Abstract
The study aims to evaluate the effectiveness of different durations of aerobic exercise on hypertensive patients. Four electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) were searched from their inception until July 2018. English publications and randomized controlled trials involving aerobic exercise treatment for hypertensive population were included. Two reviewers independently extracted the data. The Cochrane's Risk of Bias tool was used to assess the quality of included studies. In this systematic review, a total of 14 articles were included, involving 860 participants. The quality of the included studies ranged from moderate to high. The results of the meta‐analysis showed that compared with the control group, significant effects of aerobic exercise were observed on reducing systolic blood pressure (SBP) (mean difference [MD] = −12.26 mm Hg, 95% confidence interval [CI] = −15.17 to −9.34, P < 0.05), diastolic blood pressure (DBP; MD = −6.12 mm Hg, 95% CI = −7.76 to −4.48, P < 0.05), and heart rate (MD = −4.96 bpm, 95% CI = −6.46 to −3.43, P < 0.05). In addition, significant reductions were observed in ambulatory DBP (MD = −4.90 mm Hg, 95% CI = −8.55 to −1.25, P < 0.05) and ambulatory SBP (MD = −8.77mm Hg, 95% CI = −13.97 to −3.57, P < 0.05). Therefore, aerobic exercise might be an effective treatment for blood pressure improvement in hypertensive patients. However, the effectiveness between the duration of different treatment needs to be well‐designed and rigorous studies will be required to verify the dataset.
Keywords: aerobic exercise, hypertension, randomized controlled trial, systematic reviews
1. INTRODUCTION
Hypertension is a major worldwide public health concern because of its high prevalence and concomitant risk of cardiovascular and kidney disease. In addition, hypertension is frequently associated with diabetes mellitus, dyslipidemia, and obesity, which lead to negative outcomes, such as stroke, myocardial infarction, renal failure, atherosclerosis, and heart failure.1, 2 In 2015, roughly 1.13 billion individuals were affected by hypertension worldwide,3 and it has been estimated that in 2025, ~1.56 billion individuals will be affected by hypertension.4 It has previously been shown by major pharmacological trials that it is a challenge for single‐drug therapy to control and maintain the blood pressure of hypertensive patients within the normal range, and in only 25%‐62% of patients proper control is achieved.5 In order to control the blood pressure, many individuals require treatment with more than one antihypertensive drug, however, such practice increases the financial burden and may generate side effects.6 Therefore, inexpensive, safe, and strategies that can be easily implemented are of utmost importance for the prevention of hypertension.
Aerobic exercise (AE) has proven to be an effective nonpharmacological method to treat and prevent coronary artery disease,7 cardiovascular disease,8 type 2 diabetes mellitus,9, 10 and hypertension. Moreover, it has been widely recommended by both European and American hypertension guidelines that AE can be used as an adjunct to the treatment of hypertension.11 In previous studies, it has been demonstrated that AE produces the positive effects on systolic blood pressure (SBP) and diastolic blood pressure (DBP).12, 13, 14, 15, 16 Furthermore, increasing evidence has indicated that AE has favorable effects on cardiovascular risk factors, cardiac autonomic function, and endothelial pathophysiology in individuals with hypertension.17, 18 Joint guidelines from the American Heart Association (AHA) and American College of Sports Medicine (ACSM) have recommended moderate‐intensity AE for a minimum of 30 minutes per day, 5 days a week or vigorous‐intensity AE for a minimum of 20 minutes per day, 3 days a week.19 However, the duration of different exercises has different effects on the treatment outcome of hypertension patients.20 Therefore, it is of importance to further discuss which type of exercise and which duration can produce optimal treatment effects in hypertension patients.
Considering the potential benefits of AE on health outcomes, such as blood pressure and heart rate, we performed a comprehensive systematic review to evaluate the effectiveness of AE in hypertensive patients and analyzed the relationship between changes in blood pressure and the duration of exercise, so as to provide reliable clinical evidence for the treatment of hypertension.
2. METHODS AND MATERIALS
2.1. Search strategy
PubMed, Embase, Cochrane Library, and Web of Science databases were searched from inception until July 2018. Our search was not restricted based on the basis of publication type, or year of publishing. The search terms and basic search strategy were as follows: (hypertension OR “high blood pressure” OR “hypertensive”) AND (“aerobic exercise” OR “aerobic sport” OR “aerobic sports” OR “aerobic exercises” OR “endurance exercise” OR “endurance exercises”) AND random*. In addition, to ensure a comprehensive data collection, references of relevant reviews were searched manually to identify additional eligible studies.
