Abstract
High renal resistive index (RI) is observed in diabetes and is associated with poor patient survival, but whether it is primarily due to renal vascular resistance or systemic vascular alterations is unclear. The respective impact of kidney transplant from diabetic donors or to diabetic recipients on RI would shed some light on this issue. The objective of the study was to analyze the impact of donor and recipient diabetes on RI in order to understand the respective impact of the kidney and the vascular environment. The authors conducted a retrospective study in 1827 renal transplant recipients who received a kidney between 1985 and 2017, and had Doppler measurements at 3 months after transplant. Donor and recipient characteristics at the time of transplant and at 3 months were reviewed. Both donor diabetes and recipient diabetes were associated with RI in univariate analysis, but only recipient diabetes remained significantly associated in stepwise multivariate analyses (effect estimate on RI: +0.03 ± 0.005, P < 0.001). These findings were confirmed when RI was expressed as a binary variable using a cutoff of 0.75 (OR = 2.50 [1.77, 3.54], P < 0.001). Other determinants of RI were recipient characteristics (age, sex, systolic and diastolic blood pressure, and duration of dialysis). Donor characteristics were not associated with RI. Our results suggest that high RI observed in diabetic recipients shortly after transplant is primarily due to the new vascular environment, rather than to characteristics of the transplanted kidney. Therefore, RI reflects systemic rather than intra‐renal changes.
Keywords: diabetes mellitus, kidney transplant, renal resistive index, ultrasonography, vascular resistance
1. INTRODUCTION
More than a decade ago, high renal resistive index (RI) was identified as a powerful predictor of the risk of death in renal transplant recipients1, 2 and in atheromatous renovascular disease.3 However, the meaning of RI is not clearly understood. This parameter was originally interpreted as a reflection of renal vascular resistance, and studies indicated that it would increase as a result of decreasing cross‐sectional area of renal arterial bed.4 But it was also reported that changes in renal interstitial pressure, nephrosclerosis, interstitial fibrosis/tubular atrophy, or loss of peritubular capillaries and arteriolosclerosis result in RI changes.5, 6, 7, 8
In marked contrast, it was recently stated, based on theoretical concepts as well as experimental and clinical findings, that in recipients of renal transplants, RI primarily reflects recipient aortic stiffness rather than donor kidney characteristics.4, 9, 10 However, things are probably more complicated since it was found that donor age was associated with recipients RI11, 12 and with recipient aortic stiffness.13
It is interesting to notice that high RI is usually observed in patients with diabetes mellitus and in the setting of diabetic nephropathy.3, 6, 14, 15 Whether it could be the result of higher arterial stiffness or renal vascular or of parenchymal changes is not known.6 Transplant of kidneys from diabetic donors and/or to diabetic recipients could help to better understand the pathophysiology of RI by finding whether high RI is primarily due to (donor) kidney microvascular damage or (recipient) marked arterial stiffness.
In this retrospective study, we analyzed the impact of donor and recipient diabetes on RI at 3 months after transplant in a large cohort of renal transplant recipients; we also evaluated the role of other donor and recipient characteristics.
2. PATIENTS AND METHODS
2.1. Patient selection
We conducted a retrospective analysis of 2362 consecutive patients who received a renal transplant from October 1985 to October 2017 at the Tours University Hospital, France. Among them, 113 died or returned to dialysis within the three first months following transplant, and 422 patients were excluded because renal Doppler ultrasonography at 3 months was not available. Thus, 1827 patients were included in this study. Data were collected from the prospectively maintained institutional database of transplant patients of our hospital and the ASTRE database (CNIL agreement number: DR‐2012‐518). The study protocol was validated by the Ethics Committee in Human Research (Hôpital Bretonneau, CHU Tours, France).
At the time of transplant, the following variables were reviewed: donor age, sex, diabetes, double or single transplant, machine perfusion, and recipient age, sex, diabetes, graft rank, body mass index (BMI), and hemodialysis time before transplant. At the 3‐month visit after transplant, the following variables were reviewed: systolic, diastolic, and pulse arterial pressure, serum creatinine level, eGFR, (using MDRD equation), proteinuria (by a 24‐hour urine collection16), immunosuppressive induction and maintenance treatments, delayed graft function (DGF) after transplant, and renal resistive index. For double transplant, RI was the mean of both left and right graft RI value. Recipient diabetes was defined as diabetes diagnosed before the graft and did not include new‐onset diabetes after transplant (NODAT).
