Abstract
This paper describes the multilevel factors that contribute to hypertension disparities in 2052 hypertensive African Americans (mean age 52.9 ± 9.9 years; 66.3% female) who participated in a clinical trial. At the family level, participants reported average levels of life chaos and high social support. However, at the individual level, participants exhibited several adverse clinical and behavioral factors including poor blood pressure control (45% of population), obesity (61%), medication non‐adherence (48%), smoking (32%), physical inactivity (45%), and poor diet (71%). While participants rated their provider as trustworthy, they reported high levels of discrimination in the health care system. Finally, community‐level data indicate that participants reside in areas characterized by poor socio‐economic and neighborhood conditions (eg, segregation). In the context of our trial, hypertensive African Americans exhibited several adverse risks and protective factors at multiple levels of influence. Future research should evaluate the impact of these factors on cardiovascular outcomes using a longitudinal design.
Keywords: African American, hypertension, multilevel framework, risk factors
1. INTRODUCTION
The excess burden of uncontrolled hypertension in African Americans remains one of the most vexing public health problems in the United States (US).1 The age‐adjusted prevalence of hypertension, a major cardiovascular risk factor in African Americans, is 42% vs 28% for European Americans (ie, whites),2 making the prevalence in African Americans the highest in the United States.3 Among African Americans, hypertension develops at an earlier age, is more aggressive, and less well managed than in whites.4, 5 Partly owing to this, African American have a 1.8 times greater rate of fatal stroke, a 1.5 times greater rate of heart disease death, and a 4.2 times greater rate of end‐stage kidney disease than whites.6, 7, 8, 9, 10 An increased cardiometabolic risk profile including having uncontrolled diabetes, hypercholesterolemia, being obese, physical inactivity and having poor dietary behaviors is also more common among African Americans11 and a significant contributor to the racial/ethnic disparities in cardiovascular mortality.12
Using an ecological model, Mueller et al13 recently described the barriers to poor hypertension control in African American as complex and occurring at multiple levels including the following: the individual patient (poor adherence to self‐management behaviors); family (social support; family dynamics); provider (quality of communication, trustworthiness); health care system (access and use of care); and the local community (neighborhood level poverty and racism). While previous research has examined risk and protective factors for health disparities in hypertensive African Americans at one or two of these levels, there lacks a comprehensive examination of the multilevel influences that contribute to health inequities in hypertension control in African Americans.13, 14
In this article, we leverage the baseline data from a recently completed clinical trial to confirm and extend previous research by describing the individual, family, provider, health care system, and community‐level factors associated with cardiovascular risk in a sample of 2052 African American adults with hypertension who receive primary care from diverse practices in New York City (NYC).15
2. METHODS
2.1. Participants
The current descriptive study was conducted as part of the Genetic testing to Understand and Address Renal Disease Disparities (GUARDD) trial.16 GUARDD was a randomized trial designed by an academic‐community partnership15 to determine the effects and challenges of incorporating genetic renal risk information into primary care management (eg, systolic blood pressure [BP] reduction and renal screening) of African American adults with hypertension who receive care from academic, community, and safety‐net practices in NYC.16
Recruitment for GUARDD occurred at 15 academic, community, and safety‐net practices in NYC.16 Trained study coordinators enrolled and consented patients at participating clinical sites. Patients were eligible if they: (a) self‐identified as African American/Black or having African ancestry; (b) were age 18‐70 years; (c) had an ICD‐9 diagnosis of hypertension (401.XX, 402.XX AND 405.XX) and/or were taking antihypertensive medications, and/or had two systolic BP readings >140 or two diastolic readings >90 at least 6 months apart; and (d) were receiving primary care at one of the participating clinical sites. Exclusion criteria included the following: (a) diagnosis of diabetes and/or CKD; (b) pregnancy; (c) non‐English speaking; (d) planning on moving out of the area during the study period; (e) having cognitive impairment; and (f) not community dwelling. All participants provided written informed consent approved by the Institutional Review Boards at participating sites (Figure 1).
Figure 1.
