Abstract
Aortic stiffness is a marker of vascular aging and may reflect occurrence of cardiovascular (CV) diseases. Aortic pulse wave velocity (PWV), a marker of aortic stiffness, can be measured by applanation tonometry. A nomogram of aortic stiffness was evaluated by the calculation of PWV index. Theoretical PWV can be calculated according to age, gender, mean blood pressure, and heart rate, allowing to form an individual PWV index [(measured PWV – theoretical PWV)/theoretical PWV]. The purpose of the present cross‐sectional study was to investigate the determinants of the PWV index, by applying a decision tree. A cross‐sectional study was conducted from 2012 to 2017, and 597 individuals were included. A training decision tree was constructed based on seventy percent of these subjects (N = 428). The remaining 30% (N = 169) were used as the testing dataset to evaluate the performance of the decision trees. The input variables for the models were clinical and biochemical parameters. The different input variables remained in the model were diabetes, tobacco status, carotid plaque, albuminuria, C‐reactive protein, total cholesterol, BMI, and previous CV diseases. For the validation decision model, the sensitivity, specificity, and accuracy values for identifying the related risk factors of PWV index were 70%, 78%, and 0.73. Since determinants of PWV index were all well‐accepted CV risk factors, a nomogram of aortic stiffness could be considered as an integrator of CV risk factors on their duration of exposure and could be utilized to develop future programs for CV risk assessment and reduction strategies.
Keywords: aortic stiffness, data mining, decision tree, diabetes, hypertension, pulse wave velocity
1. INTRODUCTION
Large artery stiffness seems to be a common denominator in target organ damage in high‐risk population.1 In population studies, several noninvasive arterial parameters have been shown to be biomarkers of aortic stiffness.2 The stiffness of aorta is the ability of large vessel to dampen the pulsatility of ventricular ejection, and then, the transformation of a pulsatile pressure (and flow) at the ascending aorta into a continuous pressure (and flow), in arterioles, to lower the energy expenditure of organ perfusion.3 Numerous studies have presented that aortic stiffness is a marker of vascular aging and could reflect modification in mechanical wall properties responsible for vascular disease and occurrence of cardiovascular (CV) diseases.4 Furthermore, in daily practice, its measurement may become a main routine assessment for patients.5
One of the main reproducible methods used to determine aortic stiffness is carotid‐femoral pulse wave velocity (PWV).6 PWV is defined as the velocity of arterial pulse moving along the vessel wall.7 Numerous studies have shown that PWV is a predictor of CV events.2
However, PWV measurement is not currently used in routine clinic, due to the difficulty of determining a nonpathological threshold value. Although some European consortium have reported normal references values for PWV, it is still difficult to interpret elevated values of PWV independent of age or BP groups.8 These data allow identification of people in whom PWV is abnormal and whom might warrant more intensive follow‐up; however, whether the reference values should be used as cut‐off values for treatment remains to be discussed.
Though, individually, simple PWV measurement does not appear relevant. Nevertheless, numerous studies have shown that age, gender, blood pressure (BP), and heart rate (HR) are strong determinants of aortic stiffness.9 A nomogram of aortic stiffness could be determined by the calculation of a PWV index and could better represent an individual parameter of CV management. A theoretical PWV, based on these determinants, was calculated to determine the individual relevance of aortic stiffness assessment. Also, a PWV index was calculated as ([measured PWV– theoretical PWV]/theoretical PWV) to determine those patients with increased aortic stiffness, independently of age, gender, mean BP, and HR.10 Although PWV is a predictor of CV risk, the factors associated with PWV index have been poorly studied, in particular through a decision tree model. Study of these potential factors could provide a better understanding of consistent data enabling to control and thus correct CV risk.
Data mining is a retrospective computational method for extracting knowledge from large databases. Data mining algorithms were applied to define new models for predicting the risk factors of hypertension.11 Decision tree is easy to implement and interpret. It provides a tree‐based classification for developing a predictive model according to independent variables.12 Indeed, decision tree may appear to be one of the main algorithms among data mining tools in CV diseases.13
Study of the potential factors of PWV index could provide a better understanding of consistent data enabling to control and thus correct CV risk. Thus, the purpose of the present cross‐sectional study was to highlight biochemical and clinical factors of the aortic pulse wave velocity index through a decision tree.
2. METHODS
2.1. Overall population
The present study included 597 consecutive individuals from December 2012 to September 2017, both genders, with or without previous CV events. The individuals were eligible in this cross‐sectional study during their follow‐up at the Paris Hôtel‐Dieu University Hospital. The individuals were recruited after visit in the Diagnosis and Therapeutic Center of Hôtel‐Dieu University Hospital. Most of the individuals were in‐hospital source of patients, with routine CV follow‐up, and the others were referred by their general practitioner for a CV checkup because of the presence of one or more CV risk factors.
