Abstract
Beside the well‐known complications of poorly controlled, long‐standing hypertension, milder abnormalities induced by early‐stage hypertension have also been described. In our study, the authors examined the reversibility of changes induced by early‐stage hypertension. The authors performed laboratory testing, ambulatory blood pressure monitoring, carotid intima‐media thickness (IMT) measurement, evaluation of stiffness parameters, assessment of various cardiac and cerebral hemodynamic parameters during head‐up tilt table (HUTT) testing, and neuropsychological examinations in 49 recently diagnosed hypertensive patients. Following baseline assessment, antihypertensive therapy was commenced. After one year of therapy, lower IMT values were found. Pulse wave velocity showed a borderline significant decrease. During HUTT, several hemodynamic parameters improved. The patients performed better on neuropsychological testing and reached significantly lower scores on questionnaires evaluating anxiety. The present study shows that early vascular changes and altered cognitive function observed in newly diagnosed hypertensive patients may improve with promptly initiated antihypertensive management.
Keywords: arterial stiffness, cognitive function, hypertension, intima‐media thickness, neuropsychological tests
1. INTRODUCTION
Hypertension is one of the leading risk factors of cardio‐ and cerebrovascular diseases. The devastating, long‐term complications of untreated or ineffectively controlled hypertension are well known.1 Moreover, the importance of asymptomatic organ damage has also been recognized and thoroughly discussed previously.2
Manifest cardiovascular events are preceded by progressive, subclinical deterioration of the arterial wall, which can be visualized by measuring the intima‐media thickness (IMT) by B‐mode ultrasonography.3 Hence, IMT is a marker of structural vessel wall property and indicator of hypertensive target organ damage.4, 5 The change in IMT represents one of the earliest manifestations of atherosclerotic disease and its measurement greatly contributes to the timely diagnosis of target organ damage. Importantly, IMT was found to decrease or progress slower when various antihypertensive agents were applied in hypertensive patients.6, 7
While IMT reflects to the morphological characteristics of the vessel wall, functional features of the arteries may be described by stiffness parameters. Previously, it has been shown that aortic stiffness provides an independent predictive value for fatal and non‐fatal cardiovascular events in hypertensive patients.8, 9 The "gold standard" method for measuring aortic stiffness is the detection of the carotid‐femoral pulse wave velocity (PWV).10 Hence, the latest European Society of Hypertension (ESH) / European Society of Cardiology (ESC) guideline recommends the evaluation of PWV, as the marker of asymptomatic organ damage.2
In addition to the structural and functional changes induced in the arteries, hypertension also associates with a higher incidence of cognitive decline.11 Previously, a consistent association between high midlife blood pressure and late‐life cognitive decline and incident dementia has been suggested by large epidemiological studies.12
We have earlier investigated the association of hypertension with neuropsychological performance by evaluating the cardio‐ and cerebrovascular reactivity, cognitive function and affectivity in newly diagnosed hypertensive patients. While no differences were found in the cerebral blood flow velocities during head‐up tilt table (HUTT) testing, several cardiovascular parameters (blood pressure‐BP, heart rate‐HR, stroke index, total peripheral resistance‐TPR index) differed significantly when comparing hypertensive patients to healthy control individuals.13 Moreover, the sum of standardized test scores of the neuropsychological battery was significantly lower, while state and trait anxiety scores were significantly higher among HT patients.13 Additionally, analysis of the adjusted effect of mean arterial pressure shed light on the gradual elevation of IMT, augmentation index (AIx) and PWV values with increasing BP.14
In the current work, we hypothesize that hypertension‐induced subclinical vascular and cognitive changes are reversible. Therefore, we aim to examine the morphological (IMT) and functional (AIx and PWV) changes in the vascular wall, as well as cognitive function in the same population after 1 year of antihypertensive therapy. We also evaluate tilt induced changes in cardiac and cerebral hemodynamic parameters.
2. MATERIAL AND METHODS
In this prospective, human, open, observational cohort study, patient recruitment took place at the Department of Neurology, University of Debrecen Medical Center, Debrecen, Hungary.
2.1. Participants
Patients with primary hypertension were enrolled. The diagnosis was based on international guidelines.2 After the baseline evaluation, antihypertensive therapy was suggested by the internist. The study protocol was approved by the local Ethical Committee of the University of Debrecen Medical Center (DEOEC RKEB/IKEB 2425‐2005); therefore, the study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients were informed about the details of the study. Inclusion criteria of patient recruitment were the following: recent diagnosis of hypertension, subject considered mentally capable of adhering to the protocol, and written informed consent signed. Exclusion criteria were the following: untreatable, uncontrolled hypertension with hypertensive crisis episodes, secondary hypertension, explicit target organ damage, diabetic patients, extreme obesity, alcohol dependence, previous stroke, previous orthostatic hypotension or syncope, abnormal cerebral computed tomography (silent brain infarction, bleeding, atrophy or tumor), significant carotid stenosis (>70%), peripheral artery disease, pregnancy, malignancies, any severe comorbidity, antidepressant or psychotropic drug use.
