1. INTRODUCTION
Despite significant advances in health care over the last 50 years, the global cardiovascular disease burden continues to increase with dire implications. It is well known that cardiovascular disease is now the leading cause of mortality, resulting in approximately one third of all deaths globally.1 This chilling statistic is projected to worsen in years to come and therefore needs to be urgently addressed. Importantly, a large proportion of these cardiovascular disease‐related deaths is being driven by hypertension, a risk factor that is widely present, affecting approximately 30% of all adults worldwide and is poorly controlled.2, 3, 4, 5 In order to address the role that hypertension plays in cardiovascular disease morbidity and mortality, there is an urgent need for a paradigm shift in the management and treatment of hypertension on a global scale.
While lifestyle modification (non‐pharmacologic therapy) is important, it has been very difficult to apply on an individual and population level to date. Hence, effective pharmacologic management is central to hypertension control. However, with the increasing number and diversity of pharmacologic agents available, spanning several key and complementary drug classes, treatment options are now complex and need to be simplified.6, 7 One potential untapped means of simplifying the pharmacologic management of hypertension is through the use of fixed‐dose combination (FDC) agents, in which two or more drugs are present in a single pill or capsule. This approach, which is not novel, has been widely underutilized. Increasing the use of FDC therapy could significantly and rapidly improve hypertension control rates and clinical outcomes in hypertension in both high‐ and low‐ to middle‐income countries (LMICs). It is important to note that two or more antihypertensive agents could also simultaneously be administered in the management of hypertension, as separate pills or capsules, where FDC agents are not available, do not come in optimal doses, or are too costly.
To discuss the role of FDC therapy in hypertension, this manuscript will first highlight the importance of incorporating the strategic use of FDC pharmacologic therapy to improve control rates and outcomes in hypertension. Second, it will outline in detail the key processes in the selection of preferred and acceptable FDC medications for the inclusion in institutional, national, regional, and global formularies. While this manuscript will focus on two‐drug antihypertensive FDCs in the treatment of hypertension, it is important to note that other forms of FDC therapy may also be considered in the treatment of hypertension, such as three or more drug antihypertensive FDCs or FDCs with agents such as statins to address other concomitant cardiovascular risk factors.
2. BARRIERS TO THE PHARMACOLOGIC MANAGEMENT OF HYPERTENSION
One of the major challenges currently fueling the high burden of cardiovascular disease is the poor pharmacologic treatment rates of hypertension, which on a global scale are as low as 47% among people who know their condition.8, 9 This astonishingly low use of pharmacologic agents in the management of hypertension is the product of several factors on multiple fronts. For instance, patients frequently lack education regarding hypertension and the importance of treatment. This lack of education results in poor medication adherence and a lack of engagement in their care.
On the health care provider front, factors include a lack of a full understanding of the appropriate use of pharmacologic classes and individual agents, reluctance to use standardized treatment algorithms, and the presence of “clinical or therapeutic inertia” (the phenomena of not initiating therapy promptly, delaying dose up‐titration, or adding other pharmacologic agents when indicated). At the health care system level, factors such as a lack of clinic and provider access, poor availability of quality, affordable and reliable medications, the inability to sustain recognition and treatment programs once initiated, and budgetary constraints all prevent the widespread use of antihypertensive medications which contributes to poor treatment rates. While all or some of these factors are present in high‐income countries, their role as barriers to the treatment of hypertension is even more dramatic in LMICs.10
3. THE NEED FOR A MULTI‐DRUG APPROACH TO ACHIEVE HYPERTENSION CONTROL
For most persons with hypertension, multiple pharmacologic agents are often required to achieve blood pressure control.11 This need for multiple agents has been demonstrated in many large‐scale landmark hypertension clinical trials across different geographic regions and patient populations. Such landmark studies include the following: The United Kingdom's Prospective Diabetes Study (UKPDS), the Hypertension Optimal Trial (HOT), the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), the Action to Control Cardiovascular Risk in Patients with Diabetes—Blood pressure trial (ACCORD), Heart Outcomes Prevention Evaluation (HOPE)‐3 Trial, and the Systolic Blood Pressure Intervention Trial (SPRINT).12, 13, 14, 15, 16, 17 In these well‐designed trials, participants required on average two or more drugs, with some individuals requiring as many as four drugs, to reach the goal blood pressure.
The use of more than one drug in the initial management of hypertension under certain instances has been recognized as paramount and is featured in several clinical guidelines for hypertension, including those in the United States, Canada, Latin America, the United Kingdom, and Europe.4, 5, 18, 19, 20
In general, these guidelines recommend starting two‐drug therapy, either with two single separate agents in a separate pill or with a FDC (two different agents combined into a single pill), in patients with a blood pressure equal to or>160/100 mm Hg, or in patients with a baseline blood pressure >20/10 mm Hg above the treatment blood pressure target goal.4, 5, 20, 21 In addition to these guideline recommendations, there has been increased interest in initiating combination drug therapy earlier in the treatment process, including the initial treatment of hypertension. For example, Kaiser Permanente in their large‐scale hypertension treatment program uses FDCs as initial therapy.5 Additionally, current Canadian guidelines recommend replacing multiple‐pill antihypertensive combinations with FDCs.18
4. FIXED‐DOSE SINGLE‐PILL COMBINATION THERAPY IN HYPERTENSION
Given the need for a multi‐drug approach to the management and control of hypertension, the inclusion of multiple drugs in one pill (FDC) is logical. These combinations should include drug classes and individual agents within each class, which have been shown to be safe and effective in the treatment of hypertension. Beyond this principle, FDCs should include classes of agents, which, when used together, possess either additive or synergistic effects in lowering the blood pressure.22
Presently, most hypertension guidelines recommend the use of three major classes of antihypertensive agents, either alone or together: (a) renin‐angiotensin‐aldosterone system (RAAS) inhibitors, which includes two sub‐classes: angiotensin receptor blockers (ARB) or angiotensin‐converting enzyme inhibitors (ACE‐Is); (b) calcium channel blockers (CCB); (c) thiazide and thiazide‐like diuretics (DIU). Thus, two‐drug combination therapy could theoretically include the use of a two‐class combination of any of the three major classes, including the two sub‐classes of RAAS inhibitors. The steps to choosing the two‐class combinations (including agents within each class) will be discussed in detail below.