2.2. Study selection
Two reviewers (CLJ and LR) independently reviewed the title and abstracts of initially selected studies. The full texts of articles were retrieved if there was any doubt about inclusion of the study. Disagreements were resolved through discussion or by consulting a third reviewer (LXX). Studies were included if the following criteria were met: (a) randomized controlled trials (RCT); (b) enrolled participants between the ages of 30 and 85 years, who were diagnosed with hypertension based on clinical and laboratory studies (SBP ≥ 140 mm Hg and DBP ≥ 90 mm Hg), not accompanied by other metabolic or cardiovascular diseases, no alcohol use and nonsmoking, able to voluntary join exercise; (c) the exercise group only performed regular AE and the control group did not receive any type exercise, and participants in neither group received any type of special intervention, such as an improved diet or a change in lifestyle; (d) the study included at least one type of quantitative outcome data (blood pressure, heart rate, ambulatory pressure blood, or quality of life). The exclusion criteria were as follows: (a) the study included too little information or data could not be obtained, such as review articles, editorials, comments, and protocols; (b) duplicate reports of the same study.
2.3. Data extraction and quality assessment
After selecting studies based on the inclusion and exclusion criteria, two reviewers (LMX and CLJ) independently conducted the data extraction by using a self‐developed data extraction form. In case of any disagreement between the two reviewers, a final decision was obtained by consensus after discussion and consultation with a third reviewer (YPJ). General information about the study included the following: (a) basic character of the included research object (author, publication year, study country, the participant numbers in the aerobic group and control group, duration of follow‐up); (b) general demographic characteristics (gender ratio, heart rate, body mass index [BMI], and SBP and DBP at baseline and at the end of study); (c) intervention group and control group of time and intensity of exercise. If the information present was unclear or if information was missing, the corresponding author of the study was contacted via email.
Two reviewers (LR and CLJ) independently assessed the quality of included studies. Risk of bias was assessed for each study, and included using the Cochrane Risk of Bias Tool for RCT,21, 22 which evaluated seven sources of bias, including randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective outcome reporting, and other potential bias. Each study was examined based on the above seven aspects and subsequently judged as being low risk, high risk, or unclear risk. Studies that were scored as high risk of bias for more than one key domain were considered as high risk of bias. In addition, studies that were scored as low risk of bias for all key domains were considered as low risk of bias. In all other cases, studies were considered to have an unclear risk of bias.23
2.4. Statistical analyses
Blood pressure (SBP, DBP) and heart rate were considered primary outcomes of the study, and ambulatory blood pressure and quality of life were considered secondary outcomes. Statistical analysis was performed by Cochrane Review Manager (RevMan 5.3) software (Cochrane). For continuous outcomes, the mean difference (MDs) and 95% confidence intervals (CIs) were calculated. P < 0.05 was considered statistically significant. Heterogeneity between studies was assessed using the Higgins I 2 test and P values. If P < 0.05 and I 2 > 50%, the random effects model was selected to calculate the pooled effective size. In other cases, the fixed‐effects model was employed. Potential moderating factors of SBP/DBP were evaluated by subgroup analysis, and publication bias was tested by funnel plot analysis.
3. RESULTS
3.1. Literature selection
Based on the search strategy, a total of 16 553 studies were selected from the initial database search. Of those studies, 5356 studies were excluded because of duplication, therefore 11 192 studies were selected for further analysis. By screening of titles and abstracts, 10 751 studies were excluded. After reading the full text of the remaining 446 studies, another 433 were excluded, which included review articles and guidelines (n = 45), nonrandomized trials (n = 31), other intervention studies (n = 136), studies with no mention of subjects with hypertension (n = 131), other outcomes (n = 79), and studies from which obtain related data could not be obtained (n = 5). Finally, a total of 14 studies were included in the meta‐analysis. The detailed flowchart showing screening process is presented in Figure 1.
Figure 1.
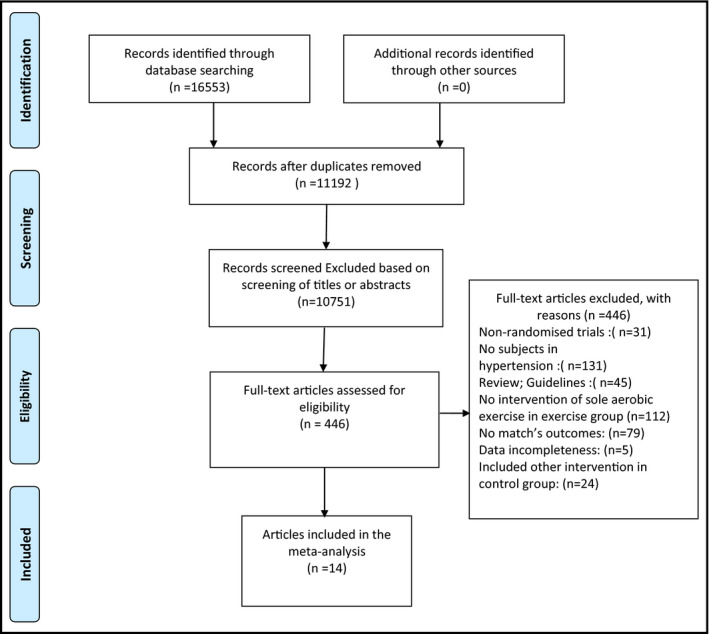
Flow diagram regarding the article selection for the meta‐analysis
3.2. Study characteristics
Table 1 shows the main characteristics of all included studies. The 14 included studies involved a total of 860 hypertensive patients were enrolled, and the sample size of the included studies ranged from 16 to 217, including 444 cases in the aerobics group and 416 cases in the control group. The included fourteen studies were published between 1985 and 2018. In one study, only female participants were enrolled, in one study only male participants were enrolled, and in seven studies, both male and female participants were enrolled. The mean age of the subjects ranged between 39.67 and 83.4 years of age. In 13 study groups, the baseline BMI was reported, which ranged from 22.48 to 29.6 kg/m2, 12 study groups reported SBP and DBP at baseline, and the SBP at baseline ranged from 130.3 to 170.45 mm Hg and the DBP at baseline ranged from 67.5 to 95.2 mm Hg. The duration of intervention ranged from 40 minutes to 6 months. Regarding nationality, patients included in both the AE group and control group were mainly from Germany, Iran, Taiwan, Ibadan, Japan, and Nigeria. Most participants in the control groups were instructed not to change their usual lifestyle, including physical activity. The duration of exercise training in the 13 studies was <8, 8‐12, and >12 weeks. Among them, 3 studies had an exercise duration of ≤8 weeks, in 8 studies, the exercise duration was between 8‐12 weeks, and in two studies, the exercise duration was more than 12 weeks. No significant differences in baseline age and BMI were observed between the aerobics group and the control group.