2.2. Doppler ultrasonography studies
For the measurement of renal resistive index, three ultrasound systems were used: Toshiba Aplio XG with PVT‐375BT probe, Esaote Technos MPX with probe, and Siemens Antares Premium Edition with CH5‐2 probe with vascular program for each examination.17 Peak systolic velocity (PSV) and end‐diastolic velocity (EDV) were measured during Doppler ultrasonography spectral analysis in renal interlobar arteries in each pole. RI was calculated with PSV and EDV by the following equation:
The mean of three consecutive measurements was used. Doppler ultrasonography studies were routinely performed at 3 months after transplant. Renal artery stenosis was ruled out at the time of measurement18 (the incidence of renal artery stenosis in our center is around 6% as previously published19). The results of other Doppler studies were not considered in this report.
2.3. Statistical analyses
All the variables had a normal distribution. Results are expressed as percentages or means ± standard deviations. Qualitative variables were compared using chi‐square test. Continuous variables between two groups were compared using Student's t test, after verifying equal standard deviations in each group.
In order to find potential confounding factors for differences in RI, characteristics of diabetic recipients and nondiabetic recipients, and characteristics of recipients of kidney from diabetic donors and nondiabetic donors were compared.
For multivariate analysis, we examined the effect of recipient and donor diabetes, as well as potential confounding factors, on RI as a continuous parameter using ANCOVA, and on RI as a binary variable using logistic regression. We used a multiple stepwise regression analysis, in which variables with a P‐value of 0.1 or more were removed from the models. To avoid collinearity between the variables, Pearson's correlation was assessed.
For a better estimation of the effect of blood pressure, we evaluated in multivariate analysis the effect of a variation of 10 mm Hg.
For RI as a binary variable, a cutoff of 0.75 was used. Indeed, studies consider 0.70 as the upper threshold of normal RI,20 whereas others showed that a RI greater than 0.80 was associated with poor allograft survival and death.1 Furthermore, 0.75 was the median of RI in patients who received a kidney from a diabetic donor.
A P‐value < 0.05 was considered statistically significant. Analyses were performed using the statistical software RStudio (RStudio Team, 2015, v1.0.153).
3. RESULTS
3.1. Baseline characteristics
Among these 1827 renal transplant recipients, 293 (16.3%) had diabetes mellitus before transplant and 105 (5.8%) received a kidney from a diabetic donor. Diabetes status was missing in 30 renal transplant recipients and 14 kidney donors. It was the first transplant for 1554 patients (85.1%), and 1732 patients (94.8%) received a cadaveric graft (Table 1). Regarding immunosuppression, induction was performed with anti‐interleukin‐2 receptor (45.5%) or thymoglobulin (53.2%), and methylprednisolone 250 mg before and after transplant. Maintenance immunosuppressive treatment included prednisone with a gradual tapering and mycophenolate mofetil (82.2%) or azathioprine (14.9%), associated with ciclosporin (39.4%), tacrolimus (56.2%), or mechanistic target of rapamycin (m‐TOR) inhibitors (6.4%; Table 1).
Table 1.