The clinical use of FDCs offers several advantages and the potential to solve some of the challenges and barriers in the effective management of hypertension. One significant advantage is the potential for FDCs to reduce clinical or therapeutic inertia in the control of hypertension, since two drugs would be introduced or up‐titrated simultaneously.23 Additional key advantages and benefits include increased efficacy, decreased pill burden, improved medication adherence, and reduction (mitigation) in side effects and adverse events of a given single agent when it is used as monotherapy.24, 25 In practical terms, clinical data have shown that the use of FDCs in the form of a single pill or capsule, when compared to combination therapy involving separate single agents, in the long term can be cost‐effective.27 Indeed, this results in decreased expenditure to the health care system due to increased adherence and persistence to therapy. It must be acknowledged that the initial cost of using FDCs may be higher particularly in settings where FDCs are not commonly used or widely available. The ability of FDCs to offer greater adherence resulting in enhanced blood pressure–lowering efficacy, reduced rates of adverse effects, and improved rates of hypertension control was demonstrated in the ACCOMPLISH trial.28 In this large randomized control trial, over 70% of participants were able to achieve a blood pressure target of <140/90 mm Hg through the use of FDC therapy. Thus, the use of FDC therapy to reduce hypertension‐related cardiovascular complications is a worthwhile strategy.
Another key benefit of the use of complementary classes of antihypertensive medications in the form of FDCs is the removal of age, race, and ethnicity in treatment algorithms. Currently, several guidelines, in their recommendations on the initiation of monotherapy, stress the importance of ethnicity, taking advantage of differences in salt sensitivity across races. However, with the use of complementary drug classes, the summative blood pressure–lowering effects of antihypertensive classes allows for reaching target blood pressure. For example, a RAAS inhibitor and CCB, when used collectively, result in successful reduction in blood pressure, even though the constituent agents, when used alone, might not have been effective. This is supported by the findings of large hypertension programs, such as that implemented by Kaiser Permanente in California, which utilized FDCs and demonstrated equal and significantly increased blood pressure control rates (to approximately 85%) across a wide range of demographics, including sex, race, and ethnicities.6
Given these benefits, why have FDCs not been more commonly used to date? In addition to the limited recommendations in current hypertension guidelines, a major reason for the delayed acceptance of FDCs stems from cost and availability. As with single‐drug therapy, FDCs have initially come on the market as expensive branded formulations. Their use has thus been restricted, especially in resource‐limited settings. When these branded formulations are used, they come with significant individual and health care system costs.29, 30 Not surprisingly, the cost factor is a major barrier to patient adherence and acceptance onto formularies. However, with time, more FDCs are becoming generic and therefore more affordable, which should help ameliorate the cost factor. Also, as the volume of FDC use increases, it is anticipated that the cost would significantly decrease.
An additional concern that has been a factor in the delayed acceptance of FDCs is the difficulty of titrating the individual components within the combination therapy with relative ease. This may pose a challenge particularly in elderly patients with significant co‐morbidities, who may require options not available in the FDC, or who may have to discontinue one of the medications due to side effects.31 However, as drug patents begin to expire, and affordable and reliable generic formulations become available, access to a wide range of FDC antihypertensive medications with increased dosing combinations is likely to improve. Beyond this, greater availability of scored tablets, which would allow ½ and even ¼ tablet dosing, could occur.
5. FIXED‐DOSE COMBINATIONS AS INITIAL THERAPY OF HYPERTENSION
As outlined above, most patients with hypertension are likely to require multiple pharmacologic agents to achieve hypertension control. Given the potential advantages and benefits of FDCs in the treatment of hypertension, it is logical to consider use of FDCs in the initial therapy of hypertension management, in addition to the current recommendations for the use of FDC (ie, baseline blood pressures at or >160/100 or 20/10 mm Hg above goal). However, using FDCs in the initial treatment of hypertension irrespective of starting blood pressure goes against present prescribing practice and until recently is not discussed or recommended in most hypertension guidelines. Of note, recently the 2018 ESC/ESH Hypertension Guideline recommended the use of FDCs for most patients with hypertension.5 As newer guidelines place emphasis on strategies to increase the treatment and control rates of hypertension, recommendations in support of the earlier use of FDC therapy are very likely to occur.
Presently, the strategy in the initial treatment of hypertension includes starting with one antihypertensive agent or “monotherapy.” Initial treatment would start with a lower dose of the agent and then gradually increase or titrate the dose of the agent until a target dose is reached. Only when a target dose of the single initial agent is reached, and blood pressure is still not at goal is a second agent added. However, most antihypertensive agents have a rather flat dose‐response curve for blood pressure reduction, while having a steeper if not exponential dose‐response curve for side effects or adverse effects.