Exclusion criteria were as follows: age under 18, acute medical conditions, presence of atrial fibrillation, and unwilling to sign the participation agreement.
The study complies with the Declaration of Helsinki. The study was registered in the French National Agency for Medicines and Health Products Safety (No. 2013‐A00227‐38) and was approved by the Advisory Committee for Protection of Persons in Biomedical Research.
2.2. Laboratory and clinical parameters
A question‐form was filled out at inclusion during the day‐hospital for CV screening and included age, gender, weight, height (respectively by a stadiometer fixed to a wall and Tanita scale with digital read‐out), body mass index (BMI) (weight [kg] divided by height [m2]) by standardized methods, family (first‐degree relatives) history of previous CV events, personal history of dyslipidemia (defined as a total/HDL cholesterol ratio >5 after an overnight fast or the presence of lipid‐lowering medications), hypertension (treated or untreated), smoking habits, previous diseases, and use of medications including antidiabetics and antihypertensive drugs. Previous CV events were retrospectively assessed by using scan imaging‐documented stroke for cerebrovascular disease; past medical history of documented myocardial infarction, coronary revascularization, or coronary heart disease diagnosed by coronary angiograms for patients with symptoms or typical electrocardiographic modifications for coronary heart disease; ankle‐brachial pressure index value <0.90, imaging‐documented atherosclerotic vascular disease, including asymptomatic severe carotid artery stenosis, peripheral vascular disease, and abdominal aortic aneurysm, arterial revascularization, or lower limb amputation. Left ventricular hypertrophy (LVH) was defined by left ventricular mass (LVM) indexed for body surface area (LVM/BSA) or for height2.7 (LVM/height2.7).
Hypertension was defined as supine systolic blood pressure (SBP) at least 140 mm Hg and/or diastolic BP (DBP) at least 90 mm Hg, according to guidelines by the European Society of Cardiology, and/or antihypertensive drug used.14 Diabetes mellitus is defined as a glycosylated hemoglobin (HbA1c) ≥6.5% and/or fasting glucose ≥7 mmol/L and/or the use of oral hypoglycemic agents or insulin therapy. Dyslipidemia was defined by the presence of lipid‐lowering medication and/or levels of LDL cholesterol and CV risk.15
Laboratory parameters included plasma glucose and glycated hemoglobin, cholesterol (total, low‐density lipoprotein, and high‐density lipoprotein) and triglycerides, plasma creatinine and calculated‐glomerular filtration rate (c‐GFR) (by MDRD formula, MDRD: modification of diet in renal disease, by mL/min/1.73m2; c‐GFR <60 mL/min/1.73 m2 defined chronic kidney disease (CKD)), and presence of albuminuria (on 24‐hour urine collection): as normo‐albuminuria (<30 mg/24 h), microalbuminuria (30‐300 mg/24 h) and proteinuria (>300 mg/24 h).
2.3. Hemodynamic parameters
Hemodynamic measurements were performed in the morning after an overnight fast in a supine position. Brachial SBP and DBP were measured in both arms using an automatic BP monitor (OMRON 705 CP II IT) with cuffs of appropriate sizes after 5 minutes of rest. Five measurements 2 minutes apart were averaged, and HR was recorded.
After BP determination, aortic PWV was performed noninvasively by applanation tonometry using an automatic device (SphygmoCor AtCor) with simultaneous three‐lead orthogonal ECG.16
This technique is considered as the standard direct and noninvasive measurement for aortic stiffness determination.17 Aortic PWV was calculated as the direct distance between carotid and femoral arteries divided by the time interval between the feet of the pressure waves at the recording sites. Direct distance was multiplied by a scaling factor of 0.8.8 The reproducibility of these measurements, in our group and in others, has been previously published in detail.10, 18
2.4. Determination of PWV index
Parameters influencing PWV measurement at baseline and during follow‐up can be evaluated independently of sex, age, mean BP, and HR.10
A nomogram of aortic PWV was constructed, by a linear regression analysis, and previously described10, 19, 20; according to the determinants of the PWV,21 to determine theoretical aortic PWV values based on age, gender (male = 1 and female = 0), mean BP and HR.
The equation derived from the multivariate analysis was then applied to the individuals to obtain a theoretical PWV value according to their age, BP, gender, and HR (Table 1).
Table 1.
Linear regression analysis of aortic PWV in the study population
| Term | Estimate | Std Error | P value |
|---|---|---|---|
| Intercept | −5.168512 | 1.18 | <.0001 |
| Age | 0.123079 | 0.01 | <.0001 |
| Gender (m = 1; f = 0) | 0.6152599 | 0.20 | .0019 |
| Mean BP | 0.0619051 | 0.01 | <.0001 |
| Heart rate | 0.0262449 | 0.01 | .0001 |
Abbreviations: BP, blood pressure; M, male; F, female.
2.5. Equation
The results were expressed as: a PWV index defined as (measured PWV − theoretical PWV/theoretical PWV) applied on these parameters for each patient.
