Abstract
Hypertension, the leading cause of cardiovascular morbidity and mortality, affects more than 1 billion people globally. The rise in mobile health in particular the use of mobile phones and short message service (SMS) to support disease management provides an opportunity to improve hypertension awareness, treatment, and control, in remote and vulnerable patient populations. The primary objective of this randomized controlled study was to assess the effect of active (with hypertension specific management SMS) or passive (health behaviors SMS alone) on the difference in blood pressure (BP) reduction between the active and passive SMS groups in hypertensive Canadian First Nations people from six rural and remote communities. Pragmatic features of the study included shifting of BP measures to non‐medical health workers. Despite an overall reduction in BP over the study, there was no difference in the BP change between groups from baseline to final for systolic 0.8 (95% CI −4.2 to 5.8 mm Hg) or diastolic −1.0 (95% CI −3.7 to 1.8 mm Hg, P = 0.5) BP. Achieved BP control was 37.5% (25.6%‐49.4%, 95% CI) in the active group and 32.8% (20.6%‐44.8%, 95% CI) in the passive group (difference in proportions −4.74% (−21.7% to 12.2%, 95% CI, P = 0.6). The study looked at changes in health services delivery, mobile health technologies, and patient engagement to support better management of hypertension in Canadian First Nations communities. The active hypertension specific SMS did not lead to improvements in BP control.
Keywords: chronic disease, disease management, health services, hypertension, indigenous, telemedicine, text messaging
Summary
The Canadian randomized trial of the DREAM‐GLOBAL study did not demonstrate that SMS specific for blood pressure (BP) control improved BP control compared to health behaviors change messages alone.
Novelty and Significance.
What is New?
Guidelines‐based blood pressure (BP) lowering study with mobile health technologies
Guidelines‐based SMS text messages to help with BP lowering
BP measurements by non‐medical health workers transmitted electronically to the health care provider and patient.
What is Relevant?
The program demonstrated a program for hypertension management that was community‐based and implementable in a low‐resource setting
Use of mobile technology and SMS to loop in members of the care team and increase patient engagement
1. INTRODUCTION
Hypertension is the leading cause of morbidity and mortality affecting more than 1 billion people worldwide, responsible for 10 million deaths annually1 as well as cardiovascular disease, kidney disease, and dementia.2, 3, 4 Despite improved awareness and treatment rates, ethnic and geographic factors can contribute to lower control rates of blood pressure (BP) associated with cardiovascular risk such as stroke.5 In Canada, improved management of hypertension nationally resulted in the awareness treatment and control of hypertension improving from 16% in 1990 to 66%,6, 7 associated with Improved cardiovascular outcomes.8 Regional variations in BP control rates exist9 and are linked to disparities in the social determinants of health, as well as disparities in health care availability, utilization, and outcomes related to race and ethnicity.10
Poorer health outcomes in Indigenous communities than in the general Canadian population are linked to inequities in the social determinants of health.11 In Canadian Indigenous populations, the hypertension prevalence is similar to or lower than the rest of the country, but average BP are higher, indicating a differential with the rest of the country for measurement and treatment.12, 13, 14, 15, 16 A framework for discussion on how to improve the prevention, management, and control of hypertension from 2011 to 2020 describes this situation and calls for Indigenous populations to have similar rates of BP awareness treatment and control as the rest of the population.17 The Global Alliance for Chronic Diseases (GACD), funds, coordinates, and facilitates global collaborations in implementation research, and their first round of sponsored research focused on improving the management of hypertension in lower‐ and middle‐income countries (LMICs) and vulnerable populations in high‐income countries such as Canada's First Nations community.18, 19
The Diagnosing hypeRtension‐Engaging Action and Management in Getting LOwer BP in Aboriginal and LMICs (DREAM‐GLOBAL) study was designed to meet the goals of the GACD to improve BP control in people with uncontrolled hypertension in Canadian First Nations communities and in Tanzania, combining innovations in health services delivery, mobile health technologies, and patient engagement. The primary objective of the study was to compare the effect on BP control of evidence‐based short message service (SMS) linked to accurately measured BP.