2.2. Measurements
After taking a detailed patient's history and performing a general physical and neurological assessment, all patients underwent the following examinations:
2.2.1. Laboratory examination
Fasting blood sample was obtained for serum lipids and glucose.
2.2.2. Ambulatory blood pressure monitoring
In those patients who were not treated yet, ambulatory blood pressure monitoring (ABPM) was started on the day when we first examined the patients, and therapy was recommended the second day, at the end of the ABPM. In case of patients who were already commenced on antihypertensive drug, ABPM was performed under the drug effect. For ABPM measurements, we used a Cardiospy ABPM device by Labtech Ltd. (Hungary, model: EC‐ABP).
2.2.3. Head‐up tilt table test
Head‐up tilt table was performed in the morning hours in a quiet room. Patients were in a fasting state. During the examination, various cardiac hemodynamic parameters (BP, HR, stroke volume, cardiac output‐CO, TPR) and the cerebral blood flow velocity (CBFV) were recorded simultaneously, continuously, and non‐invasively with a medical device (Task Force Monitor) incorporating electrocardiography, impedance cardiography, oscillometric and continuous (beat‐to‐beat) BP monitoring, and a transcranial Doppler (Multidop T2, DWL, Überlingen, Germany) being attached to the main device. Detailed presentation of HUTT was previously described.13
2.2.4. Intima‐media thickness
Intima‐media thickness was measured during carotid ultrasonography performed with a 7.5 MHz SonoSite MicroMaxx ultrasound machine (SonoSite Inc, Bothell, WA). Baseline and follow‐up IMT measurements were shared randomly between three physicians. At the time of the 1‐year follow‐up visit, the investigators were not aware of the result of the first assessment. Six measurements were taken on the far wall of both common carotid arteries (CCAs). The retrieved 12 values were subsequently averaged for statistical evaluation. The detailed method of IMT measurement was previously presented.14
2.2.5. Stiffness parameters
AIx (in %) and PWV (in m/s) were assessed with a validated, computerized, portable device (TensioClinic Arteriograph, TL1, TensioMed Ltd., Hungary).15, 16 The measurement was performed with the patients lying in supine position. A cuff was placed on the resting right arm with its lower edge located approximately 25 mm above the elbow and the air outlet directed above the brachial artery. AIx and PWV were determined by analyzing the oscillometric pressure curves registered on the upper arm.17
2.2.6. Neuropsychological examination
A series of neuropsychological tests were applied to evaluate reaction times (choice and selective), memory (Rey Auditory Verbal Learning Test, Digit Span Test), attention and vigilance (Pieron Test, Digit Span Test, Trail Making Test), visuospatial and motor skills (Block Design Test), general processing speed (Digit Symbol Test), anxiety (The Spielberger State‐Trait Anxiety Inventory), and depression (Beck Depression Inventory). The neuropsychological testing lasted for approximately 1 hour ± 10 minutes and was performed and evaluated by a trained psychologist. The tests were discussed in detail previously.13
2.3. Statistical analysis
Baseline and follow‐up levels of continuous outcome variables were compared using paired t tests if normality assumptions were satisfied, or Wilcoxon's matched‐pairs signed‐ranks tests otherwise. Neuropsychological test scores were unified into a single measure by standardizing each score, separately at baseline and at follow‐up, and taking their average (mean of standardized test scores [STS]). Missing data were not imputed.
Correlations between pairs of variables were assessed using Pearson's correlation coefficient. This involved estimation at baseline, at follow‐up, as well as for changes from baseline to follow‐up. Variable pairings for IMT included the following: mean BP, systolic BP, diastolic BP, HR, TPR, TPR index, AIx, PWV, mean of STS, Spielberger's state anxiety score, Spielberger's trait anxiety score and Beck's score; for AIx and PWV: mean of STS, Spielberger's state anxiety score, Spielberger's trait anxiety score, Beck's score, and mean BP; and for mean of STS, Spielberger's state anxiety score, Spielberger's trait anxiety score and Beck's score: mean BP, systolic BP, diastolic BP, HR, TPR, and TPR index.
Multiple linear regression was used to assess whether the linear relationship between baseline and follow‐up levels of outcome parameters was heterogeneous across baseline levels of potential modifying variables such as low‐density lipoprotein‐cholesterol (LDL‐C), total cholesterol, body mass index, age, mean BP, smoking status and, for neuropsychological test outcomes only, and presence or absence of higher education. Regression model terms included the baseline variants of the outcome and the potential modifier, with interaction terms between them to evaluate the presence of intercept (baseline‐independent modification) and slope heterogeneity (baseline‐dependent modification). Significant interactions were visualized using fitted value lines at the 75th vs the 25th percentile level of the modifier.