Baseline characteristics stratified with donor and recipient characteristics
| Overall | Recipient diabetes − | Recipient diabetes + | P | Donor diabetes − | Donor diabetes + | P | |
|---|---|---|---|---|---|---|---|
| Total patients | 1827 | 1504 | 293 | 1708 | 105 | ||
| Donor characteristics | |||||||
| Deceased donor (%) | 1732 (94.8) | 1413 (93.9) | 289 (98.6) | 0.002 | 1624 (95.1) | 105 (100.0) | 0.037 |
| Donor age (y) | 51.1 (17.6) | 49.4 (17.5) | 61.7 (13.9) | <0.001 | 50.3 (17.6) | 66.2 (10.5) | <0.001 |
| Donor with diabetes (%) | 105 (5.8) | 49.4 (17.5) | 29 (9.9) | 0.002 | 0 (0.0) | 105 (100.0) | <0.001 |
| Donor sex (% male) | 1093 (59.8) | 49.4 (17.5) | 156 (53.2) | 0.021 | 1026 (60.1) | 62 (59.0) | 0.916 |
| Recipient characteristics at time of transplant | |||||||
| Diabetes (%) | 293 (16.3) | 0 (0.0) | 293 (100.0) | <0.001 | 263 (15.7) | 29 (27.6) | 0.002 |
| Hemodialysis time (y) | 2.98 (3.40) | 3.07 (3.59) | 2.63 (2.18) | 0.061 | 3.00 (3.46) | 2.74 (2.36) | 0.462 |
| Age (y) | 51.2 (14.9) | 49.3 (14.8) | 61.8 (9.6) | <0.001 | 50.6 (14.8) | 62.6 (10.9) | <0.001 |
| Sex (% male) | 1165 (63.8) | 946 (62.9) | 199 (67.9) | 0.117 | 1086 (63.6) | 71 (67.6) | 0.465 |
| BMI (kg/m2) | 25.3 (4.90) | 24.6 (4.58) | 28.7 (5.29) | <0.001 | 25.2 (4.87) | 27.0 (5.23) | <0.001 |
| Graft rank (%) | 0.135 | 0.256 | |||||
| 0 | 1554 (85.1) | 1269 (84.4) | 260 (88.7) | 1447 (84.7) | 93 (88.6) | ||
| 1 | 232 (12.7) | 197 (13.1) | 31 (10.6) | 224 (13.1) | 8 (7.6) | ||
| 2 | 39 (2.1) | 36 (2.4) | 2 (0.7) | 35 (2.0) | 4 (3.8) | ||
| 3 | 2 (0.1) | 2 (0.1) | 0 (0.0) | 2 (0.1) | 0 (0.0) | ||
| Perfusion machine (%) | 274 (15.0) | 179 (11.9) | 95 (32.4) | <0.001 | 238 (13.9) | 35 (33.3) | <0.001 |
| Double transplant (%) | 26 (1.4) | 16 (1.1) | 10 (3.4) | 0.005 | 18 (1.1) | 8 (7.6) | <0.001 |
| Recipients characteristics at 3 mo | |||||||
| SBP (mm Hg) | 138.6 (15.9) | 137.3 (15.3) | 145.32 (17.0) | <0.001 | 138.4 (15.8) | 142.9 (16.6) | 0.011 |
| DBP (mm Hg) | 78.7 (10.8) | 79.3 (10.5) | 74.9 (11.5) | <0.001 | 78.9 (10.7) | 75.2 (11.9) | 0.002 |
| PP (mm Hg) | 59.9 (15.4) | 58.0 (14.2) | 70.4 (17.5) | <0.001 | 59.4 (15.2) | 67.7 (16.0) | <0.001 |
| DGF (%) | 342 (18.7) | 253 (16.8) | 81 (27.6) | <0.001 | 320 (18.8) | 21 (20.0) | 0.851 |
| eGFR MDRD (mL/min/1.73 m2) | 51.53 (19.56) | 52.08 (19.53) | 47.44 (19.41) | 0.001 | 52.08 (19.67) | 41.67 (14.22) | <0.001 |
| proteinuria (g/d) | 0.78 (8.04) | 0.82 (8.90) | 0.60 (0.63) | 0.734 | 0.79 (8.28) | 0.51 (0.47) | 0.785 |
| Tacrolimus (%) | 894 (56.2) | 722 (54.4) | 171 (71.5) | <0.001 | 827 (54.8) | 67 (79.8) | <0.001 |
| Ciclosporin (%) | 628 (39.4) | 548 (41.3) | 55 (23.0) | <0.001 | 616 (40.8) | 12 (14.3) | <0.001 |
| Steroids (%) | 1524 (95.8) | 1275 (96.2) | 230 (96.2) | 1.000 | 1441 (95.6) | 83 (98.8) | 0.255 |
| MMF (%) | 1308 (82.2) | 1086 (81.8) | 218 (91.2) | 0.001 | 1229 (81.5) | 79 (94.0) | 0.005 |
| Azathioprine (%) | 237 (14.9) | 201 (15.2) | 15 (6.3) | <0.001 | 235 (15.6) | 2 (2.4) | 0.002 |
| m‐TOR inhibitors (%) | 102 (6.4) | 85 (6.4) | 17 (7.1) | 0.786 | 93 (6.2) | 9 (10.7) | 0.152 |
| Thymoglobulin (%) | 971 (53.2) | 811 (54.0) | 136 (46.4) | 0.021 | 918 (53.8) | 49 (46.7) | 0.184 |
| IL‐2‐R antibodies (%) | 828 (45.5) | 670 (44.7) | 156 (53.4) | 0.008 | 762 (44.8) | 57 (54.8) | 0.058 |
| Resistive index | 0.69 (0.08) | 0.68 (0.08) | 0.76 (0.07) | <0.001 | 0.69 (0.08) | 0.74 (0.07) | <0.001 |
Values are mean (SD) or number (percentage) of patients.
