Abstract
High blood pressure is the world’s leading cause of death, but despite treatment for hypertension being safe, effective, and low cost, most people with hypertension worldwide do not have it controlled. This article summarizes lessons learned in the first 2 years of the Resolve to Save Lives (RTSL) hypertension management program, operated in coordination with the World Health Organization (WHO) and other partners. Better diagnosis, treatment, and continuity of care are all needed to improve control rates, and five necessary components have been recommended by RTSL, WHO and other partners as being essential for a successful hypertension control program. Several hurdles to hypertension control have been identified, with most related to limitations in the health care system rather than to patient behavior. Treatment according to standardized protocols should be started as soon as hypertension is diagnosed, and medical practices and health systems must closely monitor patient progress and system performance. Improvement in hypertension management and control, along with elimination of artificial trans fat and reduction of dietary sodium consumption, will improve many aspects of primary care, contribute to goals for universal health coverage, and could save 100 million lives worldwide over the next 30 years.
Keywords: clinical management of high blood pressure (HBP), combination therapy, general, hypertension, pharmacologic (drug) therapy, primary care issues
1. INTRODUCTION
High blood pressure kills 10.7 million people per year worldwide, more than all infectious diseases combined, and is the leading cause of death,1 but less than 15% of people with hypertension worldwide have it controlled.2 Together with elimination of artificial trans fat and reduction of dietary sodium consumption, improvement in hypertension management could save 100 million lives worldwide in 30 years.3, 4
Resolve to Save Lives (RTSL), a 5‐year, $225 million initiative based at the global health nonprofit organization Vital Strategies operated in coordination with the World Health Organization (WHO) and other partners, supports organizations and partners to help low‐ and middle‐income countries prevent cardiovascular disease. RTSL along with WHO is currently assisting low‐ and middle‐income countries to develop, implement, and expand national and subnational hypertension control programs (Figure 1), supporting others through the Pan American Health Organization, providing one‐time catalytic grant support through LINKS (https://www.linkscommunity.org) in 6 other countries (Bhutan, Cameroon, Philippines, Rwanda, Timor‐Leste, and Uganda), and working with WHO and other partners to provide tools for any country which wants to improve outcomes for people with hypertension (Table S1, web annex).
Figure 1.
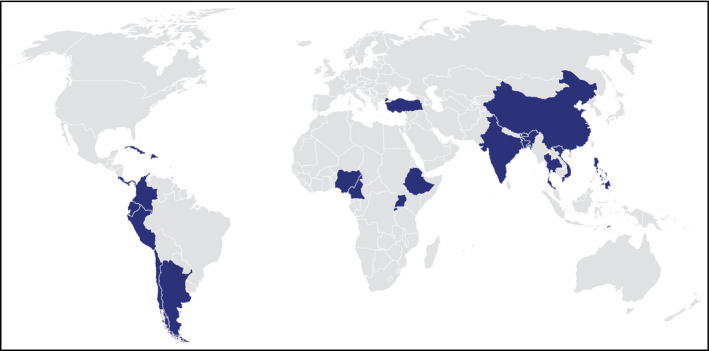
Countries Implementing the Global HEARTS Hypertension Treatment Program. (Funded by national programs and Resolve to Save Lives. Technical and other support provided by Resolve to Save Lives, the World Health Organization, the Pan American Health Organization, and other partner organizations.)
Despite treatment for hypertension being safe, effective, low cost, and the standard of care for more than half a century in high‐income countries, control rates are in the single digits in most low‐income countries, below 20% in most low middle‐income countries, and above 50% in only a few countries.2 To improve control rates, better diagnosis, treatment, and continuity of care are all needed. The five components necessary for a successful hypertension control program as recommended by WHO, the US Centers for Disease Control and Prevention, RTSL, and partners in the HEARTS technical package for treatment of hypertension in primary care are as follows: a) drug‐ and dose‐specific treatment protocols; b) quality‐assured medications and blood pressure monitors; c) team‐based care; d) patient‐centered care; and e) information systems to enable quality improvement.3
2. DRUG‐ AND DOSE‐SPECIFIC PROTOCOL
As distinct from a guideline, which provides multiple options and pathways, a drug‐ and dose‐specific protocol provides, consistent with a national guideline, a step‐by‐step schedule for initiation, titration, and addition of medications and facilitates speed, scale, and simplicity of program implementation. Practical treatment protocols can increase rates of hypertension control by reducing treatment cost; facilitate team‐based care, training, and supportive supervision; and reduce both unwarranted clinical variability and therapeutic inertia.5 They can also be useful as a model to address other noncommunicable disease issues.