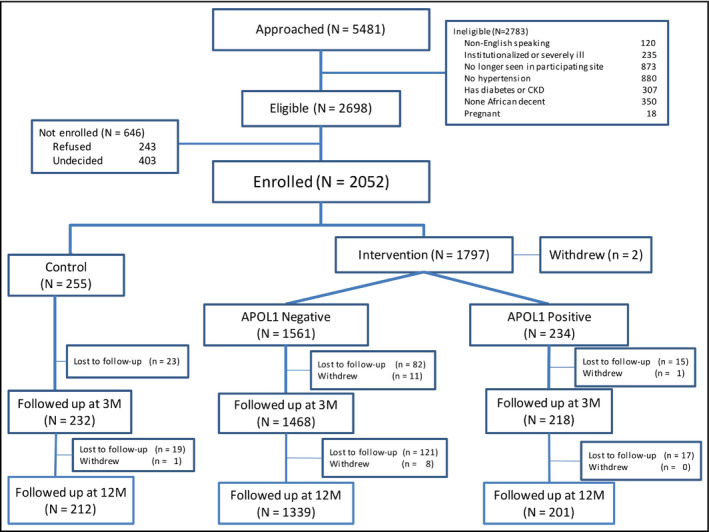
CONSORT flow diagram
2.2. Measures
The baseline survey was designed to capture multilevel risk and protective factors that have been linked to hypertension‐related outcomes in previous research.16 Questions were derived from existing validated self‐report measures. At the individual patient level, we assessed general health,17 family history of hypertension and comorbidities,18 medication adherence,19 diet and physical activity,20 patient activation,21 depression,22 general anxiety,23 perceived stress,24 beliefs about hypertension25 and medications, 26 and socio‐demographic characteristics.19 Family‐level measures included life chaos27 and social support.28 Provider and health system‐level measures included quality of the patient‐provider relationship (eg, perceived trustworthiness, communication),29 perceived discrimination in health care,30 and access to and utilization of health care services.31, 32
At the community level, we used participant zip code data to examine the percentage of GUARDD participants residing within the boundaries of the NYC District Public Health Office geographic areas characterized by poor social conditions (eg, high neighborhood poverty and unemployment), high‐risk health factors and disease burden, and premature death.33 Using publicly available data,34 we examined social‐economic characteristics (ie, % living below the federal poverty level; % unemployed and % living in crowded housing) and neighborhood conditions (ie, % of non‐white residents [proxy for residential segregation]; number of tobacco and alcohol retailers) in communities where participants reside. Finally, we also included a single‐item measure to assess the perceived health impact of institutionalized racism.35 This measure asked participants to indicate their level of agreement with the statement: “African Americans are more likely to have diseases like high blood pressure than whites because of differences in their environments, like where they live, their access to care, or pollution.”
Blood pressure was measured as three BP readings using validated, automated BpTru BPM‐200 digital monitors with the participant seated comfortably before measurement, following American Heart Association guidelines.36 The average of the last two readings served as the BP measurement recorded in the study record. Height and weight were measured using standardized procedures with portable stadiometers and high capacity platform scales, respectively, and used to calculate body mass index (BMI).
2.3. Analysis
We computed descriptive data for the baseline characteristics of the total population. Continuous variables are expressed as mean ± standard deviation (SD) and range. Categorical and binary variables are presented as frequencies and percentages. As a descriptive paper, we did not complete any inferential statistical analysis; thus, we do not present comparisons between study groups or with other outcome variables. Statistical analyses were conducted using SPSS, version 25.
3. RESULTS
3.1. Individual patient level
3.1.1. Socio‐demographic characteristics
The socio‐demographic characteristics of the total sample are displayed in Table 1. The mean age (± standard deviation) of the total sample was 52.9 ± 9.9 years, and two‐thirds of participants were female (66.3%). About 18% of the participants were born outside of United States, and among them, 28% have lived in the United States for 20 years or less. While more than half of participants reported some college education (57.1%), the majority also reported an annual income <$30 000 (55.6%). Almost half of participants were employed full time or part‐time (45.2%) and had either private insurance (40.8%) or Medicaid (47.5%).
Table 1.
Individual‐level socio‐demographic and clinical data for GUARDD participants
| Individual level | All participants (n = 2052) |
|---|---|
| Socio‐demographic characteristics | |
| Age, (mean ± SD) | 52.9 ± 9.9 |
| Sex, n (%) | |
| Male | 692 (33.7) |
| Female | 1360 (66.3) |
| Born outside the US, n (%) | 362 (17.6%) |
| Years in the US, n (%)a | |
| <10 y | 27 (7.6%) |
| 10‐20 y | 73 (20.7%) |
| >20 y | 253 (71.7%) |
| Employment status, n (%) | |
| Employed fulltime/Part‐time | 927 (45.2) |
| Unable to work | 463 (22.6) |
| Looking for work | 192 (9.4) |
| Retired | 242 (11.8) |
| Going to school | 74 (3.6) |
| Other | 153 (7.5) |
| Level of education, n (%) | |
| < High school | 327 (16.0) |
| High school degree | 549 (26.8) |
| Some college and above | 1172 (57.1) |
| Yearly income, n (%) | |
| <$30 000/y | 1014 (55.6) |
| ≥$30 000/y | 807 (44.4) |
| Insurance | |
| Private | 837 (40.8) |
| Medicare | 344 (16.8) |
| Medicaid | 974 (47.5) |
| Uninsured | 57 (2.8) |
| Military/Veterans Affairs | 8 (0.4) |
| Other | 167 (8.1) |
| Clinical characteristics | |
| Systolic BP (mean ± SD) | 134.1 ± 19.5 |
| Diastolic BP (mean ± SD) | 85.4 ± 11.9 |
| BP Control, n, % | 926 (45.1) |
| BMI (mean ± SD) | 32.5 ± 7.7 |
| Weight classification, n,% | |
| Underweight/normal | 249 (12.5) |
| Overweight | 535 (26.9) |
| Obese | 1208 (60.6) |
| Prescribed antihypertensive medications, n (%) | 1727 (84.2) |
| Family member with hypertension, n (%) | 1840 (91.8) |
| No comorbid conditions, mean (SD) | 1952 (95.1) |
| Perceived health status | |
| Excellent | 77 (3.8) |
| Very good | 453 (22.1) |
| Good | 855 (41.7) |
| Fair | 552 (26.9) |
| Poor/Very poor | 115 (5.6) |
Abbreviations: BP, blood pressure; SD, standard deviation; US, United States.