Thus, titrating the dose of the first or initial agent to a target dose may offer a small further reduction in blood pressure but at the expense of a greater increase in side effects. Furthermore, meta‐analysis has demonstrated adding a drug vs titrating a drug to full dose is five times more effective at lowering blood pressure.32 Thus, the use of FDCs as initial therapy, which is usually accompanied by lower doses of each agent, would likely achieve a greater blood pressure reduction with fewer side effects, as well as in a more timely manner. A reduced side‐effect profile with FDC therapy would be even more likely, if the two agents used were complementary in that each agent mitigated the side‐effect profile of the other agent under ideal conditions. In recent years, there has been a move to include the use of FDC therapy in the initial management of hypertension, irrespective of the starting blood pressure.
This approach was used by Kaiser Permanente in California, USA, in the development and implementation of their hypertension pharmacologic treatment algorithm, as a part of a large‐scale, hypertension improvement program. Specifically, their algorithm features the use of a lisinopril‐hydrochlorothiazide FDC, which is titrated over subsequent visits, if blood pressure control has not achieved. The use of FDC therapy as a part of the initial and continued management of hypertension was thought to be key in improving their control rate of hypertension from approximately 40%‐90% among those treated over a 13‐year period.33 As mentioned above, the European Society of Cardiology and European Society of Hypertension 2018 hypertension guidelines support the use of FDC therapy as part of the initial management of hypertension, irrespective of the stage of hypertension.5 This recommendation appears to be driven by a strategic change to address the presently low treatment and control rates, even in high‐income counties.
6. SELECTING THE IDEAL FIXED‐DOSE COMBINATION—A STEPWISE APPROACH
With the increased availability and access of FDC pharmacologic agents in the management of hypertension, the question of selecting preferred or acceptable combinations becomes important. When considering the ideal characteristics for the choice of a FDC, several of the characteristics are similar to those when choosing a single agent. However, there are some ideal characteristics that are specific to choosing combination therapy. Examples of ideal characteristics of FDCs are included in Table 1.
Table 1.
Ideal characteristics of fixed dose combination drugs in hypertension (adapted from Patel et al)34
| Characteristic |
|---|
| High efficacy (blood pressure reduction) |
| Additive/synergistic blood pressure reduction |
| Supported by clinical trials |
| Mitigation of side‐effects of either or both individual agents |
| Potential for wide availability and affordability |
| Safe and efficacious in diverse demographic settings (ie, race, ethnicity, sex, geography, salt‐sensitivity) |
| Daily dosing formulation |
| Scored tablet with multiple doses which permit split tablet dosing and easy titration |
When considering ideal FDCs for patients with hypertension, either in daily clinical practice or for inclusion on an institutional or national formulary, safety, and efficacy of each of the agents, as well as in combination, is paramount. Drugs selected should be proven to be effective, based on the findings of clinical trials, and well tolerated with few side effects or adverse effects.
From a practical standpoint, selecting agents that are widely available, with quality and reliable manufacturing and reasonable affordability, is critical. These attributes are particularly critical in LMICs where procurement of quality medications in large volumes is a challenge. In these settings, as was seen in the analysis of the PURE study, as many as 31% of households in LMICs were unable to afford to purchase two or more blood pressure–lowering medications.35 From an economic standpoint, LMICs (where 80% of global CVD deaths occur) presently only occupy approximately 21% of the market share for the acquisition of pharmaceutical products.36 This small percentage limits the negotiating power of developing countries when attempting to acquire affordable FDC medications. Programs, such as the WHO Essential List of Medicines and the PAHO Strategic Fund for medicines, are essential and welcomed in addressing and overcoming this challenge.37, 38 Adoption, integration, and scale of these tools are essential for progress in global health—and would help further drive availability and affordability gains for FDCs.
With respect to affordability, procurement and retail prices of FDCs compared to that of monotherapy single pills indicate that prices of combination therapies were similar to the sum of constituting monotherapies. As a case study, a systematic search in MedIND and MIMS‐India databases was conducted to obtain private‐sector retail prices of various FDC brands marketed in India for an application to add an FDC for hypertension to the WHO Essential Medicines List. Prices of the respective constituent single pills/monotherapies were also obtained. Table 2 summarizes the unit price comparisons for various combination medicines and respective constituent monotherapy. It was found that retail prices of FDCs were similar if not lower compared to the price of constituent monotherapies.
Table 2.
Private‐sector retail unit price (in Euros) in India: FDC vs monotherapy (adapted from Abhishek Sharma)39
| Median (range) unit price per pill (2018, Euros) | |
|---|---|
| Fixed dose combination | |
| Lisinopril 5 mg + Hydrochlorothiazide 12.5 mg | 0.060 (0.013‐0.087) |
| Constituent monotherapy pills | |
| Lisinopril 5 mg | 0.045 (0.031‐0.130) |
| Hydrochlorothiazide 12.5 mg | 0.013 (0.008‐0.026) |
| Sum of median prices of two monotherapy pills | 0.059 |
| Fixed dose combination | |
| Telmisartan 40 mg + Amlodipine 5 mg | 0.090 (0.004‐0.120) |
| Constituent monotherapy pills | |
| Telmisartan 40 mg | 0.079 (0.038‐0.100) |
| Amlodipine 5 mg | 0.026 (0.013‐0.053) |
| Sum of median prices of two monotherapy pills | 0.11 |
| Fixed dose combination | |
| Telmisartan 40 mg + Hydrochlorothiazide 12.5 mg | 0.09 (0.004‐0.190) |
| Constituent monotherapy pills | |
| Telmisartan 40 mg | 0.088 (0.038‐0.100) |
| Hydrochlorothiazide 12.5 mg | 0.013 (0.008‐0.026) |
| Sum of median prices of two monotherapy pills | 0.093 |
Selecting agents that are dosed daily must be a priority. Once‐a‐day dosing has several significant benefits to the patient, including increased adherence, a reduction in awake and nocturnal blood pressure variation, and a longer therapeutic coverage due to a longer duration of action. This longer therapeutic coverage is beneficial in blunting the impact of the early morning blood pressure surge, as well as potentially providing some degree of blood pressure coverage during episodic missed medication doses.40 Daily dosing also allows for overall cost savings.41, 42
With all the options of FDCs available, utilizing a stepwise approach is key, as outlined in Figure 1. The first step is to determine the ideal medication classes to be included in the FDC. The second step is to determine which individual drug is best within each drug class. Finally, the third step is to determine what FDCs are available locally, nationally, and/or regionally and are affordable.