The aortic PWV index was considered abnormal when positive.
2.6. Decision tree model
The target or outcome variable consisted in two classes: one class for the positive PWV index and the second for negative PWV index. Data mining leads to explore unknown patterns or prediction rules. One of the different methods of data mining is decision tree. The decision tree process is a nonparametric method which creates a tree‐based classification model.22 The major purpose of this method is to make a predictive model for the target variable regardless to predictors. Decision tree algorithms include three types of nodes, the root node, internal node, and end node.23, 24 This method uses splitting criteria to break a nod to form a tree. The main purpose of decision tree is to make a predictive model for the target variable according to predictors. Thus, the internal variables of the model represent a tree structure in which a decision is made in each branch according to the data features. Splitting criteria provide a rate for each predictor variable. Variables that have the best rate of splitting criterion are selected as staying in the model. In the decision tree, the first variable or root node is the most important factor and other variables can be classified in order to importance.11, 25 It can be stated also that the root node is the variable that can divide the whole population with the highest information gain.
2.7. Statistical analysis
Characteristics of the study population were described as the means with standard deviation (SD) for continuous variables. Categorical variables were described as numbers and proportions. Comparisons between groups were performed using Student's t test for continuous variables, which were normally distributed. Pearson's chi‐squared test was performed for categorical variables.
The study population was divided into two groups according to whether PWV index was negative (N = 343) or positive (N = 254). All the variables that were significantly different between negative and positive PWV index individuals were considered as input variables. We utilized a confusion matrix to determine the performance of the decision tree process for the presence of a positive PWV index. The accuracy and the receiver operating characteristics (ROC) curve were measured for training and testing models. ROC graph is a method for visualizing and selecting classifiers based on their performance. The area under the curve (AUC) of the classifier can be described as the probability of the classifier to rank a randomly selected positive results the higher predictive accuracy. Statistics were performed using SAS software (version 9.4; SAS Institute). A P value <.10 was considered for inclusion in the decision tree model.
3. RESULTS
The characteristics of the 597 individuals divided into the two groups (Positive PWV index and Negative PWV index are shown in Table 2. Eighty‐six individuals of our study presented no hypertension, no diabetes, and no previous CV events.
Table 2.
Clinical, biochemical and hemodynamic characteristics of the study population according to the threshold PWV index (negative or positive)
|
Study Population (n = 597) |
Positive PWV index (N = 254) |
Negative PWV index (N = 343) |
P valuea | |
|---|---|---|---|---|
| Gender/female (%) | 241 (40.4) | 108 (42.5) | 133 (38.8) | .35 |
| Age (y) | 60.8 (11.4) | 60.1 (12.9) | 61.2 (9.92) | .23 |
| BMI (kg/m2) | 27.4 (4.94) | 27.8 (4.39) | 27.0 (4.56) | .06 |
| Current smoking (%) | 41 (6.9) | 13 (5.1) | 28 (8.2) | .01 |
| Dyslipidemia (%) | 348 (58.2) | 165 (64.9) | 183 (53.4) | .11 |
| Hypertension (%) | 454 (76.0) | 215 (84.7) | 239 (69.7) | <.0001 |
| Diabetes (%) | 216 (36.2) | 122 (48.1) | 94 (27.4) | <.0001 |
| Carotid plaque (%) | 321 (53.8) | 155 (61.1) | 166 (48.4) | .01 |
| Left ventricular hypertrophy (%) | 100 (16.8) | 44 (17.3) | 56 (16.3) | .81 |
| Previous CV diseases (%) | 117 (19.6) | 61 (24.0) | 56 (16.3) | .02 |
| c‐GFR (<60) (%) | 62 (10.4) | 28 (11.0) | 34 (9.9) | .23 |
| “Healthy” patientsb | 86 (14.4) | 11 (4.3) | 75 (21.9) | <.0001 |
| Microalbuminuria (%) | 70 (11.7) | 33 (12.9) | 37 (10.8) | .06 |
| Proteinuria (%) | 11 (1.80) | 7 (2.70) | 4 (1.10) | .15 |
| Anti‐diabetic therapy (%) | 164 (27.5) | 89 (35.0) | 75 (21.9) | .001 |
| Statins therapy (%) | 261 (43.7) | 111 (43.7) | 150 (43.7) | .95 |
| Antihypertensive therapy (%) | 413 (69.2) | 193 (75.9) | 220 (64.1) | .002 |