Of the 352 participants who were born outside the United States.
3.1.2. Clinical characteristics
The mean baseline systolic BP and diastolic BP of the total sample were 134.1 ± 19.5 and 85.4 ± 11.9 mm Hg, respectively (Table 1). Approximately half (45.1%) of participants exhibited poor blood pressure control (defined as average systolic BP ≥140 mm Hg or an average diastolic BP ≥90 mm Hg). A majority (84.2%) of the participants reported being prescribed at least one antihypertensive medication. Almost all of the participants (91.8%) also reported having a family member with hypertension. Participants had a mean BMI of 32.5 ± 7.7 kg/m2. One‐quarter (26.9%) of participants were overweight, and an additional 60.6% were obese. Almost all participants (95.1%) reported having no other chronic conditions. However, two‐thirds (68.6%) of participants rated their health as only fair to good.
3.1.3. Psychosocial and behavioral characteristics
Of the participants prescribed an antihypertensive medication, 51.7% reported perfect adherence to their medications (Table 2). Two‐thirds (67.8%) reported that they were not concerned with the costs of their medications, and only 12% of participants had skipped taking their medication due to the cost. The mean score on the Patient Health Questionnaire (PHQ)‐8 depression scale was 5.3 ± 9.9 (range: 0‐24). About one‐half (46.1%) of participants reported some depressive symptomatology (PHQ score >4). On average, participants reported low levels of anxiety and stress (1.5 ± 1.8 [range: 0‐6] and 5.0 ± 3.3 [range: 0‐16], respectively).
Table 2.
Individual‐level psychosocial and behavioral characteristics for GUARDD participants
| Individual level | All participants (n = 2052) |
|---|---|
| Psychosocial characteristics (mean ± SD) | |
|
Depressive symptoms Range: 0‐24 |
5.3 ± 9.9 |
|
Anxiety Range: 0‐6 |
1.5 ± 1.8 |
|
Perceived stress Range: 0‐16 |
5.0 ± 3.3 |
| Behavioral characteristics, n (%) | |
| Perfect adherence to antihypertensive medications, n (%) | 953 (51.7) |
| Physical activity in the past week | |
| ≤2 h/wk | 919 (44.8) |
| >2 h/wk | 1126 (54.9) |
| Eat a healthy diet | |
| Not a lot/A little | 558 (27.2) |
| Somewhat | 892 (43.5) |
| Very much/Extremely | 601 (28.3) |
| Current smoker | 652 (31.8) |
| Alcohol use | |
| Never | 775 (37.8) |
| Monthly or less | 593 (28.9) |
| 2‐4 times a month | 366 (17.8) |
| 2‐3 times a week | 223 (10.9) |
| 4 or more times a week | 94 (4.6) |
| Patient activation level, n (%) | |
| Level 1 | 193 (9.7) |
| Level 2 | 594 (29.8) |
| Level 3 | 892 (44.8) |
| Level 4 | 314 (15.8) |
Abbreviation: SD, standard deviation.
Most participants (70.7%) reported “not at all” to “somewhat” eating a healthy diet, and almost half (44.8%) reported engaging in two hours of physical activity or fewer over the past week. About one‐third (31.8%) were current smokers (mean number of cigarettes per day: 5.8 ± 4.4), of which a majority (79%) expressed a desire to quit smoking. Most reported either never drinking alcohol (37.8%) or drinking alcohol monthly or less often (28.9%). Despite these adverse behaviors, approximately half (44.8%) of participants were considered in the “action stage” (level 3; total score range: 55.2‐72.4) of the Patient Activation Measure, indicating that they were starting to build self‐management skills and adopt healthful behaviors.
3.1.4. Attitudinal characteristics
Participants held mixed views about their hypertension and antihypertensive medications. More than half (58.3%) reported feeling as though they have little control over their hypertension, and almost three‐quarters (73.0%) were very to extremely concerned about their hypertension. Despite these concerns, only one‐quarter (25.5%) of participants felt that hypertension had a significant effect on their life.
A majority of participants (74.3%) reported poor outcomes expectations related to the long‐term effects of taking antihypertensive medications, and almost half (46.0%) did not think they would need their medication anymore once their BP was normal. Approximately half of participants felt that doctors prescribe too many medications (56%) that they place too much trust in medications (57.6%) and would prescribe fewer medications if they spent more time with patients (54.5%). Only one‐third of participants expected that their antihypertensive medication would protect them from a heart attack or stroke (37.7%) and that they would need to take medication for life (38.4%). However, most did expect the medications to control their hypertension (81.3%), would continue to take medications regardless of feeling symptoms (79.9%), and believed that taking the medications will help them feel better (66.5%) and live longer (55%).