We also evaluated whether the relationship between changes in BP and changes in outcome parameters was heterogeneous across levels of change in body mass index (BMI). We used linear regression models with change in outcome parameter as the outcome, and change in BP in interaction with change in BMI as the explanatory variables, and assessed whether adjustment for age and sex was necessary.
3. RESULTS
3.1. Patients
Originally, 81 hypertensive patients (43.5 ± 10.2 years, male/female ratio: 1.08) were recruited into our baseline investigation following screening for target organ damage (urine analysis, echocardiography, fundoscopy, and cerebral computed tomography) and comorbidities (diabetes mellitus, anxiety, depression, etc). After a meticulous patient selection, we excluded all patients who were already on antihypertensive therapy for more than 5 weeks, or who were treated even temporarily months/years earlier, and those who were not treated yet, but were suspected of having hypertension for several months/years based on explicit target organ damages. Hence, 72 hypertensive patients (mean [SD] age: 43.6 [10.2] years, male/female ratio: 0.95) were planned to be followed up.14 Eighteen patients (25%) did not complete the follow‐up visit at 1 year, two patients (2.8%) had to be excluded from final data analysis as diabetes mellitus was diagnosed between the baseline and 1‐year follow‐up evaluation, and another three patients (4.17%) were excluded because of taking psychotropic drug. Eventually, the final data analysis of the follow‐up study was applied in 49 (68.05%) patients.
3.2. Demographic, clinical, and social characteristics of the patients
Mean (SD) age of the 49 patients was 43.08 (10.82) years, male/female ratio was 1.45. 34.7% of patients (n = 17) were smokers, while 65.3% (n = 32) were non‐smokers or former smokers. 55.1% (n = 27) had higher education.
Thirty‐three (67.35%) patients were not treated at baseline at all, while the rest (16 patients; 32.65%) were already on antihypertensive therapy for <1 week (10 patients), for 2 weeks (four patients), or up to a maximum of 5 weeks (two patients). In the majority of patients (79.6%, n = 39), antihypertensive monotherapy was commenced with an angiotensin converting enzyme inhibitor (ACEI; n = 22; 56.4%), angiotensin receptor blocker (ARB; n = 4; 10.3%), β‐blocker (BB; n = 11; 28.2%), or calcium channel blocker (CCB; n = 2; 5.1%). Although monotherapy was preferred, combined therapy was applied when necessary (two patients on additional diuretic, one patient on two different antihypertensive agents, one patient on two agents plus additional diuretic). In three patients, switching from one to another antihypertensive agent was necessary for side effects or inefficiency. In case of three patients, lifestyle changes were solely recommended without any drug therapy. Aspirin was used in case of three patients (6.12%) and statins in case of 12 patients (24.49%), while fibrate in case of only one patient (2.04%) as co‐treatment.
At baseline, 22.45% of the patients (n = 11) had a normal body mass index (BMI < 24.9 kg/m2), 55.1% (n = 27) were overweight (BMI = 25‐29.9 kg/m2), and 22.45% (n = 11) were obese (BMI > 30 kg/m2). When evaluating the change in BMI and its influence on the different outcome measures, statistically non‐significant associations were found as follows: Changes in outcome parameters were homogeneous across the entire range of changes in BMI, the latter being close to zero on average, and non‐significant (27.18 ± 3.42 vs 27.13 ± 3.38 at baseline vs follow‐up, P = 0.7607, difference mean (95% CI) = 0.05561 (−0.3098‐0.4211).
3.3. Laboratory parameters
At baseline, the average serum cholesterol level was just above the upper normal value of our local laboratory (<5.2 mmol/L). Patients having higher serum cholesterol and LDL‐C levels were recommended on a diet free of animal fat, and they were started on a lipid lowering therapy when necessary. At follow‐up, the averages of the total serum cholesterol and LDL‐C levels showed a mild, but statistically non‐significant reduction, while the high‐density lipoprotein‐cholesterol (HDL‐C) and triglyceride (TG) levels remained unchanged. Detailed laboratory data are presented in Table 1.
Table 1.
Demographic and laboratory data of hypertensive patients
| Demographic data | |
|---|---|
| Mean age (y; SD) | 43.08 (10.82) |
| Male/female ratio | 1.45 |
| Higher education (%) | 27 (55.1) |
| Smoking habit: smoker/non‐ or former smoker (%) | 17 (34.7)/32 (65.3) |
| Laboratory parameters | |||
|---|---|---|---|
| Baseline | 1‐y follow‐up | P value, difference mean (95% CI) | |
| CHOLb (n = 47) | 5.32/1.69 | 4.93/1.72 |
0.1089, −0.253 (−0.517 to 0.012) |
| LDL‐CHOLa (n = 45) | 3.20 (0.92) | 3.09 (0.88) |
0.3958, −0.108 (−0.363 to 0.146) |
| HDL‐CHOLb (n = 45) | 1.36/0.51 | 1.36/0.49 |
0.3605, −0.052 (−0.167 to 0.064) |
| TGa (n = 47) | 1.49 (0.95) | 1.48 (0.73) |
0.9340, −0.0083 (−0.209 to 0.192) |
| Serum glucosea (n = 47) | 5.05 (0.56) | 5.19 (0.7) |
0.1046, 0.136 (−0.029 to 0.302) |
CHOL, cholesterol; CI, confidence interval; HDL‐CHOL, high‐density lipoprotein‐cholesterol; LDL‐CHOL, low‐density lipoprotein‐cholesterol; TG, triglyceride.