| Systolic BP (mm Hg) | 133 (16) | 135 (17) | 132 (15) | <.0001 |
| Diastolic BP (mm Hg) | 78 (10) | 77 (9) | 78 (10) | .06 |
| Mean BP (mm Hg) | 96 (11) | 96 (11) | 96 (11) | .89 |
| PP (mm Hg) | 56 (13) | 59 (14) | 54 (12) | <.0001 |
| HR (bpm) | 69 (12) | 69 (12) | 69 (11) | .91 |
| Aortic PWV (m/s) | 10.47 (2.82) | 12.46 (2.86) | 8.96 (1.51) | <.0001 |
| Aortic PWV index | 0.001 (0.12) | 0.21 (0.17) | ‐0.15 (0.09) | <.0001 |
| Fasting plasma glucose (mmol/L) | 6.90 (2.72) | 7.23 (2.85) | 6.68 (2.62) | .07 |
| Triglycerides (mmol/L) | 1.38 (0.99) | 1.41 (0.98) | 1.35 (0.93) | .59 |
| Total cholesterol (mmol/L) | 4.85 (1.13) | 4.95 (1.11) | 4.68 (1.16) | .04 |
| HDL cholesterol (mmol/L) | 1.09 (0.55) | 1.06 (0.56) | 1.12 (0.55) | .17 |
| LDL cholesterol (mmol/L) | 2.08 (1.14) | 2.23 (1.16) | 1.84 (1.06) | .001 |
| Glycated hemoglobin (%) | 6.32 (1.29) | 6.54 (1.33) | 6.11 (1.27) | .02 |
| CRP (mg/L) | 2.91 (4.37) | 3.72 (5.05) | 2.16 (3.24) | .0002 |
Abbreviations: BMI, body mass index; BP, blood pressure; c‐GFR, calculated‐glomerular filtration rate; CRP, C‐Reactive protein; CV, cardiovascular; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; PP, pulse pressure; PWV, pulse wave velocity.
P value difference between Negative PWV index population and Positive PWV index population.
P value, healthy patients: patients with no hypertension, no diabetes and no previous CV events.
The data were randomly divided into a training dataset (70% of the total) and testing dataset (the remaining 30%). A decision tree was built on training dataset (N = 428). Testing data (N = 169) were used to evaluate the model. In models, eight variables were used as input variables. The input variables in the models were type 2 diabetes, current smoking, carotid plaque, albuminuria, C‐reactive protein (CRP), total cholesterol, BMI, and previous CV diseases.
The variables type 2 diabetes, carotid plaque, albuminuria, CRP, total cholesterol, previous CV diseases, and BMI remained in the model.
The evaluations of the model were undertaken using a confusion matrix on testing and training dataset and are shown in Table 3.
Table 3.
Confusion matrix of training and testing dataset for decision tree and rules extracted through decision tree model
| Training model | Testing model | ||||
|---|---|---|---|---|---|
| Predicted outcome | Predicted outcome | ||||
| Positive PWV index | Negative PWV index | Positive PWV index | Negative PWV index | ||
| Actual outcome | Actual outcome | ||||
| Positive PWV index | 134 | 51 | Positive PWV index | 48 | 21 |
| Negative PWV index | 39 | 204 | Negative PWV index | 22 | 78 |
| Sensibility | 73% | Sensibility | 70% | ||
| Specificity | 82% | Specificity | 78% | ||
| AUC | 0.76 | AUC | 0.73 | ||
| Rules extracted through decision tree model |
|---|
| R1: IF diabetes, CRP ≥0.7 mg/L and previous CV disease, then class: persons with positive PWV index (85.2%) |
| R2: IF diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol ≥6.1 mmol/L, carotid plaque and micro‐albuminuria, then class: persons with positive PWV index (74.1%) |
| R3: IF diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol ≥6.1 mmol/L, no carotid plaque and micro‐albuminuria, then class: persons with positive PWV index (60.0%) |
| R4: IF diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol ≥6.1 mmol/L, no carotid plaque and normo‐albuminuria, then class: persons with positive PWV index (33.3%) |
| R5: IF diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol <6.1 mmol/L, the class: persons with positive PWV index (25.0%) |
| R6: IF diabetes, CRP <0.7 mg/L, and total cholesterol ≥5.4 mmol/L, then class: persons with positive PWV index (66.7%) |
|
R7: IF no diabetes, total cholesterol ≥4.19 mmol/L, previous CV diseases, BMI ≥26 and micro‐albuminuria, then class: persons with positive PWV index (80.0%) |
| R8: IF no diabetes, total cholesterol ≥4.19 mmol/L, previous CV diseases, and BMI <26, then class: persons with positive PWV index (27.3%) |
|
R9: IF no diabetes, total cholesterol ≥4.19 mmol/L, no previous CV diseases, and micro‐albuminuria, then class: persons with positive PWV index (66.7%) |
| R10: IF no diabetes and total cholesterol <4.19 mmol/L, then class: persons with positive PWV (21.6%) |
Abbreviations: AUC, area under the curve; BMI, body mass index; CRP, C‐reactive protein; CV, cardiovascular; PWV, pulse wave velocity.