Table 1.
Characteristics of studies included in the meta‐analysis
| Study name | Year | Country | Group | Age | Number of subjects (AE/C) | BMI, kg/m2 | Follow‐up |
|---|---|---|---|---|---|---|---|
| Farahani, A.V.40 | 2010 | Iran | AE | 48.33 ± 10.74 | (12/28) | 27.44 ± 4.27 | 10 wk |
| Control | 46.96 ± 11.58 | 28.06 ± 3.51 | |||||
| Molmen‐Hansen, H.E.26 | 2012 | Norway | AE | 52.50 ± 7.40 | (25/25) | 26.8 ± 4.10 | 12 wk |
| Control | 51.30 ± 9.20 | 28.80 ± 3.7 | |||||
| Maruf. F.A.a28 | 2013 | NA | AE | 50.80 ± 8.31 | (53/50) | 27.40 ± 4.96 | 12 wk |
| Control | 54.75 ± 8.56 | 25.39 ± 4.61 | |||||
| Maruf,.F.A.b15 | 2014 | Nigeria | AE | 50.80 ± 8.31 | (45/43) | 27.45 ± 4.99 | 12 wk |
| Control | 54.75 ± 8.56 | 25.41 ± 4.70 | |||||
| Tsai, J.C.12 | 2004 | China | AE | 48.80 ± 6.3 | (52/50) | 23.6 ± 1.8 | 10 wk |
| Control | 49.30 ± 7.2 | 23.8 ± 2.2 | |||||
| He, L.25 | 2018 | China | AE | 58.0 ± 2.0 | (20/22) | 27.41 ± 2.11 | 12 wk |
| Control | 57.0 ± 2.0 | 27.65 ± 2.61 | |||||
| Masroor, S.16 | 2018 | India | AE | 39.67 ± 4.10 | (15/13) | 29.6 ± 4.4 | 4 wk |
| Control | 41.54 ± 4.25 | ||||||
| Dimeo, F.13 | 2012 | Germany | AE | 62.80 ± 8.1 | (22/25) | 28.9 ± 4.4 | 8‐12 wk |
| Control | 67.90 ± 6.2 | 29.9 ± 4.7 | |||||
| Westhoff, T.H.14 | 2008 | Germany | AE | 66.10 ± 4.4 | (12/12) | 28.6 ± 4.4 | 12 wk |
| Control | 68.40 ± 9.1 | 26.5 ± 3.0 | |||||
| Lima, L.G.41 | 2017 | Brazil | AE | 67.80 ± 4.3 | (15/14) | 28.9 ± 3.5 | 10 wk |
| control | 69.90 ± 5.5 | 27.6 ± 3.4 | |||||
| Sikiru, L.42 | 2014 | Nigeria | AE | 58.63 ± 7.22 | (112/105) | 22.48 ± 2.89 | 8 wk |
| Control | 58.27 ± 6.24 | 24.16 ± 4.91 | |||||
| Oliveira, J.27 | 2016 | Portugal | AE | 83.40 ± 3.2 | (9/9) | 28.5 ± 2.0 | 40 min |
| Control | 82.70 ± 2.5 | 28.0 ± 2.5 | |||||
| Tsuda, K.43 | 2003 | Japan | AE | 46.2 ± 1.4 | (8/8) | 25.2 ± 0.8 | 6 mo |
| Control | 49.0 ± 5.1 | 24.9 ± 1.1 | |||||
| Duncan, J.24 | 1985 | American | AE | 21‐37 (mean: 30.4) | 56 | NA | 16 wk |
Abbreviations: AE/C: aerobic exercise/control groups; AE: aerobic exercise; BMI: body mass index.