2. METHODS
This manuscript describes the results of the Canadian branch of the study. DREAM‐GLOBAL was a multicenter double blind parallel group study conducted in First Nations populations living on reserve in six different communities in three provinces of Canada. The full protocol has been previously published.20 The details of how participating First Nations communities were identified and assessed for research readiness have been previously described.20, 21 Participants were randomized into one of two parallel groups to receive either active and passive, or only passive SMS. The passive SMS described healthy lifestyle and behavior changes. Messages were tested for cultural safety and understanding in the target population.22 The active messages included information on the management of hypertension as well as advice to follow‐up with the participant's health care provider if the measured BP was above target. Individual BP measurements were taken by community health workers using an automated BP device with Bluetooth transmission capability. This allowed for transmission of accurate BP measurements from patients in isolated and underserviced populations to their health care providers.
All SMS text messages were derived from the Hypertension Canada Clinical Practice Guidelines and modified with community input to make them culturally sensitive and specific through a process that has been published.22 There were 12 active messages explaining the importance of BP control and the rationale for medical therapy. Twenty‐six passive messages included healthy lifestyle and behavior change advice for diet. Messages were sent twice weekly at 11 am (to avoid holidays) on Mondays and Thursdays.
Participants were adults aged 18 or over living on reserve with uncontrolled hypertension, and on or off of medications. Eligible participants were stable on their current dose of antihypertensive (if treated) for at least 8 weeks and were able to complete informed consent. Participants also had to either have a mobile phone capable of receiving SMS text messages or be willing to carry and learn to use a basic flip phone for the study duration. They also had to have a current primary health care provider. Exclusion criteria included controlled BP on medication, or BP > 180/110 mm Hg, or participation in other trials. Community health workers, usually the Community Health Resource (CHR) a non‐medical health worker,23 or the Home and Community Care nurse did the recruitment. Training to carry out the study was done in each community by study team members (ST, NP) at planned education sessions. These also included sessions on clinical practice guidelines for hypertension management, open to all health care providers. Community education and recruitment efforts included posters put up in administrative offices and where possible dissemination through local media including radio information sessions, and newsletters.
To screen and exclude people with controlled BP already on antihypertensive therapy, BP was measured by the CHR or the Home and Community Care nurse with the BpTRU (BpTRU Medical Devices Ltd, Coquitlam, BC, Canada) device. This device takes 6 readings 1 minute apart, discards the first reading and averages the last 5. Appropriate training on using this device was provided by the study team (NP). Uncontrolled BP was defined as (≥140/90 mm Hg or ≥130/80 mm Hg with diabetes) on or off of medications. If the BP was controlled at the screening visit and the participant was not taking medications but was at higher risk of developing hypertension due to older age, obesity, or diabetes, or a family history of hypertension, they were also invited to participate. This was also in accordance with the community leadership's request to be as inclusive as possible with recruitment. If their BP was elevated during the first 2 months of measurements, they would be enrolled in the study. If their BP was controlled during this time, their BP results were collected as part of a pilot BP finding study, and they would have their BP measured and receive the SMS text messages as well.
Participation in the DREAM‐GLOBAL research program required registration into the study database. This was done in each community with the assistance of the CHR or Home and Community Care nurse. Registration included linking the participant's cell phone to receive the study SMS text messages. The primary care health care practitioner's fax number and phone number were also required: the fax number to allow the DREAM‐GLOBAL central server to fax information from BP measurements throughout the study and the phone number to include in text messages (for participants randomized to active messages) if a BP reading was above target. At registration, randomization occurred automatically and participants began to receive SMS twice weekly.