BMI, body mass index; DBP, diastolic blood pressure; DGF, delayed graft function; IL‐2‐R, interleukin‐2 receptor; MMF, mycophenolate mofetil; m‐TOR, mechanistic target of rapamycin; PP, pulse pressure; SBP, systolic blood pressure.
Overall, 293 recipients had diabetes mellitus, and 105 recipients received a kidney from a diabetic donor.
With RI as a binary variable, 1345 patients (74%) had a RI of 0.75 or less. Among patients with diabetes, 120 (40%) had a RI ≤ 0.75. Among patients who received a diabetic kidney, 52 (50%) had a RI ≤ 0.75 (Table 2).
Table 2.
Baseline characteristics stratified with RI as a binary variable
| RI < 0.75 | RI > 0.75 | P | |
|---|---|---|---|
| Total patients | 1345 | 482 | |
| Donor characteristics | |||
| Deceased donor (%) | 1260 (93.7) | 472 (97.9) | <0.001 |
| Donor age (y) | 47.1 (16.6) | 62.4 (15.4) | <0.001 |
| Donor with diabetes (%) | 52 (3.9) | 53 (11.0) | <0.001 |
| Donor sex (% male) | 813 (60.4) | 280 (58.1) | 0.395 |
| Recipient characteristics at time of transplant | |||
| Diabetes (%) | 120 (9.1) | 173 (36.2) | <0.001 |
| Hemodialysis time (y) | 3.02 (3.57) | 2.88 (2.91) | 0.441 |
| Age (y) | 46.9 (14.0) | 63.4 (9.5) | <0.001 |
| Sex (% male) | 872 (64.8) | 293 (60.8) | 0.126 |
| BMI (kg/m2) | 24.7 (4.71) | 26.8 (5.12) | <0.001 |
| Graft rank (%) | 0.534 | ||
| 0 | 1141 (84.8) | 413 (85.7) | |
| 1 | 170 (12.6) | 62 (12.9) | |
| 2 | 32 (2.4) | 7 (1.5) | |
| 3 | 2 (0.1) | 0 (0.0) | |
| Perfusion machine (%) | 128 (9.5) | 146 (30.3) | <0.001 |
| Double transplant (%) | 13 (1.0) | 13 (2.7) | 0.012 |
| Recipients characteristics at 3 mo | |||
| SBP (mm Hg) | 137.0 (14.9) | 143.3 (17.6) | <0.001 |
| DBP (mm Hg) | 80.4 (10.1) | 73.7 (11.1) | <0.001 |
| PP (mm Hg) | 56.56 (13.16) | 69.6 (17.2) | <0.001 |
| DGF (%) | 0.66 (0.06) | 0.79 (0.04) | <0.001 |
| eGFR MDRD (mL/min/1.73 m2) | 53.89 (20.08) | 44.59 (16.07) | <0.001 |
| proteinuria (g/d) | 0.86 (9.45) | 0.56 (0.56) | 0.574 |
| Tacrolimus (%) | 640 (53.9) | 254 (62.7) | 0.002 |
| Ciclosporin (%) | 508 (42.8) | 120 (29.6) | <0.001 |
| Steroids (%) | 1136 (95.8) | 388 (95.8) | 1.000 |
| MMF (%) | 962 (81.0) | 346 (85.4) | 0.055 |
| Azathioprine (%) | 194 (16.4) | 43 (10.6) | 0.007 |
| m‐TOR inhibitors (%) | 59 (5.0) | 43 (10.6) | <0.001 |
| Thymoglobulin (%) | 729 (54.3) | 242 (50.2) | 0.134 |
| IL‐2‐R antibodies (%) | 592 (44.2) | 236 (49.2) | 0.067 |
| Resistive index | 0.66 (0.06) | 0.79 (0.04) | <0.001 |
Values are mean (SD) or number (percentage) of patients.