3.3. Risk of bias
Figure 2 presents the summary of the risk of bias for each included study. For the item of “random sequence generation,” five studies used the methods of proper generation with a low risk of bias, three included studies were scored as unclear risk of selection bias, and in seven studies, only randomization was mentioned without any clarification of the procedures performed. Concealment of allocation to group was unclear in 12 studies. For outcome blinding, five studies adopted a single‐blind method to evaluate the intervention measures. Because of objective outcome measures, outcome data were considered low risk in 13 studies.
Figure 2.
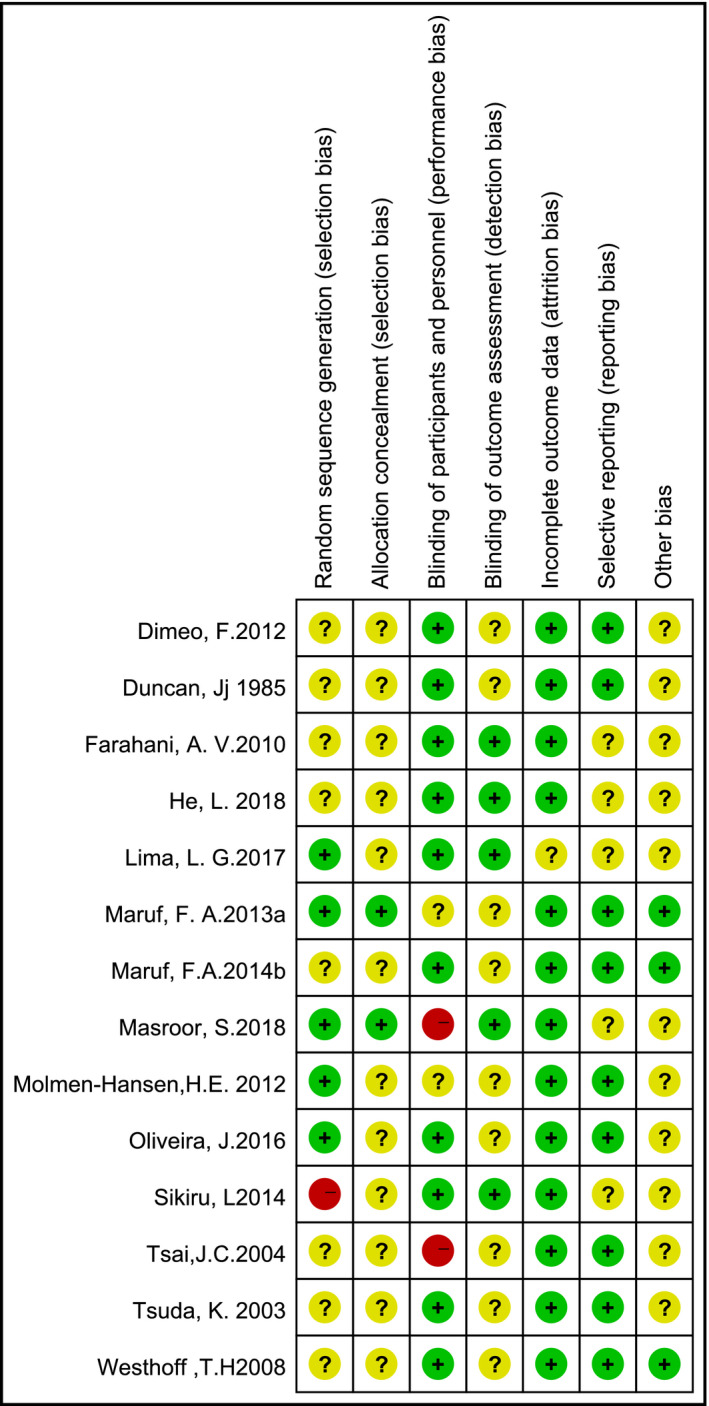
Risk of bias summary
3.4. Meta‐analysis
3.4.1. Blood pressure analysis
In a total of 13 studies (757 samples) blood pressure of participants was reported. The results of the meta‐analysis showed that, compared with the control group, SBP and DBP were significantly reduced in the AE group, and the pooled MD was −12.26 mm Hg (95% CI: −15.17 to −9.34, P < 0.05) and −6.12 mm Hg (95% CI: –7.76 to −4.48, P < 0.05), respectively. Subgroup analysis of the SBP showed that according to the exercise duration of the AE group using the random effects model (Figure 3), significant differences were observed between groups at ≤8 weeks, 8‐12 weeks, and more than 12 weeks, the pooled MD was −16.66 mm Hg (95% CI: −18.55 to −14.76, P < 0.05), −11.74 mm Hg (95% CI: −15.94 to −7.54, P < 0.05), and −8.84 mm Hg (95% CI: −13.52 to −4.15, P < 0.05), respectively. Subgroup analysis of the DBP was performed according to the duration of the AE group using the random effects model (Figure 4). The pooled MD was −6.43 mm Hg (95% CI: −7.83 to −5.03, P < 0.05), −5.44 mm Hg (95% CI: −8.22 to −2.66, P < 0.05), and −7.52 mm Hg (95% CI: −12.42 to −2.62, P < 0.05), respectively.