Paired t test: data expressed as mean (SD).
Wilcoxon matched‐pairs signed‐ranks test: data expressed as median/interquartile range.
3.4. Ambulatory blood pressure monitoring data
Ambulatory blood pressure monitoring was performed in 45 patients (91.8%) at the baseline evaluation and in 47 patients (95.9%) at the follow‐up evaluation. Forty‐four (89.8%) patients had both the baseline and follow‐up ABPM. ABPM revealed significant decrease in all measured parameters (systolic, diastolic BPs, as well as systolic and diastolic hyperbaric indices [data not shown]) during both daytime and nighttime evaluations. Detailed ABPM data are presented in Table 2.
Table 2.
Ambulatory blood pressure monitoring data
| Parameters | Baseline | Follow‐up | P value, difference mean (95% CI) |
|---|---|---|---|
| Active (daytime), n = 44 | |||
| sBP (mm Hg) | 142.2 (12)a | 129.4 (9.6) a |
<0.0001, −12.8 (−16.4 to −9.3) |
| dBP (mm Hg) | 87.8 (8.7)a | 79.8 (8.5) a |
<0.0001, −7.95 (−10.3 to −5.63) |
| Passive (night time), n = 43 | |||
| sBP (mm Hg) | 124.7 (14.2)a | 114.1 (10.1)a |
0.0001, −10.5 (−15.3 to −5.79) |
| dBP (mm Hg) | 72/ 13b | 68/ 7b |
0.0009, −5.86 (−9.06 to −2.66) |
ABPM, ambulatory blood pressure monitoring; CI, confidence interval; dBP, diastolic blood pressure; sBP, systolic blood pressure.
Paired t test: data expressed as mean (SD).
Wilcoxon matched‐pairs signed‐ranks test: data expressed as median/ interquartile range.
3.5. Intima‐media thickness measurements
A significant overall reduction in the IMT value could be observed after one year of antihypertensive therapy [median/interquartile range (iqr) 0.61/0.11 vs 0.57/0.11 mm at baseline vs follow‐up, P = 0.0003, difference mean (95% CI) = −0.034 [−0.058 to −0.011], n = 47; Figure 1A). The change was more accentuated in subjects with below‐median mean blood pressure and higher IMT at baseline. For subjects situated in the top half of the sample in terms of MBP, only a moderate tendency for improvement in their IMT value could be seen (interaction P = 0.0009; Figure 1B).
Figure 1.
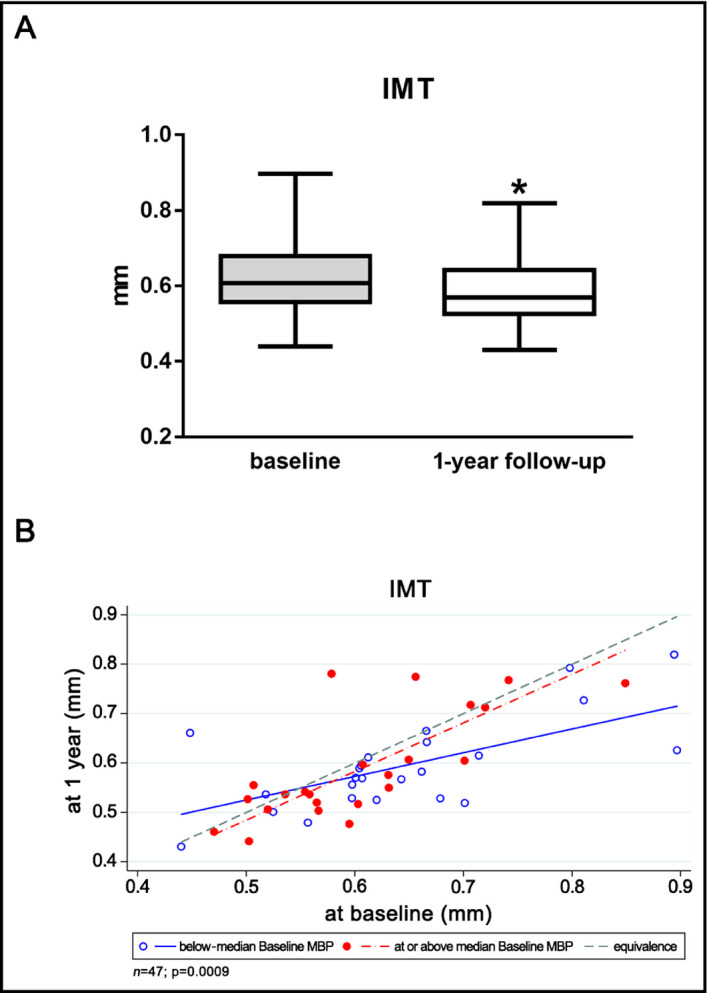
(A) IMT at baseline and after one year of antihypertensive therapy (B) IMT at the 1‐y follow‐up as a function of baseline IMT in subjects grouped by baseline MBP. Fitted regression lines below and above median represent subjects at 25th and 75th percentile, respectively, of baseline MBP. IMT, intima‐media thickness; MBP, mean blood pressure. *P = 0.0003
When analyzing the relationship of IMT with other parameters, a positive correlation was found with the TPR measured during HUTT (P = 0.0355, r = 0.270 at baseline; P = 0.0297, r = 0.281 at follow‐up) and stiffness parameters (P = 0.0261, r = 0.306 for correlation with AIx at baseline; P = 0.0321, r = 0.279 for correlation with AIx at follow‐up; P = 0.0258, r = 0.306 for correlation with PWV at baseline), while no correlation could be identified with the mean of STS (P = 0.0885, r = 0.206 at follow‐up).