3.2. Family level
Almost one‐half (40.4%) of the sample reported being single, and less than one‐quarter were married (21.4%; Table 3). The mean score on the Medical Outcome Study Social Support Index‐6 was 69.4 ± 25.5 (range: 0‐100), which is comparable to mean of 70.1 ± 24.2 reported in the Medical Outcomes Study.28 The mean score on Life Chaos Scale was 17.5 ± 2.8 (range: 10‐28), with higher scores indicating more life chaos.
Table 3.
Family‐level data for GUARDD participants
| Family‐level | All participants (n = 2052) |
|---|---|
| Marital status, n (%) | |
| Married | 440 (21.4) |
| Widowed | 126 (6.1) |
| Separated/divorced | 476 (23.2) |
| Part of an unmarried couple | 173 (8.5) |
| Single | 830 (40.4) |
|
Social support (mean ± SD) Range: 0‐100 |
69.4 ± 25.5 |
|
Life chaos (mean ± SD) Range: 10‐28 |
17.5 ± 2.8 |
Abbreviation: SD, standard deviation.
3.3. Provider and health care system level
Overall, participants reported high trust in their provider (75.6 ± 14.9, [range: 0‐100]) and rated the quality of patient‐provider communication as good (66.4 ± 15.5, [range: 0‐100]; Table 4). At the health care system‐level, most participants (81.7%) reported having no difficulty getting medical care when they needed it (Table 4). Participants reported seeing their PCPs an average of 2.6 ± 2.9 times over the past 6 months, of which they spent an average of 82.5 ± 56.9 minutes at the PCP's office (including both the time waiting and the time spent with the PCP). With respect to perceived discrimination, about one‐third of participants agreed that discrimination in a doctor's office is common (30.6%) and that doctors treat African American and white patients differently (38.7%). Moreover, half of participants agreed that African Americans and whites do not receive the same kind of care in hospitals (49.9%), or cannot get the care they want as equally as white people can (47.0%).
Table 4.
Provider and health care system‐level data for GUARDD participants
| Provider‐level | All participants (n = 2052) |
|---|---|
|
Quality of communication (mean ± SD) Range: 0‐100 |
66.4 ± 15.5 |
|
Trust in provider (mean ± SD) Range: 0‐100 |
75.6 ± 14.9 |
| Health care system level | |
| Access and use of care | |
| No difficulty getting medical care when needed, n (%) | 1673 (81.7) |
| # of visits with PCP over the past 6 mo (mean ± SD) | 2.6 ± 2.9 |
| # of minutes spent at PCP office (mean ± SD) | 82.5 ± 56.9 |
| Perceived discrimination in health care, n (%) agreed | |
| Doctors treat African Americans and whites differently | 774 (38.7) |
| Racial discrimination in a doctor's office is common | 614 (30.6) |
| African Americans and whites do not receive the same kind of care in hospitals | 1004 (49.9) |
| African Americans cannot get the care they want as equally as white people can | 952 (47.0) |
Abbreviations: PCP, primary care provider; SD, standard deviation.
3.4. Community level
Sixty‐three percent of GUARDD participants lived in neighborhoods with poor social conditions, high disease burden, and premature death (Table 5). Of these participants, 14.8% resided in the South Bronx, 42.9% resided in East and Central Harlem, and 5.5% resided in East and Central Brooklyn. The NYC Neighborhood Health Atlas34 data show that GUARDD participants lived in communities characterized by high levels of neighborhood poverty (26.1% of residents living below the federal poverty level), crowded housing conditions (11.3%), and high unemployment rates (12.2%). In addition, participants lived in communities that are residentially segregated (81.4%), with high volumes of alcohol and tobacco retailers (mean 30.8 retailers per 10 000 people). Finally, 63.3% of participants agreed that African Americans are more likely to have diseases like high blood pressure than whites are because of differences in their environments, like where they live, their access to care, or pollution.
Table 5.
Community‐level data for GUARDD participants
| Community‐level | All participants (n = 2052) |
|---|---|
| % of participants living in NYC DPHO area | 1296 (63.2%) |
| South Bronx, n (%) | 303 (14.8%) |
| East and Central Harlem, n (%) | 881 (42.9%) |
| Central Brooklyn, n (%) | 112 (5.5%) |
| Social‐economic characteristics of zip codes where GUARDD participants reside (mean ± SD) | |
| % of residents living below federal poverty level | 26.1% (15.5) |
| % of residents ages ≥16 y who are unemployed | 12.2% (6.9) |
| % of occupied housing units with more than one occupant per room | 11.3% (4.8) |
| Neighborhood conditions of zip codes where GUARDD participants reside (mean ± SD) | |
| % of non‐white population (residential segregation) | 81.4% (28.6) |
| Number of alcohol/tobacco retailers per 10 000 residents | 30.8 (10.5) |
| Perceived institutionalized racism, n (%) agreed | |
| African Americans are more likely to have diseases than whites because of differences in their environments | 1298 (63.3%) |
Abbreviation: Avg., average; DPHO, District Public Health Office; NYC, New York City; SD, standard deviation.