Blood pressure measurement throughout the study was performed by the CHR or local Home and Community Care nurses. The device used was the A&D UA‐767PBT‐C (A&D Medical, San Jose, CA) monitor and Bluetooth paired with a smartphone to identify listed study participants and to facilitate the wireless transfer of the BP to the central server. The Home and Community Care nurse or CHR after a 2‐day training session, were able to recruit, consent, register participants, and take three BP readings according to guidelines with the automated oscillometric BP device for each participant.20 The baseline BP was defined as the mean of all readings from the A&D device in the first 2 months after randomization. The BP measurement was typically performed in the office of the CHR or Nurse in the Band's health center.
To avoid regression to the mean which could arise from using the screening BP for the baseline, the baseline BP was defined as the mean of BP readings in the first 2 months and not the screening BP. All participants with uncontrolled BP in the first 2 months of the study were included in the analysis, including those participants who were not defined as hypertensive at the screening BP. The study took place on six reserves in Canada: in Northern Ontario, Quebec and New Brunswick.
Blood pressure management was provided by the patient's usual health care provider team which could be a local physician, nurse practitioner, or sometimes both. BP results from each visit were transmitted from the study server, to the participant's local health care practitioner by fax and included relevant clinical practice guidelines. For safety, the CHR was trained to be able to suggest actions based on the BP according to a list of BP levels graded by urgency.
Details on how the communities became part of the study, local health care provider engagement and support, and the training aspects have been previously reported.20 Details on how the messages were created have been previously published as has the discussion on patient engagement and integrating into community health services.20, 21, 22, 24 A summary of the DREAM‐GLOBAL intervention using the mobile health (mHealth) evidence reporting and assessment (mERA) checklist can be found in the Table S1, 25 in accordance with the CONSORT‐EHEALTH recommendations.26
The study team and researchers included expertise appropriate to the intervention including clinical trials with Indigenous communities (ST), hypertension measurement and management (NC, ST), program management and technical knowledge (CM), participatory health research, Indigenous community health and research ethics, consequences of colonization and the Indian Residential Schools on Indigenous health (MM), and clinical trials design and statistics (GW, JB).27
The primary outcome was the difference in systolic and diastolic BP from the baseline period to the last 2 months of measurement between randomized groups. Secondary outcomes included the proportion with controlled BP. To assess the balance between explanatory and pragmatic components of this controlled study, the PRECIS‐2 tool was used.28 This tool assesses how explanatory or pragmatic an intervention is and where it would be along the continuum, for example, indicating whether the intervention can work only under ideal or under usual conditions.
The sample size calculation has been previously published.20 The goal was to recruit 176 participants per group to provide 97% power to find a mean systolic BP difference of 4.8 mm Hg. Randomization was done in blocks of 2 and 4 by community. The extra power was built in to allow for under‐recruitment and a smaller difference in BP s between the groups. All patients who were hypertensive at the baseline readings in the first 2 months were included in the efficacy and safety analyses. The data analysis was performed with SAS software for windows version 9.3, SAS Institute Inc, Cary, NC, USA. Continuous results were expressed as means and standard deviations (SD). Patients were analyzed by intention‐to‐treat using analysis of variance. Ethics approval was obtained from the Sunnybrook Health Sciences Centre Research Ethics Board (approval #: 953‐2013) as well as the Cree Board of Health and Social Services of James Bay, Ontario; Manitoulin Anishnabek Research Review Committee; University of Calgary (REB13‐0573); Queen's University Health Sciences and Affiliated Teaching Hospitals (DMED‐1603‐13. The study was carried out according to the principles of Good Clinical Practice, the Declaration of Helsinki and the Tri‐Council Policy Statement on ethical conduct for research involving humans.29, 30, 31 The study adhered to the principles of Ownership, Control, Access, and Possession (OCAP).32 The technology and movement of data adhered to the principles of the Personal Information Protection and Electronic Documents Act (PIPEDA).