Based on published clinical trials, evidence‐based treatment guidelines, implementation experience in large populations, and experiences with in‐country authorities and hypertension experts, consensus conferences often preferred three core drugs for hypertension treatment protocols. These are the calcium channel blocker (CCB) amlodipine, the angiotensin receptor blocker (ARB) telmisartan (although losartan and other ARBs may be substituted), and the diuretic chlorthalidone (although hydrochlorothiazide and indapamide are more widely available).6 These drugs work synergistically, allowing for improved blood pressure control at lower dosages.7
Amlodipine is widely available in a low‐cost generic form and can be taken once daily, and laboratory testing is not required (eg, to measure potassium levels). Because of these features, almost all countries have selected amlodipine as the first drug in the protocol, generally at a dosage of 5 mg/day, with half this dose for frail elderly patients.
Telmisartan has a low rate of adverse effects, long duration of action, is well studied and is available in generic forms. Compared with some other generic ARBs, telmisartan absorption is less affected by food,8 is more effective when combined with a diuretic,9 is better documented to reduce cardiovascular events,10 and has a lower incidence of serious adverse effects.11 The addition of an ARB reduces amlodipine‐associated pedal edema and diuretic‐associated hypokalemia.12, 13 Laboratory testing is recommended prior to and after initiating treatment with an ARB because of potential medication‐related increases in potassium levels and creatinine. In every RTSL‐associated consensus conference, clinicians collectively preferred ARBs over angiotensin‐converting enzyme inhibitors (ACEi) because of the lower risk of cough and angioedema. However, because ACEi may have more evidence of efficacy reducing cardiovascular disease, and because they may be less expensive than ARBs, some areas may select ACEIs such as lisinopril or others.14, 15
Chlorthalidone is recommended as the first‐line diuretic because of its long half‐life and clinical trial‐proven reduction of cardiovascular disease.16 The evidence that chlorthalidone reduces cardiovascular events is stronger than the evidence that hydrochlorothiazide or other diuretics do so.17, 18 In some parts of the world where heat and dehydration are risks, some clinicians are reluctant to prescribe diuretics or only prescribe them in low doses.
Referral to a hypertension specialist is recommended if blood pressure is not controlled on a combination of amlodipine 10 mg, telmisartan 80 mg, and chlorthalidone 25 mg daily. Patients with a history of heart attack or stroke are also recommended to receive aspirin and a statin, and patients with a recent heart attack (within the past 1‐3 years) are recommended to receive a beta blocker. A variety of treatment protocols are acceptable, and optimal protocol design depends on local prescribing experiences, drug availability, cost, and availability of laboratory testing. The specific regimen chosen, as long as it includes drugs from classes of medications proven to reduce CVD mortality, is less important than achieving blood pressure control.
3. FIXED‐DOSE COMBINATION MEDICATIONS
Fixed‐dose combination (FDC) medications, also known as single‐pill combinations, incorporate two or more antihypertensive drugs into one pill. They are an important means to improve hypertension control, as more than 70% of patients will require at least two different drugs to achieve blood pressure control targets.19, 20, 21 FDCs decrease pill burden, simplify logistics, can improve blood pressure control rates without increasing the risk of adverse events, facilitate simpler dose schedules and team‐based care, and are effective in different populations. For some FDCs, cost is the same as or less than that of individual medicines, and costs and complexity of stocking and distribution are lower. The absence of FDCs from the WHO Essential Medications List (EML) has contributed to their noninclusion in initial protocols until now.