4. DISCUSSION
Despite therapeutic advancements in the treatment of hypertension, African Americans experience a disproportionately greater burden of hypertension‐related morbidity including stroke, heart failure, and end‐stage renal disease compared with other racial/ethnic groups.4 Evidence‐based care of African American patients with hypertension requires collaboration at multiple levels of influence—including the individual patients and their families, the provider, the health care system within which the care occurs, and the communities where patients reside. Despite these multiple levels of influence, much of the previous research in African Americans has focused on barriers to hypertension control at only one or two levels.13, 14
Recent published reports emphasize the need to identify the multilevel determinants of cardiovascular health inequities in African Americans.3, 37, 38 Such data are needed to strengthen the evidence base for the design, implementation, and evaluation of rigorous pragmatic interventions in real‐world settings.38 To achieve this goal, one report recommended analysis of large cohort studies that “may lead to understand geographic variation and evolving social and health inequities.”37 Utilizing a multilevel framework, this study sought to describe the risk and protective factors that occur at the individual, family, provider, health care system, and community levels in a sample of over 2000 African American adults with hypertension who are followed in academic, community, and safety‐net practices in NYC.
Results of our study showed that despite experiencing low barriers to accessing the health care system, a majority of participants exhibited poor clinical outcomes and maladaptive behaviors that are precursors for future cardiovascular diseases. Similar to other large‐scale studies in African Americans such as the Jackson Heart Study and Counseling African Americans to Control Hypertension (CAATCH) trial, a majority of GUARDD participants were categorized as obese or overweight.39, 40 Forty‐five percent also exhibited poor BP control.5 While this rate is comparable to national estimates of poor BP control in African Americans, it is higher than rates reported in the Jackson Heart Study and REasons for Geographic And Racial Differences in Stroke study (REGARDS; 34% poorly controlled) whose samples were comprised of African Americans from the south eastern regions of the United States (eg, Mississippi, Georgia).40, 41 It has been hypothesized that differences in cardiometabolic risk in African Americans who reside in the “stroke belt” may account for these differences (eg, higher prevalence of diabetes in the South than in the North).41
The behavioral data support our clinical findings. Approximately 50% of participants reported non‐adherence to prescribed antihypertensive medications, which is also comparable to the national estimates of non‐adherence in US adults with hypertension.42 Medication cost was not considered an adherence‐related barrier in this sample of largely insured patients. Half of participants reported getting 120 minutes or less of any kind of physical activity per week despite national guidelines recommending that American adults get at least 150 to 300 minutes of moderate‐intensity aerobic activity per week.43 Moreover, about three‐quarters of GUARDD participants reported that they infrequently eat a healthy diet. Data from the REGARDS study also showed that the African American participants spent significantly more time in sedentary behaviors than their white counterparts, when assessed by accelerometry.44 While measures differ across studies, poor diet quality has also been consistently reported in large‐scale studies of African Americans, regardless of their geographic residence.3
Thirty‐two percent of GUARDD participants reported being current smokers, which is more than double the national average smoking rate of 15.5%,45 and significantly higher than smoking rates published in the Jackson Heart Study (13.1%),46 REGARDS (16%‐19%),47 and the Multi‐Ethnic Study of Atherosclerosis (MESA; 18%).48 However, comparable rates were documented in African Americans who participated in the CAATCH trial (29.8%), which was also conducted in urban low‐resource primary care settings.39 The high prevalence of smoking in our sample is particularly concerning given recent research showing that African Americans who are current smokers are two times more likely to experience stroke than non‐smokers.49 Fortunately, a majority of participants also expressed a desire to quit smoking, which is also reflected in their high patient activation scores. This suggests that participants may be open to receiving referrals to smoking cessation resources, especially if made by one's provider, who was viewed as a trustworthy source in this study.
One noteworthy aspect of the GUARDD trial was the inclusion of important psychosocial and attitudinal patient‐level factors to characterize the potential barriers and facilitators to engaging patients in their care, an important prerequisite to helping individuals to adopt recommended behavior changes. When compared to African Americans in other large‐scale studies,50, 51 GUARDD participants were more likely to report elevated depressive symptoms, which has been implicated as a major barrier to behavior change in underserved populations.52, 53 For example, approximately half (46.1%) of GUARDD participants reported experiencing depressive symptomology compared with 28.7% in the Jackson Heart Study50 and 13.6% in REGARDS.51 Alternatively, GUARDD participants reported low levels of perceived stress and anxiety. Furthermore, at the family level, participants reported high levels of social support and an average level of life chaos, which may serve as protective factors that buffer the negative effects that depressive symptoms have on health behaviors.54
Finally, participants held conflicting attitudes about their hypertension. For example, while a majority of participants reported being concerned about their hypertension, fewer participants felt that it had any effect on their life. Participants also held negative views about the long‐term efficacy, safety, and necessity of their antihypertensive medications. Negative beliefs about treatment have consistently been linked to poorer medication adherence and health outcomes in hypertensive African Americans.55, 56, 57 Regardless of these views, most participants expected that their antihypertensive medications would control their BP, would continue to take medications regardless of feeling symptoms, and believed that taking the medications would help them feel better.