BMI, body mass index; DBP, diastolic blood pressure; DGF, delayed graft function; IL‐2‐R, interleukin‐2 receptor; MMF, mycophenolate mofetil; m‐TOR, mechanistic target of rapamycin; PP, pulse pressure; SBP, systolic blood pressure.
3.2. Univariate analysis
Renal resistive index at 3 months was significantly higher in diabetic than in nondiabetic patients (P < 0.001), and in recipients of diabetic kidney than in nondiabetic donor (P < 0.001). The other determinants of RI at 3 months in univariate analysis were recipient age, recipient BMI, systolic, diastolic, and pulse arterial pressure at 3 months, serum creatinine at 3 months, double transplant, tacrolimus, recipient sex, DGF, double transplant, and deceased donor (Tables S1 and S2).
With RI expressed as a binary variable, with a cutoff of 0.75, recipient diabetes was significantly associated with high RI (P < 0.001), as well as donor diabetes (P < 0.001). Deceased donor, higher donor, and recipient age, higher BMI, perfusion machine, double transplant, high systolic and pulse arterial pressure and low diastolic arterial pressure at 3 months, high serum creatinine at 3 months, DGF, tacrolimus, m‐TOR inhibitors, absence of ciclosporin, and absence of azathioprine were also associated with high RI (Table 2).
Of note, although recipient age and diabetes were associated with RI value, the interaction between age and diabetes was not linked with RI (P = 0.69), indicating there was no significant difference in the age‐RI relationship between diabetic and nondiabetic patients (Figure 1).
Figure 1.
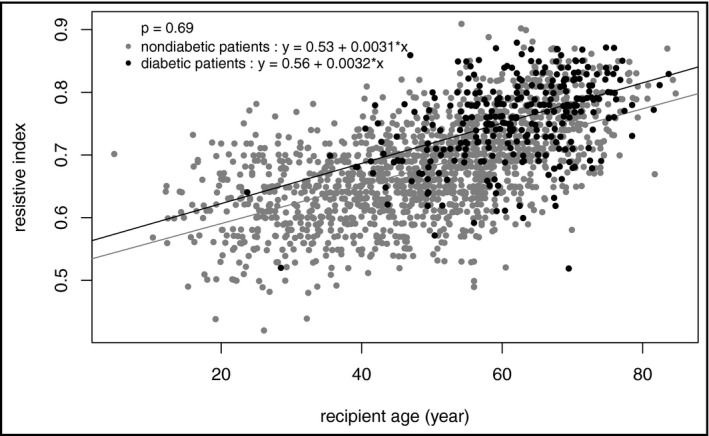
Relationship between RI and age in diabetic and nondiabetic patients
3.3. Multivariate analysis
A correlation of more than 0.7 was found between recipient age and donor age (r = 0.804), and between systolic arterial pressure and pulse pressure at 3 months (r = 0.775). Therefore, donor age and systolic blood pressure were removed from the analysis.
In multivariate analyses, after stepwise selection of the variables, RI was associated with recipient diabetes (effect estimate on RI: +0.03 ± 0.005, P < 0.001), but not with donor diabetes (Table 3).
Table 3.
Determinants of RI as a continuous variable in multivariate analysis
| Β ± SD | P | |
|---|---|---|
| Recipient diabetes | 0.03 ± 0.005 | <0.001 |
| Recipient age (y) | 0.003 ± 0.0001 | <0.001 |
| Double transplant | −0.03 ± 0.015 | 0.048 |
| PP (mm Hg) | 0.001 ± 0.0001 | <0.001 |
| DBP (mm Hg) | −0.001 ± 0.0001 | <0.001 |
| Recipient sex (male) | −0.01 ± 0.003 | <0.001 |
| Hemodialysis time (y) | 0.001 ± 0.0005 | 0.003 |
DBP, diastolic blood pressure; PP, pulse pressure.