Figure 3.
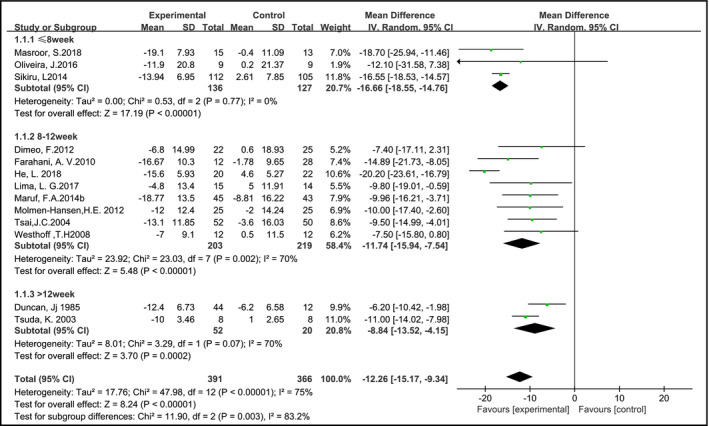
Aerobic exercise on systolic blood pressure in hypertensive patients
Figure 4.
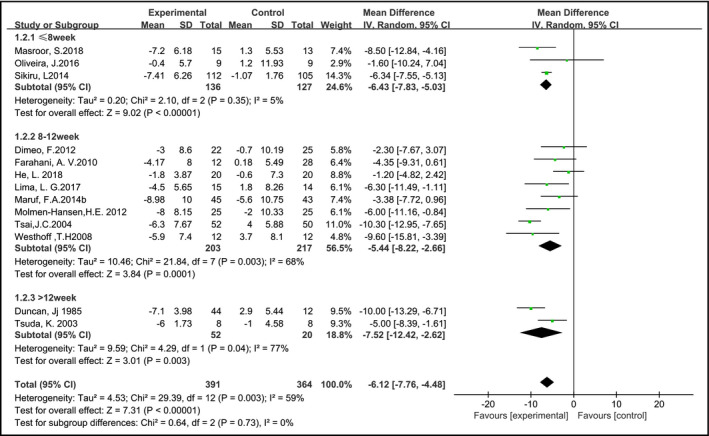
Aerobic exercise on diastolic blood pressure in hypertensive patients
3.4.2. Heart rate
In seven studies12, 14, 16, 24, 25, 26, 27 (316 samples), data for heart rate associated with each intervention was specifically reported. The heart rate of the AE group was significantly reduced compared with the control group (MD: −4.94, 95% CI: –6.46 to −3.43, P = 0.78, I 2 = 0%) (Figure 5).
Figure 5.
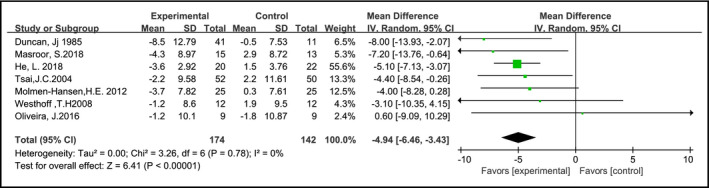
Effects of aerobic training on heart rate in hypertension
3.4.3. Ambulatory blood pressure
In two studies,13, 26 the ambulatory blood pressure was addressed as a specific outcome. The meta‐analysis of 97 participants indicated that AE reduced ambulatory SBP and ambulatory DBP with a pooled MD −8.77 mm Hg (95% CI = −13.97 to −3.57, P < 0.05) and −4.90 mm Hg (95% CI = −8.55 to −1.25, P < 0.05), respectively, when compared with individuals who did not receive exercise intervention (Figure 6).
Figure 6.
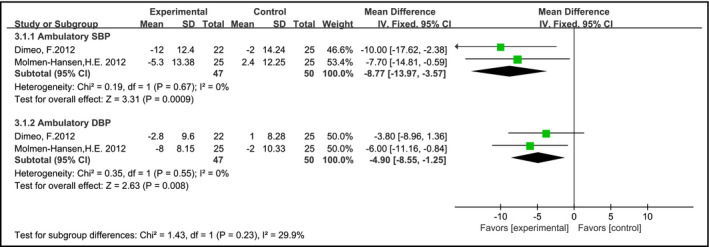
Effects of aerobic training on ambulatory blood pressure in hypertension
3.4.4. Quality of life
In only one study,28 specific data for the quality of life was reported, which showed that significant improvements were observed in the AE group in all domains of WHOQoL‐BREF (physical health: +23.33, P < 0.05; psychological health: +18.17, P < 0.05; social relationships: +14.51, P < 0.05; environment: +11.51, P < 0.05). However, in the WHOQoL‐BREF scale, the control group only showed improvements in the areas of physical health (15.42; P < 0.05), psychological health (9.70; P < 0.05), and social relationships domains (9.55; P < 0.05).
3.5. Publication bias
In a total of 13 studies, AE intervention with baseline treatment alone was performed and the effect of AE on blood pressure was evaluated. In this systematic review, a funnel plot was created to check for publication bias. Neither changes in SBP nor changes in DBP revealed potential publication bias (Figure 7).