At the provider and health care system level, participants held different views about their quality of care, depending on whether they were reporting on the relationship with their specific provider or the broader health care system. For example, although participants rated their provider as trustworthy, they also reported that African Americans are more likely to experience discrimination in health care than whites. Prior cohort studies in African Americans have documented the insidious effects of perceived discrimination on all aspects of health and well‐being including clinical outcomes (eg, hypertension, obesity) and adverse health behaviors (eg, smoking) as well as subclinical disease processes and incident cardiovascular events.58, 59, 60, 61
The community‐level data substantiate these perceptions as GUARDD participants resided in neighborhoods that experience rates of crowding and unemployment that are more than three times the national average.62, 63 GUARDD participants also viewed institutionalized racism to be an impediment to African Americans’ health status, due to exposure to differential environments. The high levels of neighborhood poverty (26.1%) in our study are nearly double the levels reported in the Jackson Heart Study (17.6%), REGARDS (17%), and MESA (13%).64, 65, 66 Finally, GUARDD participants resided in neighborhoods characterized by high levels of residential segregation and high volume of tobacco/alcohol retailers. These data are in line with previous research, which has shown that even when African American patients perceive themselves as highly activated in their care, exposure to harsher environmental and social conditions may prohibit their ability to enact behavior change and can increase their risk of incident cardiovascular events.67, 68, 69
5. STUDY LIMITATIONS AND STRENGTHS
Strengths of this trial should be highlighted. First, we were able to recruit a large socio‐demographically diverse sample of African Americans utilizing a collaborative partnership approach. Specifically, we were able to recruit our total sample of 2052 participants in two years with a low refusal rate (4% declined participation).70 Successful recruitment of this high‐risk population is a necessary prerequisite to identifying and addressing the multilevel barriers to BP control. Second, the assessment of risk and protective factors at multiple levels of influence in an exclusively African American sample is noteworthy. Gaining a comprehensive understanding of the diverse array of factors, from the individual to the community/systems level that contribute to BP control in African Americans is needed to develop meaningful interventions that can address the root causes of hypertension‐related disparities. Based on our findings, future practice‐based research should examine whether interventions that target the patient‐provider relationship may help African American patients initiate behavior changes that can be implemented in disadvantaged communities and reinforced through established social support networks.
Limitations of this study also warrant mention. The baseline survey administered in the GUARDD trial was an amalgamation of items from validated scales used to capture risk and protective factors at multiple levels of influence. The validity of these individual items, in an urban African American population, has not been demonstrated. Some findings may be due to social desirability bias, whereby participants may have over reported their responses on some measures. Study staff were trained to normalize adverse behaviors and use patient‐centered communication techniques to minimize this bias. Finally, it also possible that an enrollment bias may affect our findings whereby individuals who participate in a research study may have different characteristics than the average individual in the general population. However, three‐quarters of participants in the GUARDD trial reported never participating in research prior to this experience.
6. CONCLUSIONS
In recent years, a focus on eradicating health inequities in racial/ethnic minority populations has played a prominent role on the national research agenda.71 Research that seeks to address inequities in health requires the utilization of transdisciplinary, multilevel approaches that identify and address the complex and interacting individual, family, provider, health care system, and community‐level factors.71 In this study, we identified a variety of risk and protective factors at multiple levels of influence that are associated with hypertension‐related health and morbidity in a high‐risk population of African Americans. Future research should examine the impact of these factors on cardiovascular outcomes using a longitudinal design. Our findings may inform the development of multilevel interventions that simultaneously support patients within a clinical environment (eg, through collaborative patient‐provider relationships) and within their community of residence (eg, through positive social support networks), to help patients adopt healthy behaviors that lower their risk for future hypertension‐related disease.
CONFLICT OF INTEREST
This study was supported by NHGRI (5U01HG007278, U01HG006380) and the National Center for Advancing Translational Sciences (NCATS) (UL1TR000067). Neither NHGRI nor NCATS was involved in the study design, collection, analysis or interpretation of data, writing of this article, or decision to submit it for publication. The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors would like to thank the GUARDD team of academic, community, and clinical partners, study coordinators, staff at study sites, and their partners in the IGNITE Network, a consortium of genomic medicine pilot demonstration projects funded by the National Human Genome Research Institute (NHGRI), for their valuable contributions to this project.
Schoenthaler A, Fei K, Ramos MA, Richardson LD, Ogedegbe G, Horowitz CR. Comprehensive examination of the multilevel adverse risk and protective factors for cardiovascular disease among hypertensive African Americans. J Clin Hypertens. 2019;21:794–803. 10.1111/jch.13560
REFERENCES
- 1. Whelton PK, Einhorn PT, Muntner P, et al. Research needs to improve hypertension treatment and control in African Americans. Hypertension. 2016;68(5):1066‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043‐2050. [DOI] [PubMed] [Google Scholar]
- 3. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393‐e423. [DOI] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67‐e492. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention .Age‐specific excess deaths associated with stroke among racial/ethnic minority populations – United States, 1997, 2000. [PubMed]
- 7. Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black‐white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study. Arch Intern Med. 1995;155(12):1319‐1324. [PubMed] [Google Scholar]
- 8. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End‐stage renal disease in African‐American and white men. 16‐year MRFIT findings. JAMA. 1997;277(16):1293‐1298. [PubMed] [Google Scholar]
- 9. Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner‐city African‐American sample. J Hum Hypertens. 1997;11(5):277‐283. [DOI] [PubMed] [Google Scholar]
- 10. Singh GK, Kochanek KD, MacDorman MF. Advance report of final mortality statistics, 1994. Monthly vital statistics report; vol 45 no 3, supp. Hyattsville, Maryland: Public Health Service. 1996.