3. RESULTS
Recruitment was initiated in the first community February of 2014 and in the last community June 2014. Recruitment continued until December 2015, and participants were followed for 1 year or until January 2017. A total of 301 participants were assessed for eligibility at the six participating communities. Fifty‐eight were excluded for controlled BP, six on no therapy with no risk factors for hypertension and the rest on hypertensive therapy. Two hundred and forty‐three were registered to the program (see Figure 1 consort diagram). One hundred and sixteen were hypertensive at the time of screening. Within the first 2 months of follow‐up, nine of these participants had consistently controlled BP. Thirty‐five of the participants who had controlled BP at screening were found to be hypertensive during the first 2 months leaving 142 participants (see Table 1 for breakdown by randomization group). No participants withdrew from the study. Over the course of the study, 20 participants were lost to follow‐up with no BP readings after the first 2 months. This left 122 participants, 64 in the active and 58 in the passive treatment groups. There were two deaths and two hospitalizations in the study participants and no reports of hypotension. In the treatment arm, one patient died from a pre‐existing cancer and one from a car accident (as a passenger). There were two hospitalizations: a stroke and an MI in patients in the control and treatment arm, respectively. Both made complete recoveries and remained in the study.
Figure 1.
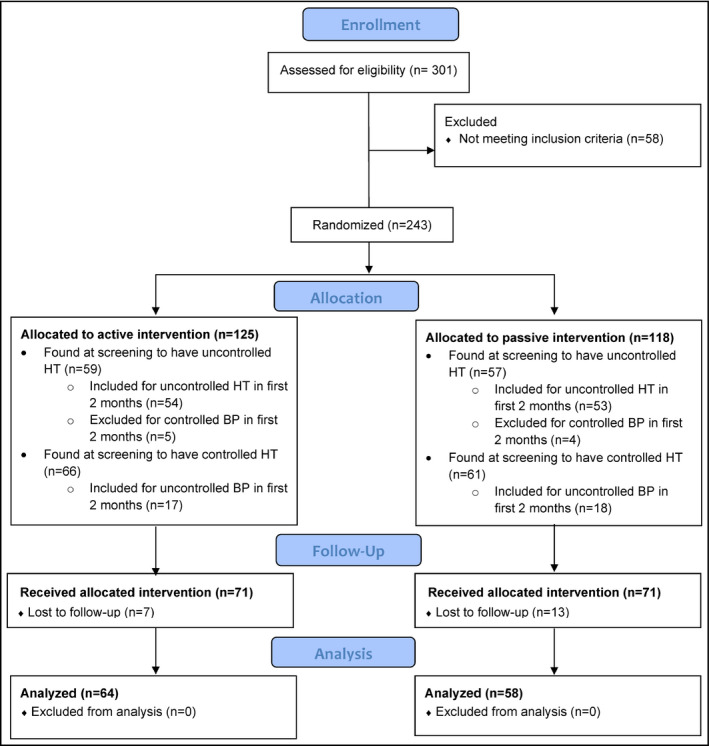
CONSORT Flow Diagram—DREAM‐GLOBAL Study (Canada)
Table 1.
Participant breakdown by randomization group
| Active messages N = 71 | Passive messages N = 71 | |
|---|---|---|
| Female/male | 34/37 | 36/35 |
| Age, years | 48.7 ± 12.8 | 49.1 ± 13.1 |
| Known hypertension | 56% | 58% |
| Family history of hypertension | 63% | 62% |
| Diabetes | 38% | 34% |
| Family history of diabetes | 70% | 63% |
| Nonsmoker % | 40% | 18% |
| Current smoker % | 44% | 69% |
| Former smoker % | 14% | 12% |
| Height, cm | 167.9 ± 9.3 | 166.5 ± 10.2 |
| Weight, kg | 88.8 ± 19.1 | 86.5 ± 24.6 |
| Waist, cm | 108.9 ± 15.9 | 109.5 ± 15.1 |
| Hip, cm | 113.6 ± 13.1 | 111.2 ± 13.2 |
No statistical significance between groups.