3.6. Stiffness parameters
Both the AIx and the PWV values decreased non‐significantly after 1 year of antihypertensive therapy (−15.9 [31.8] vs −19.2 [31.9] % for mean [SD] AIx, P = 0.7411, difference mean [95% CI] = −0.873 [−6.17 to 4.42] and 9.6 [2.3] vs 9.2 [2.7] m/s for mean [SD] PWV, P = 0.0557, difference mean [95% CI] = −0.59 [−1.2 to 0.015], respectively; Figure 2). When analyzing the relationship of stiffness parameters with other variables, a positive correlation was found between the change in AIx and PWV and the change in mean arterial pressure (P = 0.0453, r = 0.268 for AIx and P = 0.0027, r = 0.423 for PWV, respectively). Moreover, a negative correlation was found between AIx and mean of STS at the baseline evaluation (P = 0.0009, r = −0.468), as well as between the change in PWV and that observed in the Spielberger trait anxiety score (P = 0.022, r = −0.328).
Figure 2.
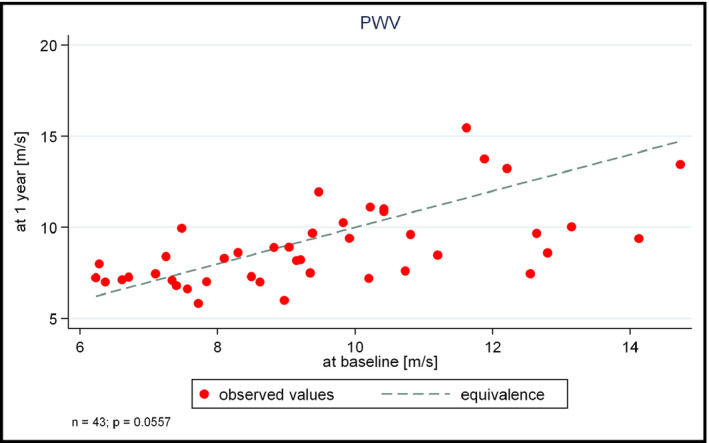
PWV at the one‐year follow‐up as a function of baseline PWV. PWV, pulse wave velocity
3.7. Head‐up tilt table testing
At 1‐year follow‐up, significantly lower values were obtained in BP, HR, and TPR index in both supine position and passive orthostasis during HUTT compared to baseline data. Data are presented in detail in Table 3.
Table 3.