Figure 1.
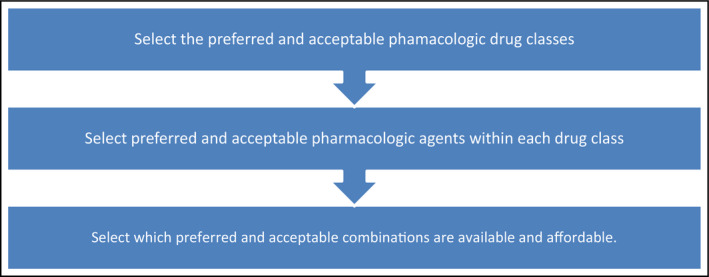
Steps in selecting the ideal fixed‐dose combination drug
7. STEP 1—SELECTING THE PREFERRED AND ACCEPTABLE PHARMACOLOGIC CLASSES
Of the three classes of antihypertensive drugs (RAAS inhibitors, CCB, and DIU) which could be included in an FDC, RAAS inhibitor‐CCB and RAAS inhibitor‐DIU combinations are preferred. This preference is based on their efficacy, tolerability, side‐effect profile, and support from clinical trials (Table 2). The combination of a CCB‐DIU, while efficacious, is non‐preferred largely due to less evidence‐based medicine supporting their use and a less desirable side‐effect profile. However, this combination may have a role, if a RAAS inhibitor is not well tolerated and/or contraindicated, such as in women or in childbearing age, desiring to become pregnant, or who are already pregnant.
Of the RAAS inhibitor‐CCB and the RAAS inhibitor‐DIU options, formulations comprising a RAAS inhibitor‐CCB are preferred given that this combination is well tolerated and supported by evidence‐based medicine including clinical cardiovascular outcomes. The landmark ACCOMPLISH trial showed that a RAAS inhibitor‐CCB (ACE‐I: benazepril and CCB: amlodipine) was superior at reducing cardiovascular outcomes compared to the RAAS inhibitor‐DIU combination (ACE‐I: benazepril and DIU: hydrochlorothiazide).28 It should be noted that some experts argue that the diuretic dose used was too low, making such comparisons controversial.43 More recently, a position paper the Latin American Society of Hypertension, which features a systematic review and meta‐analysis published in the Journal of Hypertension, highlighted the benefit of two agent management of hypertension compared to placebo. In this analysis, the combined findings of the ADVANCE trial and the PROGRESS trial demonstrated significant reductions in all‐cause mortality with the use of a perindopril‐indapamide combination when compared to placebo.44
Renin‐angiotensin‐aldosterone system inhibitors consist of two sub‐classes, the angiotensin receptor blockers (ARB) and angiotensin‐converting enzyme inhibitors (ACE‐I). Between these two sub‐classes, ARBs have significantly fewer side effects (such as a markedly less incidence of cough) and thus are better tolerated compared to ACE‐Is. Overall, a better safety profile is also seen with ARBs, given that they also have a markedly lower incidence of angioedema, a potentially life‐threatening event, when compared to an ACE‐I. Though head‐to‐head comparisons of ARBs vs ACE‐Is are limited, meta‐analyses have shown no difference in cardiovascular outcomes when comparing ARBs and ACE‐Is.45 Given their better side‐effect profile, improved tolerability, and better safety profile, a FDC containing an ARB may be preferred compared to a FDC containing ACE‐I, when available. Thus, four FDCs are preferred or acceptable and they are as follows in the order of preference: (a) ARB‐CCB (Preferred), (b) ACE‐I‐CCB (Preferred), (c) ARB‐DIU (Acceptable), and (d) ACE‐DUI (Acceptable; Table 3).
Table 3.
Treatment recommendations by drug class for fixed‐dose combination therapy
| Option | Drug class combination | Advantage | Disadvantage |
|---|---|---|---|
| 1. Preferred | RAAS inhibitor‐CCB |
|
|
| 2. Acceptable | RAAS inhibitor‐thiazide/thiazide‐like diuretic (DIU) |
|
|
| 3. Non‐preferred | CCB‐thiazide/thiazide‐like diuretic combination (DIU) |
|
|
CCB, calcium channel blocker; DIU, thiazide/thiazide‐like diuretic; RAAS inhibitor, renin‐angiotensin‐aldosterone system inhibitor.
7.1. Non‐preferred fixed‐dose combination therapy by drug classes
Other FDC options are available, including CCB‐diuretic, diuretic‐diuretic, diuretic‐vasodilator, CCB‐beta‐blocker, and diuretic‐beta‐blocker combinations, as well as others. A recent meta‐analysis has shown that a thiazide/thiazide‐like diuretic‐CCB combination is well tolerated and may reduce cardiovascular outcomes when compared to other combinations.46 However, further clinical trials and solid evidence‐based medicine (greater number of long‐term clinical trials) are needed and thiazide/thiazide‐like‐CCB combinations are not widely available. Thus, at the present time, they are considered non‐preferred.