Overall, the BP lowering for both groups over the study was similar (see Table 2). The mean systolic BP difference between groups from baseline to final was 0.8 (95% CI −4.2 to 5.8 mm Hg) and diastolic −1.0 (95% CI −3.7 to 1.8 mm Hg, P = 0.5). Achieved BP control was 37.5% (25.6%‐49.4%, 95% CI) in the active group and 32.8% (20.6%‐44.8%, 95% CI) in the passive group (difference in proportions −4.74% (−21.7% to 12.2%, 95% CI, P = 0.6).
Table 2.
Blood pressure change through study by randomization to active or passive short message service text messages
| Active messagesN = 64 | Passive messagesN = 58 | P‐value | |
|---|---|---|---|
| Change in systolic blood pressure mm Hg | −5.3 (±14) | −5.9 (± 14) | 0.9 |
| Change in diastolic blood pressure mm Hg | −2.9 (±8) | −1.9 (±9) | 0.5 |
| Baseline systolic blood pressure mm Hg | 143 (±12) | 145 (±16) | 0.4 |
| Follow‐up systolic blood pressure mm Hg | 137 (±14) | 138 (±13) | 0.6 |
| Baseline diastolic blood pressure mm Hg | 84 (±12) | 86 (±10) | 0.2 |
| Follow‐up diastolic blood pressure mm Hg | 81 (±10) | 84 (±10) | 0.06 |
Results for the PRECIS‐2 analysis of balance between explanatory and pragmatic components can be found in Table 3. The study falls midway between explanatory and pragmatic in the continuum for most domains with methodologic domains more explanatory and the primary outcome of interest to participants scoring more pragmatic. None of the variables were very explanatory, or very pragmatic. The results can be visualized on the PRECIS‐2 wheel in Figure 2.
Table 3.
PRECIS‐2—Scoring for DREAM‐GLOBAL Study
| Domain | Criteria | Score | Rationale |
|---|---|---|---|
| 1. Eligibility Criteria | To what extent are the participants in the trial similar to those who would receive this intervention if it was part of usual care? | 3 | Participants are the same as part of usual care, and there are exclusion criteria listed |
| 2. Recruitment | How different are the settings of the trial from the usual care setting? | 2 | Community screening efforts for hypertension were held to recruit. CHRs also referred individuals to the program |
| 3. Setting | How different are the settings of the trial from the usual care setting? | 2 | Task shifting with BP measurement in community not HCP's office, some satellite sites for follow‐up visits |
| 4. Organization | How different are the resources, provider expertise, and the organization of care delivery in the intervention arm of the trial from those avail‐ able in usual care? | 2 | Due to nature of task shifting, the care is organized and delivered differently than usual care (usual care and resource has been shifted), extra training for those taking BPs |
| 5. Flexibility (delivery) | How different is the flexibility in how the intervention is delivered and the flexibility anticipated in usual care? | 3 | Flexibility in delivery of care is similar as usual care, particularly with follow‐up visits and delivery |
| 6. Flexibility (adherence) | How different is the flexibility in how participants are monitored and encouraged to adhere to the intervention from the flexibility anticipated in usual care? | 3 | Flexibility in adherence is elevated from usual care with task shifting and calls from CHR's for BP monitoring visits |
| 7. Follow‐up | How different is the intensity of measurement and follow‐up of participants in the trial from the typical follow‐up in usual care? | 2 | Follow‐up every 3 mo is likely more frequent than usual care |
| 8. Primary Outcome | To what extent is the trial's primary outcome directly relevant to participants? | 4 | Primary outcome of achieving BP control is important to participants; knowledge gained from patient perspective is also relevant |
| 9. Primary Analysis | To what extent are all data included in the analysis of the primary outcome? | 2 | BPs in primary analysis were included (no patients loss to follow‐up). First 2 and last 2 BPs used. Intermediate measures were not used for baseline or final BP. Some data not used to exclude patients with controlled BP on therapy |
1, More explanatory; 5, More pragmatic.