The decision tree of the training dataset had an accuracy of 0.76 of the 243 individuals with negative PWV index in training dataset, 204 were classified correctly using the decision tree with a specificity of 82%. For the 185 participants with positive PWV index in the training dataset, the decision tree correctly classified 134 participants, with a sensitivity of 73%. The decision tree of the testing model had an accuracy of 0.73. Of the 100 individuals with negative PWV index in testing dataset, 78 were classified correctly using the decision tree with a specificity of 78%. For the 69 participants with positive PWV index in the testing dataset, the decision tree correctly classified 48 participants, with a sensitivity of 70%.
The final decision tree model is shown in Figure 1.
Figure 1.
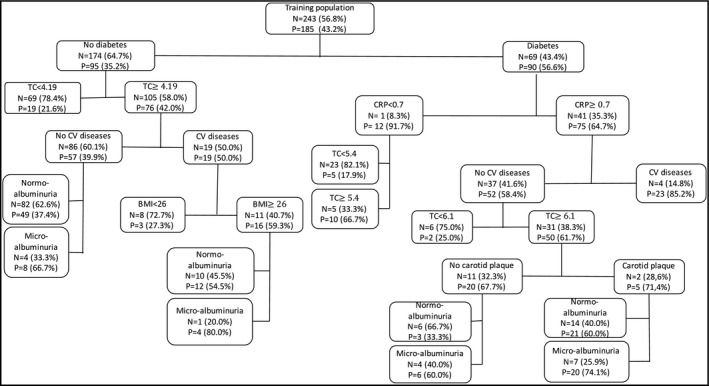
Final decision tree model (N: negative PWV index, P: positive PWV index). TC: total cholesterol; BMI: body mass index; CV: cardiovascular
The if‐then rules created by the model are shown in Table 3 . ROC curves were obtained by applying decision tree on training and testing dataset that are shown in Figure 2.
Figure 2.
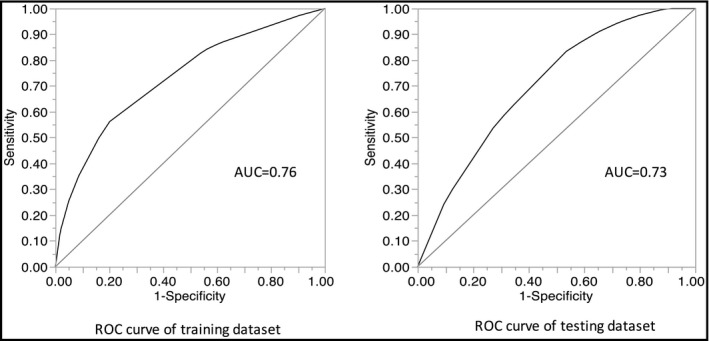
Receiver operating characteristics curve for the decision tree model in training dataset and testing dataset
The decision tree model showed that in a subgroup with diabetes, CRP ≥0.5 mg/L and previous CV disease, the probability of positive PWV index was 85.2% whereas in the subgroup with diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol ≥6.1 mmol/L, carotid plaque and micro‐albuminuria, the probability of PWV index was 74.1%. In the subgroup with diabetes, CRP ≥0.7 mg/L, no previous CV diseases, total cholesterol ≥6.1 mmol/L, no carotid plaque and normo‐albuminuria, the probability of PWV index was 33.3%. In the subgroup with diabetes, CRP <0.7 mg/L and total cholesterol ≥5.4 mmol/L, the probability of positive PWV index was 66.7% whereas in the same subgroup but with total cholesterol <5.4 mmol/L, the probability of positive PWV index was 17.9%.
In the subgroup with no diabetes, total cholesterol ≥4.19 mmol/L, previous CV diseases, BMI ≥26 and micro‐albuminuria, the probability of positive PWV index was 80%, whereas in the same group but with BMI <26, the probability of positive index was 27.3%. In the subgroup with no diabetes and total cholesterol <4.19 mmol/L, the probability of positive PWV index was 21.6% (Figure 1, Table 3).
4. DISCUSSION
According to previous studies, we have determined a PWV index based on a theoretical PWV calculated through a linear regression on major factors correlated with aortic stiffness, such as age, gender, mean BP, and HR.10, 19, 21
Few studies have investigated the different risk factors associated with PWV index.10, 21 In our study, we constructed a decision tree model, based on data from a cross‐sectional study to investigate the different factors associated with PWV index (positive or negative values). A decision tree is a modeling method that has several advantages, such as the ability to handle nonlinear relationships, creating rules, and being easy to interpret.26, 27, 28 Currently, no decision tree model methodology has been constructed to determine the different factors of PWV index neither the PWV values.
The main advantage of using a decision tree analysis is the ability to convert complicated risk equations into an organized flowchart, which can be easily navigated to identify appropriate risk factors. This is important in clinical and practice to obtain risk stratification tools to better manage diseases. A simple, practical, and user‐friendly approach can help clinicians to make more valid risk‐based decisions. Furthermore, this tool can help to minimize the need for unnecessary factors with a view to better understand risk factors associated with aortic stiffness.