Figure 7.
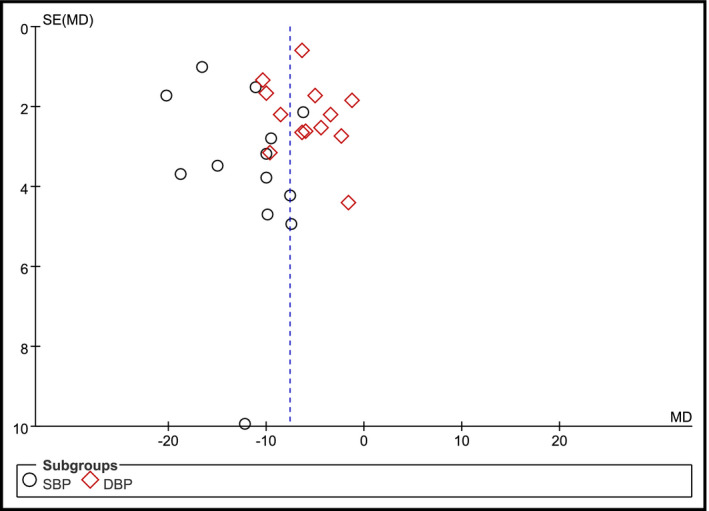
Funnel plot of comparison aerobic exercise intervention on blood pressure
4. DISCUSSION
In this systematic review and meta‐analysis, the data from 14 studies were pooled and analyzed, thereby evaluating the effects of AE training interventions on blood pressure, heart rate, and ambulatory blood pressure in a total of 860 hypertensive subjects. Overall, the results of the meta‐analysis showed that the blood pressure and heart rate were improved by AE training. Subsequently, subgroup analyses were performed to evaluate the influence of training duration on the efficacy of AE. Subgroup analysis indicated that the degree of blood pressure reduction did not significantly differ among trials in which a different duration of AE was used.
The effect of AE on blood pressure has been shown in previous meta‐analyses,20, 29, 30 which suggested that the AE intervention decreased the risk of incident hypertension or had an effect on blood pressure reduction. These findings were in accordance with our results, showing that AE can significantly reduce blood pressure (both SBP and DBP) in hypertensive patients. Moreover, in this review, we showed that the degree of blood pressure reduction significantly differs among studies for all durations of exercise (less than 8 weeks, 8‐12 weeks, and more than 12 weeks), and AE that lasts for about 8 weeks may have a better antihypertensive effect. However, the small number of included studies and the high risk of bias of the original studies may have impacted this conclusion. Furthermore, in some studies,31, 32 it was pointed out that several important variables, including differences in AE intensity, frequency, ethnicity, and hypertensive status, might have some inevitable influences on the benefits of AE in individuals with hypertension. Therefore, considering the above factors, additional studies with larger sample sizes to compare those factors should be considered to help understand these findings.
It is worth noting that the pooled SBP after AE showed a significant heterogeneity. The sensitivity analysis showed a relatively stable result for SBP after excluding two individual studies,24, 25 which indicated a relatively higher heart rate, longer duration of aerobic training or a younger age. The study presented by He et al25 demonstrated that a relatively high heart rate with a long‐term AE (12 weeks) might induce a more significant reduction on SBP, which was significantly different from that of other studies, therefore this study was removed from the dataset, which dramatically changed the results. Hypertension is a chronic age‐related disease,33 and in a study by Duncan et al,24 the overall age of study participants was significantly younger compared to that in other studies, which might be a contributing factor to the change in results.
We also observed that AE intervention played an active role in reducing heart rate. These effects have been discussed in several reviews34 and clinical reports, and similar results were obtained. Kingsley et al35 concluded that postexercise heart rate recovery was influenced by parasympathetic reactivation and sympathetic recovery to resting levels, thereby reducing the resting heart rate by increasing parasympathetic tone improvement in autonomic modulation with exercise. In another report by Cornelissen and colleagues,32 it was stated that the effects on heart rate were more pronounced after higher intensity AE. Taken together, these above reports showed evidence that supported our findings. Since only six studies (316 participants) have been included, which is a relatively small sample size, therefore, the reliability of the results is relatively small. To confirm these effects, additional trials will be required in the future.
Regarding the design of RCTs involving AE for hypertension, high risk of bias existed in random sequence generation and allocation concealment, which may have resulted in potential selection bias. In the design and reporting of included RCTs, allocation concealment was arguably the weakest link and may potentially affect the reliability of the study results.36, 37 In addition, our judgment on the quality of inclusion studies was primarily based on their reports, therefore, future studies will be required to improve the quality of the original study research and avoid the occurrence of various biases, thereby suggesting that is strictly referred to the CONSORT statement.38, 39
Compared with previous meta‐analyses presented by Montero et al20 and Wen et al,29 in the current study, strict inclusion and exclusion criteria were employed, including a comprehensive search strategy, which takes into account a wider range of outcome indicators (blood pressure, heart rate, ambulatory blood pressure, quality of life). In addition, the current study focused on the duration of AE and subgroup analysis was performed, in which different durations of AE were used to assess the blood pressure of hypertensive patients. Thus, our results might be of great value for providing references for the control of blood pressure and heart rate via AE.