Figure 2.
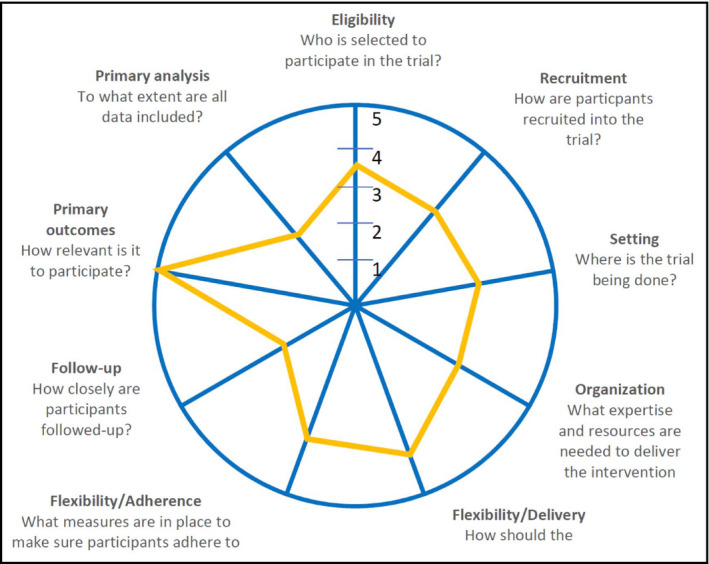
PRECIS‐2 Wheel
4. DISCUSSION
The results of the Canadian arm of the DREAM‐GLOBAL study demonstrated that guidelines‐based BP lowering with mobile health technologies, with changes in health service delivery, could be evaluated in a randomized controlled study in First Nations communities. While recruitment was below that targeted, participants were recruited, consented, and registered into the study's technology by community members, under direction from community leadership.
Blood pressure results overall showed a fall throughout the study in both groups but there was no difference between the groups. The main study hypothesis that active text messages would lead to more BP lowering than the passive messages alone was not proven.
Efforts were made to reduce the impact of regression to the mean. This phenomenon can happen when large or small measurements are followed by measurements closer to the mean and make natural variation in repeated measures look like real change.33 To reduce this effect, the baseline BP was not the screening BP reading. The baseline BP was the mean of readings taken over the first 2 months. The BP fall across all participants from this baseline period to study end is therefore more clinically significant.
The DREAM‐GLOBAL study tested whether a BP lowering program using mobile technologies and reassigning the responsibility of BP measurement to non‐medical health workers could be implemented successfully in real world conditions. Real world challenges included communities across a vast geography, reliance on trained community personnel to manage recruitment, measurement, and follow‐up. The study was able to maintain good internal and external validity and by adapting to real world conditions with pragmatic components increased the potential for sustainability and scalability of the intervention.34 Although SMS text messaging is more widespread in research since the DREAM‐GLOBAL protocol was initiated in 201235, the DREAM‐GLOBAL study is unique in comparing disease‐specific active messages compared to passive health behaviors text messages alone. It was hoped that the active messages would help to supplement participant knowledge and understanding provided by the local health care providers, and subsequently improve BP lowering through better adherence and greater engagement with health care providers. Both types of text messages had an equal impact and may have been in part responsible for the BP fall in the study, but their impact cannot be separated from the context of the entire study setup.