Other combinations are non‐preferred, as their constituent drug classes are not considered first‐line therapy in the management of hypertension. These options should be reserved for specific concomitant clinical indications, when warranted. Caution should be exercised when considering beta‐blocker‐diuretic combinations as this combination is associated with increased risk for the development of glucose intolerance and diabetes mellitus.47, 48
8. STEP 2—SELECTING THE PREFERRED AND ACCEPTABLE PHARMACOLOGIC AGENT(S) WITHIN EACH DRUG CLASS
Notably, some constituent individual pharmacologic agents within each drug class offer advantages over others. One distinct advantage is in greater efficacy in overall blood pressure reduction, which could result in a greater reduction in cardiovascular end points or outcomes (Table 4). An additional consideration should be placed on the duration of action, in addition to the potency of individual constituent drugs. For instance, within the ARBs, long‐acting ARBs such as azilsartan (also the most potent ARB),49 telmisartan, and irbesartan would be preferred individual agents. When not available, other ARBs, such as valsartan and other long‐acting, once‐daily agents, are acceptable alternatives. Of note, non‐preferred ARBS include losartan, due to a shorter duration of action, and olmesartan, due to a rare—but serious—incidence of enteropathy. It is important to note, however, that this adverse effect of olmesartan is indeed rare and that it has wide availability and clinical use.
Table 4.
Preferred and acceptable pharmacologic agents (in order of preference) within each drug class
| Drug class | Agent | Preference | Characteristics |
|---|---|---|---|
| ARB | Azilsartan | Preferred |
Most potent ARB Long duration of action |
| Telmisartan | Preferred | Long duration of action | |
| Irbesartan | Preferred | Long duration of action | |
| All other long‐acting ARBs (candesartan, valsartan) | Acceptable | ||
| Losartan and olmesartan | Non‐preferred |
Losartan—shorter duration of action Olmesartan—association with enteropathy |
|
| ACE‐I | Lisinopril, ramipril, benazepril | Preferred | Long duration of action and widely available |
| All other long‐acting agents (perindopril, trandolapril, quinapril, fosinopril, ramipril, moexipril) | Acceptable | Long duration of action | |
| Enalapril and captopril | Non‐preferred | Short duration of action | |
| CCBS | Amlodipine | Preferred | Solid evidence, long duration of action, and widely available |
| Other long‐acting, once‐daily dihydropyridines (nifedipine, felodipine, nisoldipine) | Acceptable | Long duration of action | |
| Non‐dihydropyridines: verapamil and diltiazem | Non‐preferred | Less evidence and more complicated side‐effect profile | |
| Diuretics | Chlorthalidone | Preferred |
Long duration of action Supported by evidence Most potent thiazide‐like diuretic |
| Hydrochlorothiazide | Acceptable | Shorter duration of action, widely available | |
| Indapamide | Acceptable | May be more costly, less widely available |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
The second sub‐class within the RAAS inhibitors is the ACE‐Is. Within this sub‐class, the ACE‐Is, lisinopril, benazepril, and ramipril are the preferred agents. This preference is based on the extent of the major clinical trials of which these agents were employed, as well as wide availability and affordability. However, any other long‐acting, once‐a‐day ACE‐I is clearly acceptable. Non‐preferred ACE‐Is include the agents with shorter duration of action such as enalapril and captopril.
Within the CCBs, dihydropyridines are preferred, with amlodipine as the preferred agent. This preference is based on the large number of clinical studies with amlodipine and its wide availability. Other long‐acting once‐daily dihydropyridines would be acceptable, if they were the only agents available and/or had significant cost advantages. Non‐dihydropyridine CCBs, such as verapamil and diltiazem, are non‐preferred largely due to less available robust clinical studies and a more complex side‐effect profile, in particular cardiac conduction disturbances and negative inotropic effects. However, there may be concomitant clinical situations, where either of these may advantageous such as when heart rate control is needed.
Within the thiazide and thiazide‐like diuretic class, chlorthalidone is the preferred agent due to its longer duration of action, greater potency in lowering blood pressure compared to hydrochlorothiazide, and significant body of favorable outcome evidence.50 However, chlorthalidone is less available compared to hydrochlorothiazide and many fixed‐dose combinations do not include chlorthalidone.51 Indapamide is also an acceptable thiazide‐like diuretic; however, it may not be widely available and could be more costly.
Taken together, Tables 3 and 4 could form a matrix of potential FDC combinations to choose (Table 5). Not all the preferred combinations are readily available, affordable, or meet all or most of the ideal characteristics mentioned in Table 1. For instance, an ideal FDC would be the ARB, azilsartan and the CCB, amlodipine in a pill form that is scored for ease of splitting and in a convenient dosage. However, at least in the United States, it is not available. However, this combination, as with all other combinations of agents, could be used as individual pills.
Table 5.
| ARB + CCB | |
|---|---|
| Azilsartan OR Telmisartan OR Irbesartan | Amlodipine |
| ACE‐I + CCB | |
| Lisinopril OR Ramipril OR Benazepril | Amlodipine |
| ARB + Thiazide/Thiazide‐like diuretic | |
| Azilsartan OR Telmisartan OR Irbesartan | Chlorthalidone OR Hydrochlorothiazide |
| ACE‐I + Thiazide/Thiazide‐like diuretic | |
| Lisinopril OR Benazepril | Chlorthalidone OR Hydrochlorothiazide |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
9. STEP 3—IDENTIFY AVAILABLE AND AFFORDABLE FIXED‐DOSE COMBINATION AGENTS
Having selected the ideal drug classes and best individual agents within each class, the next step is to explore what fixed‐dose combinations exist and are available in each setting. Paying attention to practical considerations such as cost, dosing options, and whether formulations are available in tablet or capsule form is important. Specifically, knowing whether the medication is available in the form of a capsule (which cannot be split) or tablet (scored or unscored) can affect formulary decisions. Understandably, the flexibility provided by using scored tablet which can be more easily split by patients and pharmacies allows multiple dosing options with one tablet strength, as well as potential cost savings. On the flip side, splitting of unscored FDCs may present a challenge. In some instances, the distribution of active medications in unscored pills may not be even. As such, splitting may result in daily fluctuations in the amount of active medicine received. It should be noted, at least in the United States, that many pharmacies do not offer pill splitting and local or states laws may prohibit pill splitting by pharmacies.
