cost reductions from adverse cardiovascular disease events might be expected from such a program.10
Finally, as highlighted in two meta‐analyses of several randomized controlled studies, in terms of effectiveness of hypertension management, a more vs a less intensive BP control, with the achievement of more strict BP target (or control), may count more than a generic BP reduction.14, 15 As can be inferred from the studies summarized in Table1, the cost per person achieving BP control with BPT may be substantially larger than that to be sustained to reduce BP by 1 mmHg. Future studies should take into account differences in cost‐efficacy according to the therapeutic target (possibly including hard endpoints).
In conclusion, although BPT programs involving a pharmacist require investment in laboratory monitoring and technologies, and are associated with a larger use of medications and more contacts with patients than standard care, they still produce significantly improved BP control at relatively low cost or with an only modest increase in healthcare costs compared to usual care. If clinical gains suggested by the economic analyses available at the moment are maintained, these additional costs would be very likely compensated for by reductions in the cost for future cardiovascular events.
The simulation provided by Padwal and coworkers extends the current evidence to high‐risk patients with stroke, evaluating the longer‐term impact and cost‐effectiveness of BPT with pharmacist case management. The analysis could not take into account potential cost savings from a reduction in cardiac events and long‐term complications, nor could account for indirect or intangible costs such as travel time to clinic or time missed from work that would be relevant to an economic analysis from the societal perspective, particularly over several years. It seems straightforward that future studies should properly address all these open questions and their results help boost the implementation of effective pharmacist‐led telehealth programs in hypertension management.16
CONFLICT OF INTEREST
The author reports no specific funding in relation to this research. He is scientific consultant of Biotechmed Ltd, provider of telemedicine services in community pharmacies.
REFERENCES
- 1. Padwal RS, So H, Wood PW, et al. Cost‐effectiveness of home blood pressure telemonitoring and case management in the secondary prevention of cerebrovascular disease in Canada. J Clin Hypertens. 2018. 10.1111/jch.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAlister FA, Majumdar SR, Padwal RS, et al. Case management for blood pressure and lipid level control after minor stroke: PREVENTION randomized controlled trial. CMAJ. 2014;186(8):577‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost‐effectiveness of home blood pressure telemonitoring: meta‐analysis of randomized controlled studies. J Hypertens. 2013;31(3):455‐467. [DOI] [PubMed] [Google Scholar]
- 5. Stoddart A, Hanley J, Wild S, et al. Telemonitoring‐based service redesign for the management of uncontrolled hypertension (HITS): cost and cost‐effectiveness analysis of a randomised controlled trial. BMJ Open. 2013;3(5):e002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaambwa B, Bryan S, Jowett S, et al. Telemonitoring and self‐management in the control of hypertension (TASMINH2): a cost‐effectiveness analysis. Eur J Prev Cardiol. 2014;21(12):1517‐1530. [DOI] [PubMed] [Google Scholar]
- 7. Upatising B, Wood DL, Kremers WK, et al. Cost comparison between home telemonitoring and usual care of older adults: a randomized trial (Tele‐ERA). Telemed J E Health. 2015;21(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fishman PA, Cook AJ, Anderson ML, et al. Improving BP control through electronic communications: an economic evaluation. Am J Manag Care. 2013;19(9):709‐716. [PMC free article] [PubMed] [Google Scholar]
- 9. Billups SJ, Moore LR, Olson KL, Magid DJ. Cost‐effectiveness evaluation of a home blood pressure monitoring program. Am J Manag Care. 2014;20(9):e380‐e387. [PubMed] [Google Scholar]
- 10. Dehmer SP, Maciosek MV, Trower NK, et al. Economic evaluation of the home blood pressure telemonitoring and pharmacist case management to control hypertension (Hyperlink) trial. J Am Coll Clin Pharm. 2018;1(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tucker KL, Sheppard JP, Stevens R, et al. Self‐monitoring of blood pressure in hypertension: a systematic review and individual patient data meta‐analysis. PLoS Med. 2017;14(9):e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green BB, Anderson ML, Ralston JD, Catz SL, Cook AJ. Blood pressure 1 year after completion of web‐based pharmacist care. JAMA Intern Med. 2013;173(13):1250‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Margolis KL, Asche SE, Dehmer SP, et al. Long‐term outcomes of the effects of home blood pressure telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension. Follow‐up of a cluster randomized clinical trial. JAMA Netw Open. 2018;1:e181617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2. Effects at different baseline and achieved blood pressure levels–overview and meta‐analyses of randomized trials. J Hypertens. 2014;32(12):2296‐2304. [DOI] [PubMed] [Google Scholar]
- 15. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels ‐ updated overview and meta‐analyses of randomized trials. J Hypertens. 2016;34(4):613‐622. [DOI] [PubMed] [Google Scholar]
- 16. Omboni S, Tenti M. Telepharmacy for the management of cardiovascular patients in the community. Trends Cardiovasc Med. 2018. pii: S1050‐1738(18)30131‐2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