Hemodynamic parameters during HUTT
| Parameters | Baseline | 1‐y follow‐up | P value, difference mean (95% CI) |
|---|---|---|---|
| sBP (mm Hg) | |||
| supinea | 134.6 (12.6) | 122.8 (10.7) | <0.0001, −11.83 (−15.19 to −8.464) |
| during tilta | 140.7 (12.7) | 127.9 (11.6) | <0.0001, −12.81 (−16.4 to −9.224) |
| change (%)a | 4.6 (4.4) | 4.2 (4.2) | 0.5814 |
| dBP (mm Hg) | |||
| supinea | 91.6 (9.4) | 82.8 (8.7) | <0.0001, −8.822 (−11.4 to −6.246) |
| during tilta | 100.7 (10.1) | 90.9 (8.6) | <0.0001, −9.741 (−12.24 to −7.241) |
| change (%)a | 10 (5.3) | 10 (4.3) | 0.9799 |
| mBP (mm Hg) | |||
| supinea | 103.6 (10) | 94.4 (8.4) | <0.0001, −9.245 (−12.01 to −6.477) |
| during tilta | 112.6 (10.4) | 101.8 (8.8) | <0.0001, −10.74 (−13.48 to −7.993) |
| change (%)a | 8.8 (4.8) | 8 (4.2) | 0.3710 |
| HR (beats/min) | |||
| supineb | 72.4/13.8 | 67.9/11 | 0.0019, −3.629 (−6.423 to −0.8347) |
| during tilta | 83.8 (10.7) | 80.2 (11) | 0.0358, −3.57 (−6.893 to −0.2464) |
| change (%)a | 15 (8.4) | 15.9 (9.4) | 0.4713 |
| SI (ml/m2) | |||
| supinea | 40.6 (9.7) | 41.1 (9.4) | 0.6402, 0.4388 (−1.437 to 2.315) |
| during tilta | 29.9 (5.7) | 30.9 (5.8) | 0.0632, 1.054 (−0.0605 to 2.168) |
| change (%)a | −24.7 (12.3) | −23.3 (11.6) | 0.3628 |
| TPRI (dyn × sec/cm5) | |||
| supineb | 2839/1268 | 2722/1045 | 0.0937, −187.2 (−358 to −16.47) |
| during tilta | 3728 (902) | 3383 (813) | 0.0003, −345.1 (−525.1 to −165.2) |
| change (%)b | 26.4/26.3 | 24.5/29 | 0.1082 |
| CBFVm (cm/s) | |||
| supineb | 67.5/13.1 | 67/12.3 | 0.7564, −0.4512 (−3.369 to 2.466) |
| during tiltb | 61.4/12.8 | 59.6/11.2 | 0.1881, −1.759 (−4.412 to 0.8947) |
| change (%)b | −8.8/6.3 | −10.8/4.8 | 0.0852 |
CBFVm, mean cerebral blood flow velocity; CI, confidence interval; dBP, diastolic blood pressure; HR, heart rate; HUTT, head‐up tilt test; mBP, mean blood pressure; sBP, systolic blood pressure; SI, stroke index; TPRI, total peripheral resistance index. n = 42 for CBFVm, n = 49 for the rest of the parameters.
Paired t test: data expressed as mean (SD).
Wilcoxon matched‐pairs signed‐ranks test: data expressed as median/interquartile range.
3.8. Neuropsychological tests
After 1 year of antihypertensive therapy, patients performed significantly better in many of the tests (reaction time, Rey Auditory Verbal Learning Test, Digit Span Test, Digit Symbol Test), while showing a non‐significant improvement in the remaining tests (Table 4). Hence, the mean of the STS also improved significantly after 1 year (Figure 3). In addition, the patients reached significantly lower scores on the tests evaluating anxiety (Table 4). When analyzing the correlation of these tests with other parameters, a negative correlation was found between the Spielberger trait anxiety score and blood pressure values at 1 year (P = 0.035, r = −0.28 for MBP; P = 0.018, r = −0.321 for SBP and P = 0.0272, r = −0.296 for DBP, respectively).
Table 4.
Neuropsychological tests
| Neuropsychological test | Baseline | 1‐y follow‐up | P value, difference mean (95% CI) |
|---|---|---|---|
| Choice reaction time (s), n=46a | 0.56 (0.08) | 0.56 (0.08) |
0.6448, 0.0054 (−0.018 to 0.029) |
| Selective reaction time (s), n=47b | 0.65/0.14 | 0.61/0.12 |
0.0116, −0.036 (−0.062 to −0.009) |
| Rey auditory verbal learning test (no.), n = 46a | 13.5/4.5 | 15/1 |
0.0001, 1.74 (0.924 to 2.55) |
| First recognition (no.), n = 47a | 13/3 | 12/2 |
0.2777, 0.681 (−0.096 to 1.46) |
| Pieron test (%), n = 46a | 92.21 (6.66) | 93.46 (6.64) |
0.1395, 2.52 (−0.266 to 5.32) |
| Trail making test (s), n = 47a | 29.33 (11.21) | 28.47 (9.76) |
0.5721, −0.83 (−3.76 to 2.11) |
| Digit span test (no.: forward + backward), n = 47a | 10/2.5 | 11/3 |
0.0076, 1.23 (0.235 to 2.23) |
| Block design test (s), n = 44a | 25.36 (3.57) | 25.62 (3.72) |
0.4065, 0.545 (−0.767 to 1.86) |
| Digit symbol test (no.), n = 45a | 46.85 (10.95) | 51.5 (10.73) |
<0.0001, 4.2 (2.68 to 5.72) |
| Mean of standardized test scores, n = 47a | 1.98/0.55 | 3.19/0.63 |
<0.0001, 1.23 (1.09 to 1.38) |
| Spielberger state anxiety inventory (score), n = 46a | 40.5/15 | 34/10 |
0.0008, −4.24 (−6.52 to −1.95) |
| Spielberger trait anxiety inventory (score), n = 44a | 38.83 (8.18) | 32.4 (6.66) |
<0.0001, −5.5 (−7.4 to −3.6) |
| Beck depression inventory (score), n = 47a | 4.5/6.5 | 3/6 |
0.1752, 0 (−1.87 to 1.87) |
CI, confidence interval.