In the United States, several options for FDCs exist and are listed below (Table 6). This table is largely meant as an example or blueprint. Some of these options, as well as others, may be available in different jurisdictions around the world.
Table 6.
First‐line combination antihypertensive options (approved in USA for hypertension)
| Medication | Strengths | Formulation | Advantages | Disadvantages | Overall recommendation | |
|---|---|---|---|---|---|---|
| ARB‐CCB | Telmisartan‐Amlodipine |
40/5 mg 80/5 mg 40/10 mg 80/10 mg |
Non‐scored tablets |
Strong EBM Long half‐life of telmisartan |
Non‐scored tablets would make it difficult to obtain amlodipine 2.5 mg if needed | Preferred |
| Valsartan‐Amlodipine |
160/5 mg 320/5 mg 160/10 mg 320/10 mg |
Non‐scored tablets | Strong EBM | Non‐scored tablets would make it difficult to obtain amlodipine 2.5 mg if needed | Acceptable | |
| ACE‐I‐CCB | Benazepril‐Amlodipine |
10/2.5 mg 10/5 mg 20/5 mg 20/10 mg 40/5 mg 40/10 mg |
Capsules | Strong EBM | Capsule formulation does not allow for splitting option | Preferred |
| Perindopril‐Amlodipine |
3.5/2.5 mg 7/5 mg 14/10 mg |
Non‐scored tablets | Currently not in production in the United States (no release date); brand name only product; non‐scored tablets | Non‐preferred (unavailable) | ||
|
ARB‐Thiazide/Thiazide‐like diuretic Only 1 ARB‐Chlorthalidone option in the United States |
Azilsartan‐Chlorthalidone |
40/12.5 mg 40/25 mg |
Non‐scored, coated tablets | Chlorthalidone‐increased duration of action and potency i | Non‐scored tablets and fixed‐dose ARB portion; brand name only in the United States | Preferred |
| Valsartan‐ HCTZ |
80/12.5 mg 160/12.5 mg 320/12.5 mg 160/25 mg 320/25 mg |
Non‐scored tablets | Non‐scored tablets | Acceptable | ||
| Candesartan‐HCTZ |
16/12.5 mg 32/12.5 mg 32/25 mg |
Scored tablets | Scored tablet would allow for splitting option | Unable to reach HCTZ dose of 50 mg | Not preferred | |
| Telmisartan‐HCTZ |
40/12.5 mg 80/12.5 mg 80/25 mg |
Non‐scored tablets | Unable to reach HCTZ dose of 50 mg | Not preferred | ||
| Irbesartan‐HCTZ |
150/12.5 mg 300/12.5 mg |
Non‐scored tablets | Unable to reach HCTZ dose of 50 mg | Not preferred | ||
|
ACE‐I‐Thiazide/Thiazide‐like diuretic Only ACE‐I/HCTZ are options in the United States—would prefer option with chlorthalidone |
Benazepril‐HCTZ |
10/12.5 mg 20/12.5 mg 20/25 mg |
Scored tablets | Scored tablet would allow for splitting option | Preferred | |
| Lisinopril‐HCTZ |
10/12.5 mg 20/12.5 mg 20/25 mg |
Non‐scored tablets | Non‐scored tablet | Acceptable | ||
| Quinapril‐HCTZ |
10/12.5 mg 20/12.5 mg 20/25 mg |
Scored (10/12.5 mg, 20/12.5 mg), non‐scored (20/25 mg) tablets | Non‐scored 20/25 mg tablet | Acceptable | ||
| Moexipril‐HCTZ |
7.5/12.5 mg 15/12.5 mg 15/25 mg |
Scored tablets | Scored tablet would allow for splitting option | Medication must be taken on an empty stomach | Acceptable | |
| Fosinopril‐HCTZ |
10/12.5 mg 20/12.5 mg |
Non‐scored (10/12.5 mg), scored (20/12.5 mg) tablets | Scored 20/12.5 mg tablet | Unable to reach HCTZ dose of 50 mg | Not preferred |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
10. INCLUDING FIXED‐DOSE COMBINATION THERAPY IN TREATMENT ALGORITHMS
Most current major hypertension guidelines do not recommend specific agents to use in the initiation of pharmacologic therapy, nor how to manage dosage titration. Realizing these limitations, several groups have developed drug‐specific algorithms, including which specific agents or combination of agents to use, for the management of hypertension. Success has been seen in improving control rates of hypertension by some groups using this approach.6 This approach has been utilized as a choice in the formation of the management algorithms recommended by HEARTS in the Americas Initiative, a regional application of the Global HEART Program.52 This initiative is focused on improving hypertension treatment and control rates and ultimately clinical outcomes in hypertension by strengthening the health care system.53, 54 For example, in the HEARTS in the Americas Initiative, dual combination or FDCs (when available) are the foundation in each treatment algorithm for the initial treatment of hypertension in each of the four initial demonstration sites, Barbados, Chile, Colombia, and Cuba. In all four sites, hypertension control rates have increased markedly (data available upon request).