In July 2019, WHO added 2‐drug rational, synergistic combination FDCs to its EML based on an application submitted by Resolve to Save Lives and partners22; these are already used commonly in private practice treatment of hypertension. The combinations suggested were a renin‐angiotensin system inhibitor (ARB or ACEi) with either a diuretic or calcium channel blocker. A possible protocol could start with amlodipine and then add an FDC of telmisartan and chlorthalidone, which could minimize the risk of hyper‐ or hypokalemia and may reduce the need for laboratory monitoring, especially for lower‐dose initial treatment combinations. FDCs are effective, are associated with 27% improvement in hypertension control,22 and are affordable. China has included an FDC on its national essential medicines list.
4. DRUG SUPPLY
In many low‐ and middle‐income countries, medication supply is inconsistent. Lack of funding, procurement delays, and distribution gaps are common. Scarcity of medications has been an important obstacle to improvement of hypertension control.23 Scaling up treatment requires increasing, often by 10‐fold or more, both the budget and the number of pills procured and distributed. In most places where the WHO HEARTS package is being implemented, scarcity of medications has been an important rate‐limiting obstacle to improvement of hypertension control. Best practices to ensure consistent medication supply include selecting locally available medications, purchasing and supplying a buffer stock, sending initial supplies to facilities and restocking from drug stores rather than having facilities request and collect medications, and implementing effective stock management to anticipate and avoid shortages.
In the longer run, using FDCs, reducing the number of medications to one per class, and establishing benchmark prices and an efficient procurement system can facilitate treating more patients with limited resources. Based on information collected from around the world, the cost per tablet of quality‐assured amlodipine may be US$0.01/tablet or lower and of quality‐assured telmisartan and chlorthalidone $0.03/tablet or lower. Governments have obtained prices that enable treatment of each patient for less than $10/year, and in some areas as low as $2‐5/year, although prices are as much as 30 times higher in some areas, often for the same medication and sometimes from the same company. Quality and shelf life of medications need to be ensured. Including hypertension protocol medicines in national and subnational essential medicines lists and formularies is an important step to promote access and reduce costs.
5. BLOOD PRESSURE MEASUREMENT
Because mercury sphygmomanometers are being phased out worldwide, and, even more importantly, because auscultatory methods of blood pressure measurement are not performed reliably on a program basis, independently clinically validated automated (digital) monitors are essential to effective hypertension management. Low‐quality, nonvalidated automated monitors have contributed, in some areas, to health care worker skepticism about the accuracy of all automated monitors. RTSL therefore established recommended guidelines for the purchase of automated monitors—external clinical validation is essential—and provided a starter set of monitors in several areas.
Arm‐in automated devices have the advantage of high throughput (up to 40 patients per hour) with limited or no staff supervision, but correctly positioning patients (such as with back supported and feet on the ground) may be difficult. Electronic transmission of blood pressure results into patient records is a promising technology, but, in practice, limitations in connectivity, interoperability, and access to functional equipment have not yet enabled this technology to be scaled up widely.
The process of measuring blood pressure has also been a hurdle to hypertension diagnosis. In many facilities, particularly at heavily utilized hospitals, only a small proportion of adult outpatients have their blood pressure measured. Ensuring that all adult patients have their blood pressure measured and recorded at each visit may require changing patient flow patterns, using automated monitors, and training a range of health care workers to measure blood pressure accurately.
6. TEAM‐BASED CARE
Medication titration or initiation by nonphysicians can increase access to treatment, improve consistency of care, reduce health care system costs, and provide treatment that is more convenient to patients.24 However, some physician groups forcefully oppose allowing nondoctors to titrate medications, and some countries require that drugs be dispensed only by licensed pharmacists.25 At a minimum, other members of the health care team can interview patients, measure blood pressure, record results, promote adherence, and provide patient education. Optimally, in the context of drug‐ and dose‐specific protocols, nonphysicians can provide a much wider array of support for patients. In one area, community health workers were authorized to continue medication regimens for patients whose blood pressure was under control, in effect refilling prescriptions that had been ordered by a prescribing clinician, as a first step toward more extensive task sharing. More than 40 years ago, a clinical trial in the United States documented that nurse‐directed care following standard protocols resulted in better outcomes than cardiologist‐provided care,26 a finding that has been replicated many times, but resistance to the evidence‐based practice of task sharing remains strong.