Paired t test: data expressed as mean (SD).
Wilcoxon matched‐pairs signed‐ranks test: data expressed as median/interquartile range.
Figure 3.
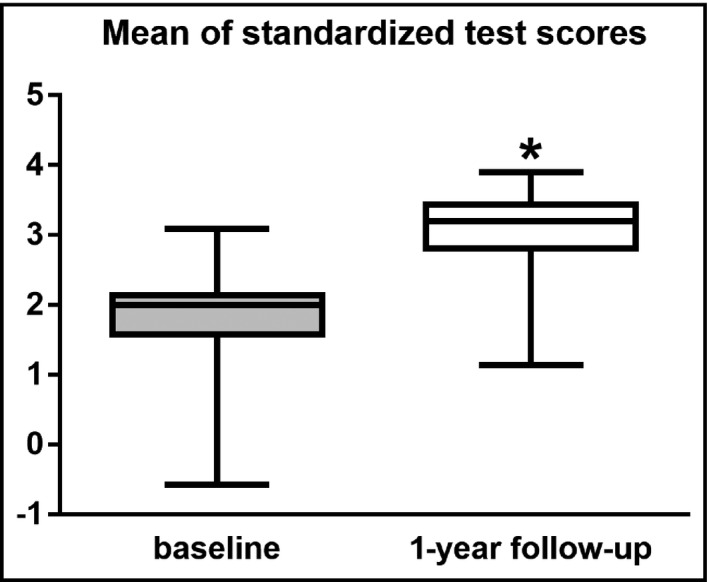
Averages of neuropsychological tests at baseline and after 1 y of antihypertensive therapy. *P < 0.0001
4. DISCUSSION
The current study focused on the reversibility of the vascular and cognitive changes in recently diagnosed hypertensive patients in response to medical management. After 1 year of antihypertensive treatment, IMT values of patients significantly decreased, while their stiffness parameters showed only a statistically non‐significant improvement. Nevertheless, the patients performed better on neuropsychological assessment at 1‐year follow‐up compared to baseline.
Previously, several investigations focused on structural vessel wall remodeling during hypertension. Disparate findings have surfaced regarding the beneficial long‐term effects of overall blood pressure control on IMT, as well as the extent of IMT progression upon various individual drug choices.6, 7, 18, 19, 20, 21, 22 In our prior work, we found higher IMT values in recently diagnosed hypertensive patients compared to controls.14 Although the numerical IMT values were within the normal range in both groups, apparently, statistical significance could be seen. The IMT values of the same hypertensive patients at 1‐year follow‐up were lower following the antihypertensive treatment, and the reduction was more pronounced in patients with a mean blood pressure level below the median value at baseline. This might draw attention to the importance of early recognition of elevated blood pressure values.
Arterial stiffness measurement may be utilized for retrieving valuable predictive information for cardiovascular outcomes, complementing standard risk factor assessments such as BP measurement.23 As hypertension is the strongest modifiable risk factor directly leading to arterial stiffness, a number of clinical trials have been carried out in the past to investigate the effect of antihypertensive medications on the change in arterial stiffness. Almost all classes of the tested antihypertensive medications effectively decreased arterial stiffness.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 In our study group, both stiffness parameters (PWV and AIx) decreased upon antihypertensive treatment; however, the reduction was statistically not significant. This might be due to the fact that the baseline average values were only borderline abnormal according to recent recommendations.35 The low number of patients may also explain why the observed changes could not reach statistical significance.
Prior investigations suggested that hypertension can affect both the structure and the function of the brain. According to more recent studies, a complex association exists between blood pressure and brain health, and this link is often dependent on other factors too, such as age, hypertension chronicity, and antihypertensive treatment. Lately, it has been suggested that hypertension‐induced alterations in brain structure and function are the results of cerebral vessel remodeling, leading to disruption in cerebral autoregulation, reduction in cerebral perfusion, and limitation of the brain's ability to eliminate potentially harmful proteins (eg, β‐amyloid).12 Previously, the effect of antihypertensive therapy on cognitive function of both the elderly and younger individuals was thoroughly investigated. While in the majority of the studies, examinations were conducted during a longer follow‐up period, shorter term effect of antihypertensive therapy on cognition was investigated in only a few studies. In the OSCAR study (Observational Study on Cognitive function And Systolic Blood Pressure Reduction) for instance, Hanon et al evaluated the effect of eprosartan in mono‐ or combination therapy in hypertensive patients older than 50 years of age during a follow‐up interval of only 6 months. They found a significant reduction in mean systolic blood pressure levels and improvement in the mean of Mini‐Mental State Examination.36 When examining older hypertensive subjects with mild executive dysfunction, Hajjar et al37 found that 1 year of ARB‐based therapy may preserve cerebral hemodynamics and improve executive function. In line with these findings, the patients in our study cohort also performed better on neuropsychological testing already after 1 year of antihypertensive therapy. Recent guidelines suggest that the various first‐line antihypertensive drug agents are more or less equivalent regarding their blood pressure lowering effect.2 However, some contradictory findings have emerged related to the ability of these drugs to prevent cognitive decline and dementia.38, 39, 40, 41 Our current study did not aim for comparing the preservative effects of the various antihypertensive drug agents on cognitive function. However, the results may indicate that an early commenced treatment may also be important for the conservation of a good cognitive battery. Nevertheless, further investigations on a higher number of patients are necessary in the future to provide additional information on the most efficient drug regimen and the importance of timing the therapy.