- 11. Ferdinand KC, Rodriguez F, Nasser SA, et al. Cardiorenal metabolic syndrome and cardiometabolic risks in minority populations. Cardiorenal Med. 2014;4(1):794‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585‐1592. [DOI] [PubMed] [Google Scholar]
- 13. Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens. 2015;28(6):699‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odedosu T, Schoenthaler A, Vieira DL, Agyemang C, Ogedegbe G. Overcoming barriers to hypertension control in African Americans. Cleve Clin J Med. 2012;79(1):46‐56. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan B, Caddle‐Steele C, Chisholm G, et al. A culture of understanding: reflections and suggestions from a Genomics Research Community Board. Prog Community Health Partnersh. 2017;11(2):161‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horowitz CR, Abul‐Husn NS, Ellis S, et al. Determining the effects and challenges of incorporating genetic testing into primary care management of hypertensive patients with African ancestry. Contemp Clin Trials. 2016;47:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stewart AL, Hays RD, Ware JE Jr. The MOS short‐form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724‐735. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 19. Voils CI, Maciejewski ML, Hoyle RH, et al. Initial validation of a self‐report measure of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12):1013‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Center for Health Statistics . National Health and Nutrition Examination Survey Questionnaire. 2013. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013. Accessed December 17, 2018.
- 21. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163‐173. [DOI] [PubMed] [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317‐325. [DOI] [PubMed] [Google Scholar]
- 24. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385‐396. [PubMed] [Google Scholar]
- 25. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631‐637. [DOI] [PubMed] [Google Scholar]
- 26. Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening pStient adherence and barriers to adherence. Patient Educ Couns. 1999;37(2):113‐124. [DOI] [PubMed] [Google Scholar]
- 27. Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV‐infected persons. J Gen Intern Med. 2007;22(9):1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705‐714. [DOI] [PubMed] [Google Scholar]
- 29. Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728‐739. [DOI] [PubMed] [Google Scholar]
- 30. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(suppl 1):146‐161. [DOI] [PubMed] [Google Scholar]
- 31. Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274(4):305‐311. [PubMed] [Google Scholar]
- 32. Bhandari A, Wagner T. Self‐reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63(2):217‐235. [DOI] [PubMed] [Google Scholar]
- 33. New York City Department of Menthal Health and Hygeine . Neighborhood health action centers. 2019. https://www1.nyc.gov/site/doh/health/neighborhood-health/neighborhood-health-action-centers.page. Accessed April 2, 2019.
- 34. New York City Department of Mental Health and Hygeine . New York City Neighborhood Health Atlas. https://www1.nyc.gov/site/doh/health/neighborhood-health/nyc-neighborhood-health-atlas.page. Accessed April 8, 2019.
- 35. Jones CP. Levels of racism: a theoretic framework and a gardener's tale. Am J Public Health. 2000;90:1212‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5):2460‐2470. [DOI] [PubMed] [Google Scholar]
- 37. Engelgau MM, Narayan KMV, Ezzati M, et al. Implementation Research to Address the United States Health Disadvantage: Report of a National Heart, Lung, and Blood Institute Workshop. Glob Heart. 2018;13(2):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampson U, Kaplan RM, Cooper RS, et al. Reducing Health Inequities in the U.S.: recommendations from the NHLBI's Health Inequities Think Tank Meeting. J Am Coll Cardiol. 2016;68(5):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernandez S, Tobin JN, Cassells A, Diaz‐Gloster M, Kalida C, Ogedegbe G. The Counseling African Americans to Control Hypertension (CAATCH) trial: baseline demographic, clinical psychosocial, and behavioral characteristics. Implement Sci. 2011;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Djousse L, Petrone AB, Blackshear C, et al. Prevalence and change over time of ideal cardiovascular health metrics among African‐Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Howard G, Prineas R, Moy C, et al. Racial and Geographic Differences in Awareness, Treatment, and Control of Hypertension. Stroke. 2006;37(5):1171–1178. [DOI] [PubMed] [Google Scholar]
- 42. Ostererg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 43. United States Department of Health and Human Services . Physical Activity Guidelines for Americans. 2nd edn. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 44. Hooker SP, Hutto B, Zhu W, et al. Accelerometer measured sedentary behavior and physical activity in white and black adults: The REGARDS study. J Sci Med Sport. 2016;19(4):336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sims M, Lipford KJ, Patel N, Ford CD, Min YI, Wyatt SB. Psychosocial factors and behaviors in African Americans: the Jackson Heart Study. Am J Prev Med. 2017;52(1S1):S48–S55. [DOI] [PubMed] [Google Scholar]