Study limitations included a lower recruitment than planned. Recruitment was conducted by non‐study personnel on reserves and reflected the interest, abilities, and time available by the CHR and local Home and Community Care nurse as well as other competing priorities on each reserve that would arise over the course of the study. In one respect, the recruitment and registration process demonstrated the initial community commitment to the study but over time, in all but two of the communities, recruitment lagged targets, and was only boosted when study personnel were on site during periodic visits. The intervention was delivered as it would have been in normal practice by staff with typical experience and the use of routinely available equipment.27
In conclusion, the Canadian randomized trial of the DREAM‐GLOBAL study demonstrated that a BP lowering program combining innovations in health services delivery, mobile health technologies, and patient engagement could be implemented in real world conditions, in First Nations communities. There was, however, no difference in BP lowering with the active compared to the passive text messages.
CONFLICT OF INTEREST
NRCC is a paid consultant to the Novartis Foundation to support their program to improve hypertension control in low‐ to middle‐income countries which includes travel support for site visits and a contract to develop a survey (2016‐2017). NRCC has provided paid consultative advice on accurate BP assessment to Midway Corporation (2016) and is an unpaid member of World Action on Salt and Health (WASH).
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the health care staff and leadership of the following individuals for their contributions to this participatory study and their insightful advice and wisdom: Eric Contant, Marcia Barron, Roger Beaudin, Valerie Beaudin, David Dannenbaum, Amit Garg (DSMB), Margaret Moy Lum‐Kwong, Marlene Nose, Gail Shawanda, Mary Jo Wabano, Olvie Li, Joan Wentworth, and Pamela Williamson.
Tobe SW, Yeates K, Campbell NRC, et al. Diagnosing hypertension in Indigenous Canadians (DREAM‐GLOBAL): A randomized controlled trial to compare the effectiveness of short message service messaging for management of hypertension: Main results. J Clin Hypertens. 2019;21:29‐36. 10.1111/jch.13434
Funding information
The DREAM‐GLOBAL Study was funded by the Canadian Institute of Health Research (CIHR), Grand Challenges Canada (GCC), and International Development Research Centre (IDRC).
Trial Registration: Clinicaltrials.gov NCT02111226; https://clinicaltrials.gov/ct2/show/NCT02111226
[Correction updated on December 17, 2018, after initial online publication: The last author's degree was updated from “PhD” to “MSc”.]
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1· 25 million people. Lancet. 2014;383(9932):1899‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23(2):116‐124. [DOI] [PubMed] [Google Scholar]
- 4. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934‐2941. [DOI] [PubMed] [Google Scholar]
- 5. Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37(5):1171‐1178. [DOI] [PubMed] [Google Scholar]
- 6. Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10(10 Pt 1):1097‐1102. [DOI] [PubMed] [Google Scholar]
- 7. McAlister FA, Wilkins K, Joffres M, et al. Changes in the rates of awareness, treatment and control of hypertension in Canada over the past two decades. Can Med Assoc J. 2011;183(9):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell NRC, Brant R, Johansen H, et al. Increases in antihypertensive prescriptions and reductions in cardiovascular events in Canada. Hypertension. 2009;53(2):128‐134. [DOI] [PubMed] [Google Scholar]
- 9. Mohan S, Chen G, Campbell NR, Hemmelgarn BR. Regional variations in not treating diagnosed hypertension in Canada. Can J Cardiol. 2010;26(8):409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens. 2017;19(10):1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tobe SW, Maar M, Roy MA, Warburton DER. Preventing cardiovascular and renal disease in Canada's Aboriginal Populations. Can J Cardiol. 2015;31:1124‐1129. [DOI] [PubMed] [Google Scholar]
- 12. Foulds HJ, Warburton DE. The blood pressure and hypertension experience among North American Indigenous populations. J Hypertens. 2014;32(4):724‐734. [DOI] [PubMed] [Google Scholar]
- 13. King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374(9683):76‐85. [DOI] [PubMed] [Google Scholar]