7. PATIENT‐CENTERED SERVICES
Because hypertension is usually asymptomatic, complications far in the future, and treatment lifelong, patients need effective support to increase adherence. Providing medications for up to 180 days at a time to patients whose blood pressure is stably controlled can increase adherence, as can use of once‐daily medications, reducing the number of pills, using FDCs, eliminating all copayments, making health facility locations and hours of operation convenient, and providing effective information and emotional support for patients.
Enabling monitoring of blood pressure outside of health care facilities, through home blood pressure monitoring and/or at community blood pressure monitoring sites, as well as patient support by community health workers and others, can further increase control rates. Telephoning patients within a week of being prescribed new medications also improves adherence.27 Ensuring medications are available at no charge to patients is associated with improvements in adherence and overall control rates.28 In Kerala, India, community leaders provided blood pressure monitors to shopkeepers at heavily utilized stores so patients could have their blood pressure checked while going about their daily routines.
8. MONITORING AND ACCOUNTABILITY
Perhaps the single most important aspect of an effective hypertension management program is an information system which enables providers to know the control rate of their patient panel, with lists of specific patients requiring follow‐up. Even in areas with electronic health records, accurately recording and analyzing blood pressure control rates and acting to bring patients back into care if they have interrupted treatment has generally not been possible.
The World Health Organization has established three core indicators for hypertension control: first, the proportion of patients whose blood pressure is under control among patients in care in all facilities in an area (reported monthly or quarterly and based either on analysis of cohorts of patients or a cross‐section of all patients being treated); second, the number of patients whose blood pressure is controlled in areas being monitored, divided by the estimated number of hypertensive patients in the catchment area of the area (an estimated community‐wide control rate, calculated at least annually); and third, a gold standard population‐based control rate based on community survey approximately every 5 years.
RTSL developed Simple, a fast, free, open‐source mobile application, currently in use in India, for clinicians to track their patients with high blood pressure. This allows health care workers to create longitudinal records of patient blood pressures and hypertension medications and generate SMS text reminders, interactive voice response telephone calls, patient contact lists, and other follow‐up methods to help return patients to care and keep them on treatment. The Simple app requires minimal training, has been designed to fit into the workflows of front‐line health workers and can be used offline in places with limited connectivity.
The best health systems use standardized treatment protocols and regular information feedback to improve quality. Kaiser Permanente Northern California, an integrated care delivery system with more than 4 million members, increased hypertension control rates from 42% to nearly 90% from 2001 to 2013.29 Canada improved the national hypertension control rate from 13% to 66% between 2000 and 2007.30
9. PRIVATE SECTOR
In some low‐ and middle‐income countries, a large proportion of patients are treated in the private health sector. Private sector care ranges from world‐class hospital facilities to unlicensed, untrained informal providers practicing alone in the community. The private sector is sometimes more convenient for patients (closer geographically, with evening and weekend hours of operation, and more culturally acceptable), but generally provides episodic rather than ongoing care. In many countries, private providers neither maintain health records nor have systems to recall patients for follow up care; along with financial barriers, this results in low rates of hypertension control.
Possible means to improve control rates in the private sector include provider training, dissemination of simple protocols, subsidies for medication costs, reimbursement that incentivizes control, a franchise model of effective chronic disease care, public education about the importance of blood pressure treatment, and electronic tools that enable providers to know and improve their control rates. RTSL and partners have begun exploring or piloting several of these approaches to determine the potential for large‐scale impact.
10. LESSONS LEARNED
In scaling up treatment for high blood pressure in the first 2 years of the RTSL‐WHO initiative, key lessons have been learnt (Table). Most hurdles to hypertension control are limitations in the health care system, not those related to patient behavior (Figure 2). The three core areas are diagnosis, treatment, and continuity of care. Variations in screening standards and policies have led to many patients who attend health facilities not having their blood pressure measured, many who have it measured not having it measured correctly, and many who have elevated readings not being diagnosed or treated for hypertension.31 Therapeutic inertia (not initiating or intensifying treatment despite elevated blood pressure) is a recognized problem,32 but diagnostic inertia is another, largely unrecognized, problem. Requiring ambulatory blood pressure monitoring is an additional potential cause of diagnostic inertia.
Table 1.