More recently, the Resolve to Save Lives Cardiovascular Health Initiative published a treatment protocol in partnership with the World Hypertension League, which features use of a single‐pill FDC, as a first‐line choice in the management of hypertension.7 The Resolve to Save Lives is a non‐profit organization with the mandate of providing organizations in low and middle‐income countries with tools to implement strategies which reduce cardiovascular disease burden.55 In their protocol, the single‐pill FDC of choice is telmisartan 40 mg‐amlodipine 5 mg, which they recommend be initiated as half of a tablet per day and titrated up to a maximum of two tablets. If two tablets are reached and blood pressure is still uncontrolled, then it is recommended that a thiazide/thiazide‐like diuretic be added. It is a simple approach, which could allow rapid titration and a corresponding quicker reduction in blood pressure to goal. Although to best of our knowledge, this combination is not available in a scored pill, if demand significantly increases it is likely that the pharmaceutical industry will make such an agent available.
11. IMPROVING THE AVAILABILITY OF FIXED‐DOSE COMBINATION THERAPY
With the numerous benefits of using FDC therapy in the management of hypertension, organizations have focused their efforts on making the availability of FDCs more widespread. In some cases, the lack of available FDCs is not due purely to cost, but to other factors, such as the current small number of preferred FDCs, the current FDCs available, the difficulty of titrating doses, the lack of scored tablet formulations, and legislative barriers.
12. CONCLUSIONS
Incorporating an earlier and wider use of FDC drug therapy is a practical and effective strategy which has clear policy implications targeted to improve hypertension treatment and control worldwide. The overarching benefits to the patient, provider, and the health care system are numerous and apparent. Understanding the steps in the selection of preferred and acceptable FDCs is key to providers and health care system leaders, as we move forward. Increased efforts to place these medications on formularies and to make them more available and affordable are likely to reap noteworthy benefit. At this juncture, the inclusion of FDCs for the treatment of hypertension by local, regional, and national drug formularies would be welcomed. Given that cardiovascular disease burden is on the rise, continuing to plan, develop, and implement more innovative strategies to improve clinical outcomes at all areas of the prevention, diagnosis, and treatment spectrum of hypertension is paramount. The increasing role of FDC therapy in the treatment of hypertension, including in the initial treatment, is a new and key strategy to address this complex public health disease burden.
CONFLICT OF INTEREST
NRCC was a paid consultant to the Novartis Foundation (2016‐2017) to support their program to improve hypertension control in low‐ to middle‐income countries which includes travel support for site visits and a contract to develop a survey. NRCC has provided paid consultative advice on accurate blood pressure assessment to Midway Corporation (2017) and is an unpaid member of World Action on Salt and Health (WASH).
PO is staff member of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization or affiliate institutions.
[Corrections updated on Dec 08, 2018, after initial online publication: A paragraph has been added to the Conflict of Interest section.]
REFERENCES
- 1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 3. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control ‐ global disparities of hypertension prevalence and control a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Committee W, Carey RM, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2017;2017(17):735‐1097. [Google Scholar]
- 5. Williams B, Mancia G, Desormais I. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 6. Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large‐scale hypertension program. JAMA. 2013;310(7):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaffe MG, Frieden TR, Campbell N, et al. Recommended treatment protocols to improve management of hypertension globally: a statement by resolve to save lives and the World Hypertension League (WHL). J Clin Hypertens. 2018;20(5):829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melgarejo JD, Maestre GE, Thijs L, et al. Prevalence, treatment, and control rates of conventional and ambulatory hypertension across 10 populations in 3 continents novelty and significance. Hypertension. 2017;70(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310(9):959. [DOI] [PubMed] [Google Scholar]
- 10. Ordunez P, Martinez R, Niebylski ML, Campbell NR. Hypertension prevention and control in Latin America and the Caribbean. J Clin Hypertens. 2015;17(7):499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension. Drugs. 2002;62(3):443‐462. [DOI] [PubMed] [Google Scholar]
- 12. Turner R, Matthews D, Neil A, Mcelroy H. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703‐713. [PMC free article] [PubMed] [Google Scholar]
- 13. Cruickshank JM. Hypertension optimal treatment (HOT) trial. Lancet. 1998;352(9127):573‐574. [DOI] [PubMed] [Google Scholar]
- 14. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288(23):2981‐2997. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the action to control cardiovascular risk in diabetes (ACCORD) trial. Circulation. 2010;122(8):847‐849. [DOI] [PubMed] [Google Scholar]
- 16. Lonn EM, Bosch J, López‐Jaramillo P, et al. Blood‐pressure lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2009‐2020. [DOI] [PubMed] [Google Scholar]
- 17. SPRINT Research Group , Wright JT, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34(5):506‐525. [DOI] [PubMed] [Google Scholar]
- 19. Task Force of the Latin American Society of Hypertension . Guidelines on the management of arterial hypertension and related comorbidities in Latin America. J Hypertens. 2017;35(8):1529‐1545. [DOI] [PubMed] [Google Scholar]
- 20. National Clinical Guideline Centre (UK) . Hypertension: clinical management of primary hypertension in adults (NICE clinical guideline 127). https://www.ncbi.nlm.nih.gov/books/NBK83274/. Accessed July 2018.