There were seven predictors in our developed model. One key feature in our developed model was the classification of the different predictors. First, the main sensitive predictors (ie, diabetes, CRP, total cholesterol) appear in the decision tree model, and in the end the main specific predictors (ie, albuminuria, carotid plaque, BMI). A hierarchical classification of the different parameters of positive PWV index can be done using this model. For example, a subject without diabetes but a total cholesterol ≥4.19 mmol/L, presence of previous CV diseases, a BMI ≥26 and the presence of micro‐albuminuria has a probability of positive PWV index around 80%. Moreover, using decision tree yields threshold values for the variables that have the highest classification accuracy where tree branching take places. This is an evident advantage of decision tress compared to regression models. Moreover, the decision tree model yields a machine learning classifier that can accurately discriminate variables based on retrospectively acquired training data and assess positive PWV index risk with a prospective testing dataset.
Clinical and biochemical parameters should be sensitive to the signs and symptoms presented by the population that may evidence positive PWV index. Nevertheless, there are no studies in the literature using decision tree models for PWV index or PWV values, and therefore it is difficult to compare with other samples.
However, several studies have shown that the different input variables (as diabetes, carotid plaque, total cholesterol, previous CV disease, BMI, albuminuria, and CRP) included in our decision tree have been associated with increased PWV. Thus, the predictors shown in our classification decision tree model are similar as the factors observed in the literacy.
Indeed, aortic stiffness may be accelerated by pathophysiological conditions related to diabetes mellitus (a sensitive variable in our model)29 through several pathways, such as calcification, inflammation, and oxidative stress.30
CRP level appears as a sensitive variable in our decision tree model. Several studies have shown that CRP levels are associated with aortic stiffness, atherosclerosis, and CV events.31 CRP is one of the markers of chronic low‐grade inflammation and is considered as a mediator of atherothrombotic disease.32 CRP is the only circulating biomarker related to vascular wall biology33 and increased levels of CRP are linearly associated with PWV and PWV index values.10, 34 High levels of CRP are associated with arterial and aortic stiffness in hypertensive and type 2 diabetes patients.35 However, the direct etiological role of CRP in arterial dysfunction and atherosclerosis remains contradictory and would require other studies to better understand it.35
Moreover, numerous studies have shown that albuminuria levels (ie, a specific variable in our model) were associated positively with the increase of PWV values and PWV index.10, 36 Micro‐albuminuria is an early marker of CKD, vascular dysfunction, arterial stiffness, and CV diseases.37 However, the mechanism between albuminuria and arterial stiffness remains unclear. A possible link might associate inflammation (through CRP levels), PWV levels and the increase of albuminuria.38
Vascular calcifications and aortic stiffness are strongly correlated39 although this bidirectional association remains unknown.40 However, presence of vascular calcification is considered as the main factor of accelerated aortic stiffness with age‐dependent mechanism in hypertensive and diabetic patients and appears as a specific variable in our model.41 It is thought to primarily involve structural changes within the media, such as fatigue fracture of elastin and deposition of collagen.42 Another suggested mechanism is directly the phenomenon of vascular calcification.43
Numerous mechanisms have been described to explain the possible link between BMI and aortic stiffness. Increased BMI is correlated with low inflammation that enhances PWV.44 This link with inflammation (CRP levels) could explain the specific role of high BMI in our model for positive PWV index. Nitric oxide (NO) production is reduced by inflammatory cytokines, and then, reactive oxygen species (ROS) generated by inflammatory process consume existing NO.45 The reduction of NO production leads to increased PWV.46 Inflammatory processes may induce changes in the arterial wall by breakdown of elastin, smooth muscle cells proliferation, and changes in the composition of extracellular matrix.47
The calculation of the PWV index is mainly associated with age. Advancing age may be the main potent independent predictor of future CV events. Thus, after adjusting on age, PWV index appears associated with numerous CV risk factors, such as CRP, diabetes, hypertension, LVH, microalbuminuria and CKD. The relationship between time exposure and CV events is not fully explained by time‐related changes in CV risk factors. Even if chronological age is mainly and independently associated with CV risk, the biological age of vessels may be different. Repeated exposure to potentially CV risk factors may lead to differences in vascular function and structure and then leads to a dissociation between biological and chronological age. These observed differences may be correlated with inter‐individual differences in vascular health. Defining an integrative measure of vascular structure adjusted on age may provide prediction of the real biological age of arteries.48 PWV index calculation could appear as an interesting integrator risk factor correlated with time exposure of CV risk factors for the management and prevention of CV diseases.
4.1. Limitations
The cross‐sectional design of our study may appear as a limitation, because it presents only simple correlation between determinants and PWV index and not causal interferences. The small study sample appears as a worth‐noted limitation of the study.