Key lessons learnt in implementing hypertension control programs
| 1. Drug‐ and dose‐specific protocols: National and subnational consensus on specific protocols is possible and necessary for progress |
| 2. Fixed dose combinations: Fixed dose combinations are an evidence‐based, rational approach to hypertension control |
| 3. Drug supply: Supply of quality‐assured medication is irregular or absent in many areas, resulting from insufficient financing and suboptimal procurement models, despite availability of affordable best‐in‐class medications that minimize adverse events |
| 4. Blood pressure measurement: High‐quality, independently validated automated (digital) BP monitors are preferable but are often resisted by practitioners |
| 5. Team‐based care: Task sharing is essential, but nonphysician health workers often are not authorized to prescribe, titrate, or refill medications |
| 6. Patient‐centered services: The biggest barriers to adherence are system weakness and limitations, not patient behaviors; systems should minimize barriers to adherence, including elimination of co‐payments, provision of 3‐6 month prescriptions for patients who are stable, access to blood pressure monitoring, and convenient access to care |
| 7. Monitoring: Information systems are not easy to implement, particularly because of difficulties with patient identification, patient flow, paper‐based systems, and Internet bandwidth; the Simple app can support programs to improve patient care |
| 8. Private sector: It is necessary to improve hypertension standards and practice in the private medical sector |
| 9. Accountability: Improving hypertension treatment both requires and facilitates effective primary health care services; political will and civil society demand is critical to accelerate progress and scale up efforts |
| 10. Prioritization: The public health and clinical challenge of global hypertension control can be met, but will require giving priority to hypertension control and using simple, scalable interventions, implemented with collaboration and persistence |
Figure 2.
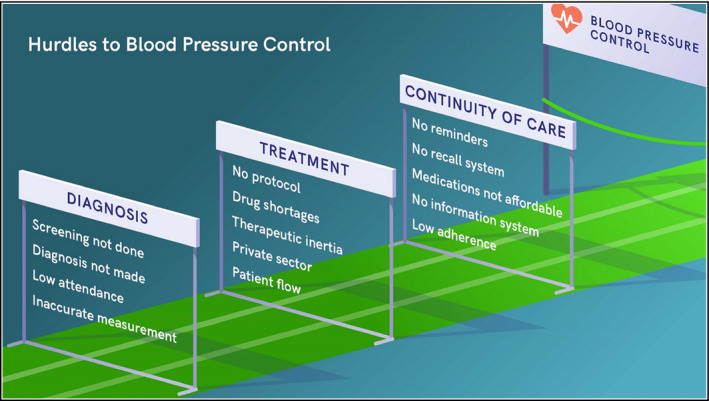
Hurdles to blood pressure control
A pragmatic approach adopted in several areas has been to diagnose hypertension if the systolic blood pressure is more than 160 mm Hg or the diastolic more than 100 mm Hg on two or more readings on a single day, or more than 140/90 mm Hg on two different days. Averaging multiple blood pressure measurements taken on the same day, as is done in community surveys, can be problematic and lead to data invention in busy and pressured clinics.
Changing patient flow so that all patients have their blood pressure measured digitally for all visits enables substantial improvement. An innovation in some areas has been, if human resources allow, to measure the blood pressure of adult relatives who accompany patients to health facilities. Increasing the measurement and follow‐up of people attending health facilities generally yields many more patients started on and continuing treatment than community screening programs.
Initiating treatment for hypertension is an important decision, as most patients need to remain on treatment for their entire lives. The recommendation to attempt lifestyle management changes for three months or more before starting treatment for patients whose blood pressure is in the 140‐159/90‐99 mmHg range results in most such patients neither adopting lifestyle changes nor receiving any indicated pharmacologic treatment for hypertension. Treatment programs in multiple countries therefore recommend that, if a patient's blood pressure is in the 140‐159/90‐99 mm Hg range on two separate days, both lifestyle management counseling and antihypertensive treatment begin on the second day that the elevation is documented.
Recent rigorous studies document that lower is better—premature cardiovascular mortality doubles for every 20 mm Hg increase in systolic blood pressure above 115 mm Hg33, 34and the far greater risk is under‐ rather than overtreatment. If the systolic pressure is below 110‐120 mm Hg on follow‐up, or if the patient is experiencing adverse effects, medications can be de‐escalated or discontinued. Attempts to implement risk‐based treatment approaches may result in complex processes which result in few patients—of any risk level—being treated.