This study has several limitations. Firstly, although a comprehensive search strategy was conducted, the current study only included studies that were written in the English or Chinese language. Therefore, it is likely that relevant published or unpublished studies were missed, however the representativeness of included studies was not affected. Secondly, because not all included trials were of high quality, the estimates of therapeutic effects may have been impacted. Furthermore, with the emergence of newly related studies, the existing results may changes. When such novel and related new studies appear after July 2018, this systematic review will be updated.
5. CONCLUSIONS
The results of this meta‐analysis showed that AE has favorable effects on blood pressure, heart rate, and ambulatory blood pressure of hypertensive patients. However, the effectiveness between the duration of different treatments is still not clear. Our finding was based on a small number of studies with evidence of considerable statistical and clinical heterogeneity, and there is insufficient evidence of high‐quality studies. Furthermore, high‐quality original studies are also warranted to confirm the magnitude of the effect of different durations of AE on changes in blood pressure and heart rate among hypertensive individuals.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
AUTHOR'S CONTRIBUTION
LJ Cao and XX Li: project development, data collection, analysis and interpretation, manuscript writing, article revised. PJ Yan: project development, analysis and interpretation, manuscript writing, article revised. XQ Wang: data collection, analysis and interpretation, manuscript writing. MX Li: data collection, analysis and interpretation. R Li: data analysis and interpretation. XE Shi: data collection, article revised. KH Yang and XR Liu: academic oversight and edited all drafts. All authors critically revised the article for important intellectual content and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Jinhui Tian and all members of Evidence‐Based Medicine Center, Lanzhou University, for their help with this study.
Cao L, Li X, Yan P, et al. The effectiveness of aerobic exercise for hypertensive population: A systematic review and meta‐analysis. J Clin Hypertens. 2019;21:868–876. 10.1111/jch.13583
Liujiao Cao and Xiuxia Li are co‐first authors.
Funding information
Supported by the Fundamental Research Funds for the Central Universities (16LZUJBWTD013, 18LZUJBWZX006, lzujbky‐2018‐14): Evidence‐based Social Sciences Research. China Medical Board Open Project Funding (CMB #17‐279): Tracking and Evaluating Quality (TEQ) of Rural Health Services in NW China: Tool kits for rural clinic quality management and capacity building. Special Fund for Soft Science in Gansu Province (18CX1ZA043): Study on the Measures to Improve the Quality of Rural Health Services in Gansu Province
Contributor Information
Xingrong Liu, Email: liuxingrong2019@163.com.
Kehu Yang, Email: kehuyangebm2006@126.com.
REFERENCES
- 1. Whelton PK. Hypertension curriculum review: epidemiology and the prevention of hypertension. J Clin Hypertens. 2004;6(11):636‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371(9623):1513‐1518. [DOI] [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389(10064):37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 5. Niiranen TJ, Kantola IM, Vesalainen R, Johansson J, Ruuska MJ. A comparison of home measurement and ambulatory monitoring of blood pressure in the adjustment of antihypertensive treatment. Am J Hypertens. 2006;19(5):468‐474. [DOI] [PubMed] [Google Scholar]
- 6. Maruf FA, Salako BL, Akinpelu AO. Can aerobic exercise complement antihypertensive drugs to achieve blood pressure control in individuals with essential hypertension? J Cardiovasc Med. 2014;15(6):456‐462. [DOI] [PubMed] [Google Scholar]
- 7. Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX‐CAD study. Int J Cardiol. 2015;179:203‐210. [DOI] [PubMed] [Google Scholar]
- 8. Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086‐3094. [DOI] [PubMed] [Google Scholar]
- 9. Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357‐369. [DOI] [PubMed] [Google Scholar]
- 10. Pan B, Ge L, Xun Y‐Q, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta‐analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;16(1):14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai J‐C, Yang H‐Y, Wang W‐H, et al. The beneficial effect of regular endurance exercise training on blood pressure and quality of life in patients with hypertension. Clin Exp Hypertens. 2004;26(3):255‐265. [DOI] [PubMed] [Google Scholar]
- 13. Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60(3):653‐658. [DOI] [PubMed] [Google Scholar]
- 14. Westhoff TH, Schmidt S, Gross V, et al. The cardiovascular effects of upper‐limb aerobic exercise in hypertensive patients. J Hypertens. 2008;26(7):1336‐1342. [DOI] [PubMed] [Google Scholar]
- 15. Maruf FA, Akinpelu AO, Salako BL. A randomized controlled trial of the effects of aerobic dance training on blood lipids among individuals with hypertension on a thiazide. High Blood Press Cardiovasc Prev. 2014;21(4):275‐283. [DOI] [PubMed] [Google Scholar]