Logistic regression was also performed with RI as a binary variable, with a cutoff of 0.75. High RI was associated with recipient diabetes (OR = 2.5 [1.77, 3.54], P < 0.001), but not with donor diabetes (Table 4).
Table 4.
Determinants of RI as a binary variable in multivariate analysis
| OR | 95% confidence interval | P | |
|---|---|---|---|
| Double transplant (%) | 0.30 | (0.11, 0.82) | 0.019 |
| Recipient diabetes (%) | 2.50 | (1.77, 3.54) | <0.001 |
| Recipient age (y) | 1.11 | (1.09, 1.13) | <0.001 |
| PP (×10 mm Hg) | 1.20 | (1.08, 1.33) | 0.001 |
| DBP (×10 mm Hg) | 0.70 | (0.60, 0.81) | <0.001 |
| Recipient sex (% male) | 0.68 | (0.51, 0.91) | 0.010 |
DBP, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
None of the other donor characteristics were associated with RI in multivariate analyses. The other determinants of RI in multivariate analysis were recipient age, double transplant, high PP, low DBP, and recipient sex, for RI as a continuous variable and as a binary variable. For RI as a continuous variable, hemodialysis time before graft was also associated with RI.
The results remained unchanged when using SBP instead of PP in the multivariate model.
4. DISCUSSION
The aim of this study was to analyze the impact of donor and recipient diabetes on RI after renal transplant. Whereas RI has been identified as a good prognostic marker for patients with renal artery stenosis,3 chronic kidney disease,21 and kidney transplant recipients,1, 2 its clinical signification is not clearly established. Indeed, for some authors, it is related to renal vascular resistance and to renal damages,4, 5 whereas for others, it is related to systemic vascular alterations.6, 22, 23
Some authors observed that RI was increased in patients with diabetic nephropathy.14, 15 Diabetic patients who undergo renal transplant have suffered for years from chronic glucotoxicity and therefore suffer from the vascular consequences of increased production of advanced glycation end products.24 These complications imply both systemic and renal vascularization; therefore, the impact of donor and recipient diabetes could help to understand whether high RI is related to renal or systemic vascular damages.
In the present study, we found that RI was associated with recipient diabetes in multivariate analyses, but not with donor diabetes. This finding seems consistent with the fact that RI is associated with systemic arterial disease, resulting in systemic arterial stiffness, but not with renal vascular resistance. Moreover, our results show that kidneys transplanted in a diabetic environment were associated with higher RI than kidneys transplanted in a nondiabetic environment, regardless of the donor characteristics, which confirms that the systemic vascular disease due to diabetes is associated with high RI, regardless of the renal impairment. Finally, we found that a kidney from a diabetic donor was not independently associated with higher RI than kidneys from a nondiabetic donor, which could mean that RI is not associated with renal vascular resistance or renal vascular damages due to diabetes.
Our study confirmed age as a strong determinant of RI.6, 12, 22 The association between RI and donor diabetes in univariate analyses could be explained by the fact that the patients who received diabetic kidneys were much older. However, there was no interaction between recipient age and diabetes: The age‐RI slope was not steeper in diabetic than in nondiabetic patients. The high correlation between recipient and donor age could explain the results of the studies finding an association between RI and donor age.
Systolic and diastolic blood pressures were associated with RI. This confirms the link between blood pressure and RI, although pulse pressure, which is known to be a determinant of systemic arterial stiffness, and to be associated with RI,6 was removed from our model because of the high correlation with systolic blood pressure. Hemodialysis time was also independently associated with RI, but not with RI as a binary variable. This seems coherent with the fact that hemodialysis and chronic kidney disease are a known risk factor of systemic arterial stiffness.25 We also found a relationship with female sex, and this association persisted despite numerous adjustments. This association was reported in the general population26 but not in transplant.2 The meaning of this association is unclear. Finally, RI was independently associated with double transplant, but only 26 patients in our cohort received two kidneys, which makes this result difficult to interpret. None of the other donor characteristics were associated with RI in multivariate analysis, which is consistent with the idea that it is not organ‐specific.22 Nevertheless, among other unknown determinants, it was also reported that genetics would play a role in RI and the heritability estimate of RI was 42% in the general population.26 Whether it is also true for RI in renal transplant is unknown.