11. CONCLUSION
Hypertension control rates are a key indicator of health system achievement and indicate whether primary health care and universal health coverage goals are being met. The United Nations Sustainable Development Goal (SDG 3.4.1) is to reduce by one third premature mortality from noncommunicable diseases through prevention and treatment by 203035; the WHO 13th General Programme of Work established a target of reducing prevalence of raised blood pressure by 20% (more than 200 million additional people with blood pressure controlled) by 2023 from the 2015 baseline.36 Improving hypertension treatment will improve many aspects of primary care and contribute to the goal for universal health coverage (UHC).37 Success will depend on identifying and cultivating program champions who provide technical, operational, and political support and leadership.
There is synergy between UHC and improvement of hypertension control. Treatment of hypertension can save more lives than any other adult health care intervention.38 Effective hypertension treatment can also reduce the number of patients needing expensive care for heart attacks, strokes, and kidney failure—conditions which could make UHC unaffordable in some areas. Establishment of effective hypertension management services—with protocols, evidence‐based treatment, affordable and accessible medicines and diagnostic technology, team‐based care, patient‐centered services, and robust information systems—both requires and facilitates strong primary care services, which are the most important, and, all‐too‐often, most neglected part of the health care system. The need, tools, and effective models exist—the single most important component lacking is governmental commitment to implement accountable, high‐quality primary health in order to increase, by hundreds of millions, the number of people whose blood pressure is controlled.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors of this paper made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; made final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DISCLAIMER
The views expressed in the document do not necessarily represent the views of the World Health Organization.
Supporting information
Frieden TR, Varghese CV, Kishore SP, et al. Scaling up effective treatment of hypertension—A pathfinder for universal health coverage. J Clin Hypertens. 2019;21:1442–1449. 10.1111/jch.13655
REFERENCES
- 1. GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet. 2018;391:709‐712. [DOI] [PubMed] [Google Scholar]
- 4. Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M, Danaei G. Three public health interventions could save 94 million lives in 25 years: global impact assessment analysis. Circulation. 2019;140:715‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frieden TR, King SM, Wright JS. Protocol‐based treatment of hypertension: a critical step on the pathway to progress. JAMA. 2014;311:21‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaffe MG, Frieden TR, Campbell NRC, et al. Recommended treatment protocols to improve management of hypertension globally: a statement by Resolve to Save Lives and the World Hypertension League (WHL). J Clin Hypertens. 2018;20:829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DiPette DJ, Skeete J, Ridley E, et al. Fixed‐dose combination pharmacologic therapy to improve hypertension control worldwide: clinical perspective and policy implications. J Clin Hypertension. 2019;21:4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38:33‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng Z, Lin S, Shi H. A systematic review and meta‐analysis of telmisartan vs valsartan in the management of essential hypertension. J Clin Hypertens. 2010;12:414‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dézsi CA. The different therapeutic choices with ARBs. Which one to Give? When? Why? Am J Cardiovasc Drugs. 2016;16(4):255‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mancia G, Schumacher H. Incidence of adverse events with telmisartan compared with ACE inhibitors: evidence from a pooled analysis of clinical trials. Patient Prefer Adherence. 2012;6:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makani H, Bangalore S, Romero J, et al. Peripheral edema associated with calcium channel blockers: incidence and withdrawal rate – a meta‐analysis of randomized trials. J Hypertens. 2011;29:1270‐1280. [DOI] [PubMed] [Google Scholar]
- 13. Ram CV. Angiotensin receptor blockers and diuretics as combination therapy: clinical implications. Am J Hypertens. 2004;17:277‐280. [DOI] [PubMed] [Google Scholar]