- 14. Anand SS, Yusuf S, Jacobs R, et al. Risk factors, atherosclerosis, and cardiovascular disease among Aboriginal people in Canada: the Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE‐AP). Lancet. 2001;358(9288):1147‐1153. [DOI] [PubMed] [Google Scholar]
- 15. Reading JL, Kmetic A, Gideon V. First Nations wholistic policy and planning model. Discussion Paper for the World Health Organization Commission on Social Determinants of Health. Ottawa: Assembly of First Nations; 2007. [Google Scholar]
- 16. Loppie Reading C, Wien F. Health inequalities and the social determinants of Aboriginal peoples’ health: National Collaborating Centre for Aboriginal Health. Prince George, BC; 2009. [Google Scholar]
- 17. Campbell NR, Hemmelgarn BR. New recommendations for the use of ambulatory blood pressure monitoring in the diagnosis of hypertension. CMAJ. 2012;184:633‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobe SW, the Global Alliance for Chronic Diseases Hypertension Research Teams With the World Hypertension League . The Global Alliance for Chronic Diseases Supports 15 Major Studies in Hypertension Prevention and Control in Low‐ and Middle‐Income Countries. J Clin Hypertens. 2016;18(7):600‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riddell MA, Edwards N, Thompson SR, et al. Developing consensus measures for global programs: lessons from the Global Alliance for Chronic Diseases Hypertension research program. Global Health. 2017;13(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeates K, Campbell N, Maar MA, et al. The Effectiveness of text messaging for detection and management of hypertension in indigenous people in Canada: protocol for a randomized controlled trial. JMIR Res Protoc. 2017;6(12):e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maar M, Yeates K, Barron M, et al. I‐RREACH: an engagement and assessment tool for improving implementation readiness of researchers, organizations and communities in complex interventions. Implement Sci. 2015;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maar MA, Yeates K, Toth Z, et al. Unpacking the black box: a formative research approach to the development of theory‐driven, evidence‐based, and culturally safe text messages in mobile health interventions. JMIR Mhealth Uhealth. 2016;4(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lavallée C, James CA, Robinson EJ. Evaluation of a community health representative program among the Cree of northern Quebec. Can J Public Health 1991;82(3):181‐184. [PubMed] [Google Scholar]
- 24. Maar MA, Yeates K, Perkins N, et al. A framework for the study of complex mHealth interventions in diverse cultural settings. JMIR Mhealth Uhealth. 2017;5(4):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agarwal S, LeFevre AE, Lee J, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. [DOI] [PubMed] [Google Scholar]
- 26. Eysenbach G, CONSORT‐EHEALTH Group . CONSORT‐EHEALTH: improving and standardizing evaluation reports of Web‐based and mobile health interventions. J Med Int Res. 2011;13(4):e126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454‐463. [DOI] [PubMed] [Google Scholar]
- 28. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS‐2 tool: designing trials that are fit for purpose. bmj. 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 29. Health C . International Conference on Harmonization (ICH) Guidance E6: Good Clinical Practice: Consolidated Guidelines; 1997.
- 30. World Medical Association . World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. From the 59th World Medical Association Assembly, Seoul; 2008.
- 31. Canadian Institutes of Health Research NSaERCoC, and Social Sciences and Humanities Research Council of Canada . Tri‐Council Policy Statement. Ethical Conduct for Research Involving Humans. CIHR, NSERC, SSHRC of Canada; 2014.
- 32. Boffa J, King M, McMullin K, Long R. A process for the inclusion of Aboriginal People in health research: lessons from the Determinants of TB Transmission project. Soc Sci Med. 2011;72(5):733‐738. [DOI] [PubMed] [Google Scholar]
- 33. Barnett AG, Van Der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2004;34(1):215‐220. [DOI] [PubMed] [Google Scholar]
- 34. Loudon K, Zwarenstein M, Sullivan F, Donnan P, Treweek S. Making clinical trials more relevant: improving and validating the PRECIS tool for matching trial design decisions to trial purpose. Trials. 2013;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta‐analysis. JAMA Intern Med. 2016;176(3):340‐349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