Our study has many strengths. To our knowledge, it represents one of the largest cohorts of renal transplant recipients focused on the mechanism of increased RI in diabetic patients. RI has already been shown to be associated with recipient characteristics, not with donor characteristics in kidney transplant, but these findings have never been studied with diabetes, which harms both kidney and vascular environment. Despite the small amount of patients receiving a diabetic kidney, we selected the parameters for our multivariate analysis with a stepwise method in order to limit their number. Regarding Doppler indices, they were measured by experienced physicians, as these parameters are studied in our hospital since the early seventies.27
Our study also has limitations. It is a retrospective monocentric study, and therefore, our findings would need to be replicated. Despite the large number of patients in our cohort, the number of patients who received a diabetic kidney is only around 5% (105 patients), which could be a power limit for the analysis of the impact of kidneys from diabetic donors. Besides, diabetes status was missing in 30 renal transplant recipients and 14 kidney donors. It was also not possible to provide the inter‐observer variability of the RI measure due to the fact the RI was measured over a 30‐year period.
5. CONCLUSIONS
Our results show that increased RI is associated with recipient diabetes, but not with donor diabetes or any donor characteristics. This confirms the fact RI reflects systemic vascular changes (aortic stiffness), rather than intra‐renal changes, consistently with studies reporting associations of RI with extra‐renal vascular damage.6, 9, 22, 23 From a biophysical point of view, the stiffening of large arteries increases their characteristic impedance, which gets closer to the small artery characteristic impedance. This engenders less reflected pulsatile energy, and more pulsatility transmitted to the microcirculation, and therefore increases RI. A way to further explore these mechanisms would be a comparison of pulse wave velocity (PWv) with RI in diabetic patients to directly assess its link with aortic stiffness. Besides, further studies could benefit of ours in new evaluations of the prognostic impact of RI knowing that it reflects vascular environment and aortic stiffness.
CONFLICT OF INTEREST
The authors report no specific funding in relation to this research and no conflicts of interest relevant for this Review Paper to disclose.
Supporting information
de Freminville J‐B, Vernier L‐M, Roumy J, et al. Impact on renal resistive index of diabetes in renal transplant donors and recipients: A retrospective analysis of 1827 kidney transplant recipients. J Clin Hypertens. 2019;21:382–389. 10.1111/jch.13492
REFERENCES
- 1. Radermacher J, Mengel M, Ellis S, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 2. Naesens M, Heylen L, Lerut E, et al. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369(19):1797‐1806. [DOI] [PubMed] [Google Scholar]
- 3. Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal‐artery stenosis. N Engl J Med. 2001;344(6):410‐417. [DOI] [PubMed] [Google Scholar]
- 4. Bude RO, Rubin JM. Effect of downstream cross‐sectional area of an arterial bed on the resistive index and the early systolic acceleration. Radiology. 1999;212(3):732‐738. [DOI] [PubMed] [Google Scholar]
- 5. Murphy ME, Tublin ME. Understanding the Doppler RI. J Ultrasound Med. 2000;12:304‐314. [PubMed] [Google Scholar]
- 6. Heine GH, Reichart B, Ulrich C, Köhler H, Girndt M. Do ultrasound renal resistance indices reflect systemic rather than renal vascular damage in chronic kidney disease? Nephrol Dial Transplant. 2007;22(1):163‐170. [DOI] [PubMed] [Google Scholar]
- 7. Bigé N, Lévy PP, Callard P, et al. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol. 2012;13(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura N, Kimura H, Takahashi N, et al. Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol. 2015;19(6):1114‐1119. [DOI] [PubMed] [Google Scholar]