- 16. Masroor S, Bhati P, Verma S, Khan M, Hussain ME. Heart rate variability following combined aerobic and resistance training in sedentary hypertensive women: a randomised control trial. Indian Heart J. 2018;70:S28‐S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chanudet X, de Lambert Cremeur G, Bonnevie L. Physical activity in hypertension management. Presse Med. 2006;35(6 Pt 2):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 18. Kelley GA, Kelley KS, Franklin B. Aerobic exercise and lipids and lipoproteins in patients with cardiovascular disease: a meta‐analysis of randomized controlled trials. J Cardiopulm Rehabil. 2006; 26(3):131‐139; quiz 140‐131, discussion 142‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081‐1093. [DOI] [PubMed] [Google Scholar]
- 20. Montero D, Roche E, Martinez‐Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre‐ and hypertensive subjects: a systematic review and meta‐analysis. Int J Cardiol. 2014;173(3):361‐368. [DOI] [PubMed] [Google Scholar]
- 21. Xiu‐xia LI, Ya Z, Yao‐long C, Ke‐hu Y, Zong‐jiu Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. 2015;119(4):503‐510. [DOI] [PubMed] [Google Scholar]
- 22. Yan P, Yao L, Li H, et al. The methodological quality of robotic surgical meta‐analyses needed to be improved: a cross‐sectional study. J Clin Epidemiol. 2019;109:20‐29. [DOI] [PubMed] [Google Scholar]
- 23. Higgins J, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duncan J, Farr J, Upton S, Hagan R, Oglesby M, Blair S. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA. 1985;254(18):2609‐2613. [PubMed] [Google Scholar]
- 25. He L, Wei WR, Can Z. Effects of 12‐week brisk walking training on exercise blood pressure in elderly patients with essential hypertension: a pilot study. Clin Exp Hypertens. 2018;40(7):673‐679. [DOI] [PubMed] [Google Scholar]
- 26. Molmen‐Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19(2):151‐160. [DOI] [PubMed] [Google Scholar]
- 27. Oliveira J, Mesquita‐Bastos J, Argel de Melo C, Ribeiro F. Postaerobic exercise blood pressure reduction in very old persons with hypertension. J Geriatr Phys Ther. 2016;39(1):8‐13. [DOI] [PubMed] [Google Scholar]
- 28. Maruf FA, Akinpelu AO, Salako BL. Self‐reported quality of life before and after aerobic exercise training in individuals with hypertension: a randomised‐controlled trial. Appl Psychol Health Well Being. 2013;5(2):209‐224. [DOI] [PubMed] [Google Scholar]
- 29. Wen H, Wang L. Reducing effect of aerobic exercise on blood pressure of essential hypertensive patients: a meta‐analysis. Medicine. 2017;96(11):e6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2(1):e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pitsavos C, Chrysohoou C, Koutroumbi M, et al. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hellenic J Cardiol. 2011;52(1):6‐14. [PubMed] [Google Scholar]
- 32. Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post‐exercise blood pressure, heart rate and heart‐rate variability. J Hum Hypertens. 2010;24(3):175‐182. [DOI] [PubMed] [Google Scholar]
- 33. Sabbahi A, Arena R, Elokda A, Phillips SA. Exercise and hypertension: uncovering the mechanisms of vascular control. Prog Cardiovasc Dis. 2016;59(3):226‐234. [DOI] [PubMed] [Google Scholar]
- 34. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e116‐e135. [DOI] [PubMed] [Google Scholar]
- 35. Kingsley JD, Figueroa A. Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging. 2016;36(3):179‐187. [DOI] [PubMed] [Google Scholar]
- 36. Yao L, Sun R, Chen Y‐L, et al. The quality of evidence in Chinese meta‐analyses needs to be improved. J Clin Epidemiol. 2016;74:73‐79. [DOI] [PubMed] [Google Scholar]
- 37. Ge L, Tian J‐H, Li Y‐N, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta‐epidemiological study. J Clin Epidemiol. 2018;93:45‐55. [DOI] [PubMed] [Google Scholar]
- 38. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med. 2017;167(1):40‐47. [DOI] [PubMed] [Google Scholar]
- 39. Tian J, Zhang J, Ge L, Yang K, Song F. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. 2017;85:50‐58. [DOI] [PubMed] [Google Scholar]
- 40. Farahani AV, Mansournia MA, Asheri H, et al. The effects of a 10‐week water aerobic exercise on the resting blood pressure in patients with essential hypertension. Asian J Sports Med. 2010;1(3):159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lima LG, Bonardi JT, Campos GO, et al. Combined aerobic and resistance training: are there additional benefits for older hypertensive adults? Clinics (Sao Paulo, Brazil). 2017;72(6):363‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sikiru L, Okoye G. Therapeutic effect of continuous exercise training program on serum creatinine concentration in men with hypertension: a randomized controlled trial. Ghana Med J. 2014;48(3):135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsuda K, Yoshikawa A, Kimura K, Nishio I. Effects of mild aerobic physical exercise on membrane fluidity of erythrocytes in essential hypertension. Clin Exp Pharmacol Physiol. 2003;30(5‐6):382‐386. [DOI] [PubMed] [Google Scholar]