- 47. McClure LA, Murphy HL, Roseman J, Howard G, Malarcher A. Regional and racial differences in smoking and exposure to secondhand smoke: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Prev Chronic Dis. 2011;8(5):A108–A108. [PMC free article] [PubMed] [Google Scholar]
- 48. Kramer H, Han C, Post W, et al. Racial/Ethnic differences in hypertension and hypertension treatment and control in the multi‐ethnic study of atherosclerosis (MESA). Am J Hypertens. 2004;17(10):963–970. [DOI] [PubMed] [Google Scholar]
- 49. Oshunbade A. Smoking Doubles Risk of Stroke Among African‐Americans. Houston, TX: American Heart Association's Epidemiology and Prevention/Lifestyle and Cardiometabolic Health Scientific Sessions; 2019. [Google Scholar]
- 50. O’Brien EC, Greiner MA, Sims M, et al. Depressive symptoms and risk of cardiovascular events in blacks: findings from the Jackson Heart Study. Circ Cardiovasc Qual Outcomes. 2015;8(6):552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ye S, Muntner P, Shimbo D, et al. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2013;61(6):622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bolen JC, Rhodes L, Powell‐Griner EE, Bland SD, Holtzman D. State‐specific prevalence of selected health behaviors, by race and ethnicity–Behavioral Risk Factor Surveillance System, 1997. MMWR CDC Surveill Summ. 2000;49(2):794–60. [PubMed] [Google Scholar]
- 53. Timmerman GM. Addressing barriers to health promotion in underserved women. Fam Community Health. 2007;30(1 suppl):S34–42. [DOI] [PubMed] [Google Scholar]
- 54. Reblin M, Uchino BN. Social and emotional support and its implication for health. Curr Opin Psychiatry. 2008;21(2):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gatti ME, Jacobson KL, Gazmararian JA, Schmotzer B, Kripalani S. Relationships between beliefs about medications and adherence. Am J Health‐Syst Pharm. 2009;66(7):657–664. [DOI] [PubMed] [Google Scholar]
- 56. Gagnon MD, Waltermaurer E, Martin A, Friedenson C, Gayle E, Hauser DL. Patient beliefs have a greater impact than barriers on medication adherence in a Community Health Center. J Am Board Fam Med. 2017;30(3):331–336. [DOI] [PubMed] [Google Scholar]
- 57. Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119(1):70.e79–15. [DOI] [PubMed] [Google Scholar]
- 58. Hausmann LR, Jeong K, Bost JE, Ibrahim SA. Perceived discrimination in health care and health status in a racially diverse sample. Med Care. 2008;46(9):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sims M, Diez‐Roux AV, Gebreab SY, et al. Perceived discrimination is associated with health behaviours among African‐Americans in the Jackson Heart Study. J Epidemiol Community Health. 2016;70(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Everson‐Rose SA, Lutsey PL, Roetker NS, et al. Perceived discrimination and incident cardiovascular events: the multi‐ethnic study of atherosclerosis. Am J Epidemiol. 2015;182(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kershaw KN, Lewis TT, Roux AVD, et al. Self‐reported experiences of discrimination and inflammation among men and women: the multi‐ethnic study of atherosclerosis. Health Psychol. 2016;35(4):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. United States Census Bureau . Census of housing. https://www.census.gov/hhes/www/housing/census/historic/crowding.html. Accessed April 9, 2019.
- 63. Bureau of Labor Statistics . Labor force statistics from the current population survey. 2019; https://data.bls.gov/timeseries/lns14000000. Accessed April 9, 2019.
- 64. Barber S, Hickson DA, Kawachi I, Subramanian SV, Earls F. Neighborhood disadvantage and cumulative biological risk among a socioeconomically diverse sample of African American Adults: an examination in the Jackson Heart Study. J Racial Ethn Health Disparities. 2016;3(3):444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McClellan WM, Newsome BB, McClure LA, et al. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tomey K, Diez Roux AV, Clarke P, Seeman T. Associations between neighborhood characteristics and self‐rated health: a cross‐sectional investigation in the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort. Health & Place. 2013;24:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. AuYoung M, Ponce NA, Duru OK, Bustamante AV, Mangione CM, Rodriguez HP. Patient activation is inconsistently associated with positive health behaviors among obese safety net patients. J Immigr Minor Health. 2016;18(6):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez‐Roux AV. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health. 2016;106(12):2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood‐level racial/ethnic residential segregation and incident cardiovascular disease: the multi‐ethnic study of atherosclerosis. Circulation. 2015;131(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Horowitz CR, Sabin T, Ramos M, et al. Successful recruitment and retention of diverse participants in a genomics clinical trial: a good invitation to a great party. Genet Med. 2019. 10.1038/s41436-019-0498-x [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71. Srinivasan S, Williams SD. Transitioning from health disparities to a health equity research agenda: the time is now. Public Health Rep. 2014;129(suppl 2):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