Even if HR appears as a significant determinant of PWV index, a long‐term follow‐up is needed to evaluate the potential clinical significance of increased HR. Gender, which appears as significant factor of PWV index, can influence the role of autonomic nervous system in attenuating pressure wave reflections but remains to be further established.
Calculation of an index is dependent on the theoretical evaluation, which is expected to be different in another population study and potentially depends on the method used to measure PWV. Theoretical PWV value has been estimated on individuals which were recruited in a hospital which presents a center of excellence in hypertension and a primary care with general practitioners. Thus, the study population is not representative of the general population but this double medical care center may limit population selection bias compared to hyper‐specialized centers. These life behaviors may enhance theoretical PWV values when compared to healthy populations. However, these life behaviors are also observed in the general population and can thus reflect a theoretical PWV close to reality. Moreover, the absence of ABPM could overlook cases of masked‐hypertension.
5. CONCLUSION
A decision tree analysis was applied in our study population. Clinical and biochemical factors were identified to be associated with PWV index threshold. Diabetes, carotid plaque, albuminuria, total cholesterol, CRP, BMI, and previous CV diseases remained in the construction of the decision tree. This first decision tree model remains an experimental model for PWV index, as nomogram of aortic stiffness. One main feature of our developed model is the classification of the different predictors of PWV index. Use of decision tree in research could be a means of explaining the observed relationships between variables and thus classify them according to their degree of impact on PWV index. Future studies may aim to develop prediction models with higher specificity and sensibility, which could be used for determining the threshold of PWV index more accurately. However, this study provides an easy classification rules for identifying risk factors associated with PWV index that could be useful to develop programs for CV risk management.
CONFLICT OF INTEREST
None declared.
Vallée A, Safar ME, Blacher J. Application of a decision tree to establish factors associated with a nomogram of aortic stiffness. J Clin Hypertens. 2019;21:1484–1492. 10.1111/jch.13662
REFERENCES
- 1. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111‐1117. [DOI] [PubMed] [Google Scholar]
- 2. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Safar ME, O'Rourke M. Handbook of Hypertension, Volume 23: Arterial Stiffness in Hypertension. Amsterdam, Netherlands: Elsevier; 2006. [Google Scholar]
- 4. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426‐444. [DOI] [PubMed] [Google Scholar]
- 5. Zhong QI, Hu M‐J, Cui Y‐J, et al. Carotid‐femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta‐analysis. Angiology. 2018;69(7):617‐629. [DOI] [PubMed] [Google Scholar]
- 6. Safar ME, Toto‐Moukouo JJ, Bouthier JA, et al. Arterial dynamics, cardiac hypertrophy, and antihypertensive treatment. Circulation. 1987;75:I156‐161. [PubMed] [Google Scholar]
- 7. Mitchell GF, Hwang S‐J, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values'. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nichols W, O'Rourke M. McDonald's blood flow in arteries theoretical, experimental and clinical principles (4th edn). London, UK: Edward Arnold; 2006. [Google Scholar]
- 10. Vallée A, Yannoutsos A, Temmar M, et al. Determinants of the aortic pulse wave velocity index in hypertensive and diabetic patients: predictive and therapeutic implications. J Hypertens. 2018;36(12):2324‐2332. [DOI] [PubMed] [Google Scholar]
- 11. Tayefi M, Esmaeili H, Saberi Karimian M, et al. The application of a decision tree to establish the parameters associated with hypertension. Comput Methods Programs Biomed. 2017;139:83‐91. [DOI] [PubMed] [Google Scholar]
- 12. Berry M, Linoff G. Data Mining Techniques: For marketing, sales and Customer Support. Indianapolis, US: John Wiley & Sons Inc; 1997. [Google Scholar]
- 13. Soni J, Ansari D, Sharma D, Soni S. Predictive data mining for medical diagnosis: an overview of heart disease prediction. Int J Comput Appl. 2011;17:43‐48. [Google Scholar]
- 14. Williams B, Mancia G, Spiering W, et al. 2018 practice guidelines for the management of arterial hypertension of the European society of hypertension and the European society of cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36:2284‐2309. [DOI] [PubMed] [Google Scholar]
- 15. Expert Dyslipidemia Panel , Grundy SM. An international atherosclerosis society position paper: global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7:561‐565. [DOI] [PubMed] [Google Scholar]
- 16. Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension. 1995;26:485‐490. [DOI] [PubMed] [Google Scholar]
- 17. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 18. Yannoutsos A, Ahouah M, Dreyfuss Tubiana C, Topouchian J, Safar ME, Blacher J. Aortic stiffness improves the prediction of both diagnosis and severity of coronary artery disease. Hypertens Res. 2018;41(2):118‐125. [DOI] [PubMed] [Google Scholar]