- 21. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569‐588. [DOI] [PubMed] [Google Scholar]
- 22. Kalra S, Kalra B, Agrawal N. Combination therapy in hypertension: An update. Diabetol Metab Syndr. 2010;2(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basile J, Neutel J. Overcoming clinical inertia to achieve blood pressure goals: the role of fixed‐dose combination therapy. Ther Adv Cardiovasc Dis. 2010;4(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 24. Tung Y‐C, Huang Y‐C, Wu L‐S, Chang C‐J, Chu P‐H. Medication compliance and clinical outcomes of fixed‐dose combinations vs free combinations of an angiotensin II receptor blocker and a calcium channel blocker in hypertension treatment. J Clin Hypertens. 2017;19(10):983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satsoglou S, Tziomalos K. Fixed‐dose combinations: a valuable tool to improve adherence to antihypertensive treatment. J Clin Hypertens. 2018;20(5):908‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du L‐P, Cheng Z‐W, Zhang Y‐X, Li Y, Mei D. The impact of fixed‐dose combination versus free‐equivalent combination therapies on adherence for hypertension: a meta‐analysis. J Clin Hypertens. 2018;20(5):902‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong SH, Wang J, Tang J. Dynamic view on affordability of fixed‐dose combination antihypertensive drug therapy. Am J Hypertens. 2013;26(7):879‐887. [DOI] [PubMed] [Google Scholar]
- 28. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359(23):2417‐2428. [DOI] [PubMed] [Google Scholar]
- 29. Angeli F, Reboldi G, Mazzotta G, et al. Fixed‐dose combination therapy in hypertension. High Blood Press Cardiovasc Prev. 2012;19(2):51‐54. [DOI] [PubMed] [Google Scholar]
- 30. Rabbani A, Alexander GC. Out‐of‐pocket and total costs of fixed‐dose combination antihypertensives and their components. Am J Hypertens. 2008;21(5):509‐513. [DOI] [PubMed] [Google Scholar]
- 31. Bramlage P, Schmidt S, Sims H. Fixed‐dose vs free‐dose combinations for the management of hypertension‐an analysis of 81 958 patients. J Clin Hypertens. 2018;20(4):705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290‐300. [DOI] [PubMed] [Google Scholar]
- 33. Jaffe MG, Young JD. The kaiser permanente Northern California story: improving hypertension control from 44% to 90% in 13 years (2000 to 2013). J Clin Hypertens. 2016;18(4):260‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel P, Ordunez P, DiPette D, et al. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: the standardized hypertension treatment and prevention project. J Clin Hypertens. 2016;18(12):1284‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Attaei MW, Khatib R, McKee M, et al. Availability and affordability of blood pressure‐lowering medicines and the effect on blood pressure control in high‐income, middle‐income, and low‐income countries: an analysis of the PURE study data. Lancet Public Heal. 2017;2(9):e411‐e419. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen TA, Knight R, Roughead EE, Brooks G, Mant A. Policy options for pharmaceutical pricing and purchasing: issues for low‐ and middle‐income countries. Health Policy Plan. 2015;30(2):267‐280. [DOI] [PubMed] [Google Scholar]
- 37. de L Horst MM, Soler O. [The PAHO strategic fund: a mechanism to facilitate access to medicines]. Rev Panam Salud Publica. 2010;27(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 38. Ordunez P, Ramon M, Niebylski M, Campbell NR. Hypertension prevention and control in Latin America and the Caribbean. J Clin Hypertens. 2015;17(7):499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. WHO . Fixed‐Dose Combination Antihypertensives. Geneva, Switzerland: WHO; 2018. https://www.who.int/selection_medicines/committees/expert/22/fixed-dose_combination_antihypertensives/en/. Accessed August 15, 2018. [Google Scholar]
- 40. Flack JM, Nasser SA. Benefits of once‐daily therapies in the treatment of hypertension. Vasc Health Risk Manag. 2011;7:777‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bae JP, Dobesh PP, Klepser DG, et al. Adherence and dosing frequency of common medications for cardiovascular patients. Am J Manag Care. 2012;18(3):139‐146. [PubMed] [Google Scholar]
- 42. Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta‐analysis. Patient Prefer Adherence. 2013;7:419‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whelton P, David B. Benazepril plus amlodipine or hydrochlorothiazide for hypertension. N Engl J Med. 2009;360(11):1147‐1150. [PubMed] [Google Scholar]
- 44. Coca A, López‐Jaramillo P, Thomopoulos C, Zanchetti A, Latin American Society of Hypertension (LASH) . Best antihypertensive strategies to improve blood pressure control in Latin America. J Hypertens. 2018;36(2):208‐220. [DOI] [PubMed] [Google Scholar]
- 45. Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014;(8):CD009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rimoldi SF, Messerli FH, Chavez P, Stefanini GG, Scherrer U. Efficacy and safety of calcium channel blocker/diuretics combination therapy in hypertensive patients: a meta‐analysis. J Clin Hypertens. 2015;17(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369(9557):201‐207. [DOI] [PubMed] [Google Scholar]
- 48. Stump CS, Hamilton MT, Sowers JR. Effect of antihypertensive agents on the development of type 2 diabetes mellitus. Mayo Clin Proc. 2006;81(6):796‐806. [DOI] [PubMed] [Google Scholar]
- 49. Jadhav U, Wadhwa N. Randomised controlled clinical trials of azilsartan: a systematic review and evidence based perspectives. J Am Soc Hypertens. 2016;10(4):e27‐e28. [Google Scholar]
- 50. Flack JM, Sica DA, Nesbitt S. Chlorthalidone versus hydrochlorothiazide as the preferred diuretic. Hypertension. 2011;57(4):665‐666. [DOI] [PubMed] [Google Scholar]
- 51. Springer K. Chlorthalidone vs. hydrochlorothiazide for treatment of hypertension. Am Fam Physician. 2015;92(11):1015‐1016. [PubMed] [Google Scholar]
- 52. WHO . HEARTS Technical Package. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 53. Patel P, Ordunez P, Connell K, Lackland D, DiPette D. Standardized hypertension management to reduce cardiovascular disease morbidity and mortality worldwide. South Med J. 2018;111(3):133‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no 220. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 55. Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet. 2018;391(10121):709‐712. [DOI] [PubMed] [Google Scholar]