- 14. Salvador GL, Marmentini VM, Cosmo WR, Junior EL. Angiotensin‐converting enzyme inhibitors reduce mortality compared to angiotensin receptor blockers: systematic review and meta‐analysis. Eur J Prev Cardiol. 2017;24:1914‐1924. [DOI] [PubMed] [Google Scholar]
- 15. Sindone A, Erlich J, Lee C, Newman H, Suranyi M, Roger SD. Cardiovascular risk reduction in hypertension: angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers. Where are we up to? Intern Med J. 2016;46:364‐372. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269‐1324. [DOI] [PubMed] [Google Scholar]
- 17. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689‐694. [DOI] [PubMed] [Google Scholar]
- 18. Roush GC, Buddharaju V, Ernst ME. Is chlorthalidone better than hydrochlorothiazide in reducing cardiovascular events in hypertensives? Curr Opin Cardiol. 2013. Jul;28(4):426‐432. [DOI] [PubMed] [Google Scholar]
- 19. Kishore SP, Salam A, Rodgers A, Jaffe MG, Frieden T. Fixed‐dose combinations for hypertension. Lancet. 2018;392:819‐820. [DOI] [PubMed] [Google Scholar]
- 20. Mensah GA, Bakris G. Treatment and control of high blood pressure in adults. Cardiol Clin. 2010;28:609‐622. [DOI] [PubMed] [Google Scholar]
- 21. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290‐300. [DOI] [PubMed] [Google Scholar]
- 22. Salam A, Kanukula R, Esam H, et al. An application to include blood pressure lowering drug fixed dose combinations to the model essential medicines list for the treatment of essential hypertension in adults. Geneva: World Health Organization; 2018. https://www.who.int/selection_medicines/committees/expert/22/s12_FDC-antihypertensives.pdf [Google Scholar]
- 23. Attaei MW, Khatib R, McKee M, et al. Availability and affordability of blood pressure‐lowering medicines and the effect on blood pressure control in high‐income, middle‐income, and low‐income countries: an analysis of the PURE study data. Lancet. Public Health. 2017;2:e411‐e419. [DOI] [PubMed] [Google Scholar]
- 24. Ogedegbe G, Gyamfi J, Plange‐Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low‐income and middle‐income countries: a systematic review of randomised controlled trials. BMJ Open. 2014;4:e005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim D, Emery J, Lewis J, Sunderland VB. A systematic review of the literature comparing the practices of dispensing and non‐dispensing doctors. Health Policy. 2009;92:1‐9. [DOI] [PubMed] [Google Scholar]
- 26. Hypertension Detection and Follow‐up Program Cooperative Group . Five‐year findings of the hypertension detection and follow‐up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562‐2571. [PubMed] [Google Scholar]
- 27. Márquez Contreras E, Vegazo García O, Martel Claros N, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM‐HTA study. Blood Press 2005;14:151‐158. [DOI] [PubMed] [Google Scholar]
- 28. Yu B, Zhang X, Wang G. Full coverage for hypertension drugs in rural communities in China. Am J Manag Care. 2013;19:e22‐e29. [PMC free article] [PubMed] [Google Scholar]
- 29. Jaffe MG, Young JD. The Kaiser Permanente Northern California story: improving hypertension control from 44% to 90% in 13 years (2000 to 2013). J Clin Hypertens. 2016;18:260‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell NR, Sheldon T. The Canadian effort to prevent and control hypertension: can other countries adopt Canadian strategies? Curr Opin Cardiol. 2010;25:366‐372. [DOI] [PubMed] [Google Scholar]
- 31. Wall HK, Hannan JA, Wright JS. Patients with undiagnosed hypertension: hiding in plain sight. JAMA. 2014;312:1973‐1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Egan BM, Sutherland SE, Rakotz M, et al. Improving hypertension control in primary care with the Measure accurately, Act rapidly, and Partner with patients Protocol. Hypertension. 2018;72:1320‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. SPRINT Research Group , Wright JT, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 35. Sustainable Development Goals . Goal 3: Ensure healthy lives and promote well‐being for all at all ages. Goal 3 targets. New York: United Nations, 2015. https://www.un.org/sustainabledevelopment/health
- 36. Draft WHO Impact Framework: 13th General Programme of Work. Geneva: World Health Organization, 2017. https://www.who.int/about/GPW13_-impact-framework-draft.pdf
- 37. Varghese C, Nongkynrih B, Onakpoya I, McCall M, Barkley S, Collins TE. Better health and wellbeing for billion more people: integrating non‐communicable diseases in primary care. BMJ. 2019. Jan;28(364):l327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the US by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600‐609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