- 19. Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end‐stage renal disease. Kidney Int. 2003;63:1852‐1860. [DOI] [PubMed] [Google Scholar]
- 20. Vallée A, Cinaud A, Blachier V, Lelong H, Safar ME, Blacher J. Coronary heart disease diagnosis by artificial neural networks including aortic pulse wave velocity index and clinical parameters. J Hypertens. 2019;37(8):1682‐1688. [DOI] [PubMed] [Google Scholar]
- 21. Yannoutsos A, Ahouah M, Tubiana CD, et al. Hemodynamic parameters in hypertensive diabetic patients. J Hypertens. 2016;34:1123‐1131. [DOI] [PubMed] [Google Scholar]
- 22. Vallée A, Petruescu L, Kretz S, Safar ME, Blacher J. Added value of aortic pulse wave velocity index in a predictive diagnosis decision tree of coronary heart disease. Am J Hypertens. 2019;32(4):375‐383. [DOI] [PubMed] [Google Scholar]
- 23. Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006;27:861‐874. [Google Scholar]
- 24. Shi G. Chapter 5, Decision trees. In: Shi G, ed. Data Mining and Knowledge Discovery for Geoscientists. Oxford, UK: Elsevier; 2014:113-138. [Google Scholar]
- 25. Ramezankhani A, Pournik O, Shahrabi J, Khalili D, Azizi F, Hadaegh F. Applying decision tree for identification of a low risk population for type 2 diabetes. Tehran lipid and glucose study. Diabetes Res Clin Pract. 2014;105:391‐398. [DOI] [PubMed] [Google Scholar]
- 26. Kammerer JS, McNabb S, Becerra JE, et al. Tuberculosis transmission in nontraditional settings: a decision‐tree approach. Am J Prev Med. 2005;28:201‐207. [DOI] [PubMed] [Google Scholar]
- 27. Wang C‐J, Li Y‐Q, Wang L, et al. Development and evaluation of a simple and effective prediction approach for identifying those at high risk of dyslipidemia in rural adult residents. PLoS ONE. 2012;7:e43834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Podgorelec V, Kokol P, Stiglic B, Rozman I. Decision trees: an overview and their use in medicine. J Med Syst. 2002;26:445‐463. [DOI] [PubMed] [Google Scholar]
- 29. Agnoletti D, Lieber A, Zhang YI, et al. Central hemodynamic modifications in diabetes mellitus. Atherosclerosis. 2013;230:315‐321. [DOI] [PubMed] [Google Scholar]
- 30. Stehouwer C, Henry R, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527‐539. [DOI] [PubMed] [Google Scholar]
- 31. Hage FG. C‐reactive protein and hypertension. J Hum Hypertens. 2014;28:410‐415. [DOI] [PubMed] [Google Scholar]
- 32. Ridker PM. Clinical application of C‐reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363‐369. [DOI] [PubMed] [Google Scholar]
- 33. Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European society of cardiology working group on peripheral circulation: endorsed by the association for research into arterial structure and physiology (ARTERY) society. Atherosclerosis. 2015;241:507‐532. [DOI] [PubMed] [Google Scholar]
- 34. Mattace‐Raso F, van der Cammen T, van der Meer IM, et al. C‐reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176:111‐116. [DOI] [PubMed] [Google Scholar]
- 35. Mozos I, Malainer C, Horbańczuk J, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. 2017;8:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim BJ, Lee HA, Kim NH, Kim MW, Kim BS, Kang JH. The association of albuminuria, arterial stiffness, and blood pressure status in nondiabetic, nonhypertensive individuals. J Hypertens. 2011;29:2091‐2098. [DOI] [PubMed] [Google Scholar]
- 37. Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens. 2015;28:561‐569. [DOI] [PubMed] [Google Scholar]
- 38. Liu C‐S, Pi‐Sunyer FX, Li C‐I, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects–a population‐based study (Taichung Community Health Study, TCHS). Atherosclerosis. 2010;211:315‐321. [DOI] [PubMed] [Google Scholar]
- 39. Tsao CW, Pencina KM, Massaro JM, et al. Cross‐sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34:2495‐2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stabley JN, Towler DA. Arterial calcification in diabetes mellitus: preclinical models and translational implications. Arterioscler Thromb Vasc Biol. 2017;37:205‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McEniery CM, McDonnell BJ, So A, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524‐531. [DOI] [PubMed] [Google Scholar]
- 43. Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age‐related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307‐317. [DOI] [PubMed] [Google Scholar]
- 44. Jain S, Khera R, Corrales‐Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237:381‐390. [DOI] [PubMed] [Google Scholar]
- 45. Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase‐mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:1617‐1622. [DOI] [PubMed] [Google Scholar]
- 46. Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid‐femoral pulse wave velocity and augmentation index in humans. Hypertension. 2003;42:915‐918. [DOI] [PubMed] [Google Scholar]
- 47. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251‐262. [PubMed] [Google Scholar]
- 48. Thijssen D, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol. 2016;594:2275‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]