- 9. O’Neill WC. Renal resistive index: a case of mistaken identity. Hypertension. 2014;64(5):915‐917. [DOI] [PubMed] [Google Scholar]
- 10. Schwenger V, Keller T, Hofmann N, et al. Color Doppler indices of renal allografts depend on vascular stiffness of the transplant recipients. Am J Transplant. 2006;6(11):2721‐2724. [DOI] [PubMed] [Google Scholar]
- 11. Saracino A, Santarsia G, Latorraca A, Gaudiano V. Early assessment of renal resistance index after kidney transplant can help predict long‐term renal function. Nephrol Dial Transplant. 2006;21(10):2916‐2920. [DOI] [PubMed] [Google Scholar]
- 12. Rodrigo E, López‐Rasines G, Ruiz JC, et al. Determinants of resistive index shortly after transplantation: independent relationship with delayed graft function. Nephron Clin Pract. 2010;114(3):c178‐c186. [DOI] [PubMed] [Google Scholar]
- 13. Delahousse M, Chaignon M, Mesnard L, et al. Aortic stiffness of kidney transplant recipients correlates with donor age. J Am Soc Nephrol JASN. 2008;19(4):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boeri D, Derchi LE, Martinoli C, et al. Intrarenal arteriosclerosis and impairment of kidney function in NIDDM subjects. Diabetologia. 1998;41(1):121‐124. [DOI] [PubMed] [Google Scholar]
- 15. Ohta Y, Fujii K, Arima H, et al. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23(10):1905‐1911. [DOI] [PubMed] [Google Scholar]
- 16. Halimi J‐M, Laouad I, Buchler M, et al. Early low‐grade proteinuria: causes, short‐term evolution and long‐term consequences in renal transplantation. Am J Transplant. 2005;5(9):2281‐2288. [DOI] [PubMed] [Google Scholar]
- 17. Mutinelli‐Szymanski P, Caille A, Tranquart F, et al. Renal resistive index as a new independent risk factor for new‐onset diabetes mellitus after kidney transplantation: resistive index and risk of diabetes in kidney transplantation. Transpl Int. 2012;25(4):464‐470. [DOI] [PubMed] [Google Scholar]
- 18. Halimi JM, Al‐Najjar A, Buchler M, et al. Transplant renal artery stenosis: potential role of ischemia/reperfusion injury and long‐term outcome following angioplasty. J Urol. 1999;161(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 19. Ba S, Halimi J‐M, Al‐Najjar A, et al. Prognostic value of absent end‐diastolic flow within the first week following renal transplantation. Transplant Proc. 2009;41(2):645‐647. [DOI] [PubMed] [Google Scholar]
- 20. Tublin ME, Bude RO, Platt JF. The resistive index in renal doppler sonography: where do we stand? Am J Roentgenol. 2003;180(4):885‐892. [DOI] [PubMed] [Google Scholar]
- 21. Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long‐term progression in chronic renal failure. Nephrol Dial Transplant. 1997;12(7):1376‐1380. [DOI] [PubMed] [Google Scholar]
- 22. Seiler S, Colbus SM, Lucisano G, et al. Ultrasound renal resistive index is not an organ‐specific predictor of allograft outcome. Nephrol Dial Transplant. 2012;27(8):3315‐3320. [DOI] [PubMed] [Google Scholar]
- 23. Lerolle N. Please don’t call me RI anymore; I may not be the one you think I am!. Crit Care. 2012;16(6):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Q‐F, Sheng C‐S, Liu M, Li F‐H, Li Y, Wang J‐G. Arterial stiffness and wave reflections in relation to plasma advanced glycation end products in a Chinese population. Am J Hypertens. 2013;26(6):754‐761. [DOI] [PubMed] [Google Scholar]
- 25. Hermans M, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol JASN. 2007;18(6):1942‐1952. [DOI] [PubMed] [Google Scholar]
- 26. Ponte B, Pruijm M, Ackermann D, et al. Reference values and factors associated with renal resistive index in a family‐based population study. Hypertension. 2014;63(1):136‐142. [DOI] [PubMed] [Google Scholar]
- 27. Pourcelot L. Indications of Doppler’s ultrasonography in the study of peripheral vessels. Rev Prat. 1975;25(59):4671‐4680. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