Recently, the association between anxiety and hypertension was thoroughly demonstrated in a systematic review and meta‐analysis performed by Pan et al.42 Although they concluded that an association between anxiety and increased risk of hypertension exists, they also cited a number of epidemiological studies with inconsistent results. While Muldoon et al38 found that a short time (6‐week) antihypertensive treatment did not affect mood or anxiety in middle‐aged hypertensive patients, our patients reached lower scores in the inventories evaluating both state and trait anxiety following 1 year of treatment. Taking into account the bidirectional association between anxiety and hypertension, it is hard to elucidate the sequence of events and tell which one is the “chicken” and which one is the “egg.”
Overall, our findings suggest that a timely diagnosis and adequate treatment of recently developed hypertension may revert the subclinical vascular and cognitive changes induced by early blood pressure elevation. Compared to previous studies, one strength of the present work is that we used an array of various methods to explore morphological and functional characteristics of the vasculature, cardio‐ and cerebrovascular hemodynamics, as well as a wide range of neuropsychological features. One weakness of our study is the relatively low number of patients. From a clinical perspective, our findings may be used to raise awareness to the importance of an early diagnosis and therapy at a stage of hypertension where the adverse changes are still reversible and further progression may be prevented. However, future work is essential to further scrutinize which subgroup of patients benefit the most from an early therapy, what pathomechanisms of reverting these abnormalities lay in the background and finally, to develop a tailored therapy with the most effective antihypertensive agent, taking into account patient‐specific information as well.
5. CONCLUSIONS
We demonstrated that after 1 year of effective antihypertensive therapy, the decrease in BP was accompanied not only by a more balanced hemodynamic state of our patients evident from HUTT measurements, but also by an improved cognitive battery assessed by neuropsychological testing. Anxiety level of the patients also decreased after 1 year of treatment. While a definitive improvement in vascular wall structure could be demonstrated by the reduction in IMT, the degree of improvement regarding the functional properties of the vascular wall represented by arterial stiffness parameters did not reach statistical significance. Overall, our study results may raise attention to an early diagnosis and prompt management of hypertension during the everyday clinical practice.
CONFLICT OF INTEREST
The authors report no relationships that could be construed as a conflict of interest.
AUTHOR CONTRIBUTIONS
KRC, CS, ZB, and PS were responsible for patient recruitment. KRC, CS, and ZB were responsible for data collection and source data preparation. AB was responsible for conducting the neuropsychological tests. LK was responsible for the statistical analysis and creating the figures. KRC was responsible for drafting the manuscript and creating the figures. DB, DC, KC, LC, MM, SM, LO, and PS were responsible for the critical revision of the manuscript. LC was responsible for the study design and final approval of the manuscript.
Czuriga‐Kovács KR, Szekeres CC, Bajkó Z, et al. Hypertension‐induced subclinical vascular and cognitive changes are reversible—An observational cohort study. J Clin Hypertens. 2019;21:658–667. 10.1111/jch.13537
Funding information
The study was supported by grants from the National Research, Development and Innovation Fund (K120042), the Gedeon Richter research fund (4700168520 KK/1781/2013), the Hungarian Academy of Sciences (MTA‐DE Cerebrovascular and Neurodegenerative Research Group), and the GINOP‐2.3.2‐15‐2016‐00048 project, which was also co‐financed by the European Union and the European Regional Development Fund. D. Cz. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00523/16/5) and by the ÚNKP‐18‐4 New National Excellence Program of the Ministry of Human Capacities (ÚNKP‐18‐4‐DE‐49).
REFERENCES
- 1. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591‐603. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). Eur Heart J. 2013;34(28):2159‐2219. [DOI] [PubMed] [Google Scholar]
- 3. Touboul P‐J, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness and plaque consensus (2004‐2006‐2011). Cerebrovasc Dis. 2012;34(4):290‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang L, Zhang J, Monticone RE, et al. Calpain‐1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age‐associated aortic wall calcification and fibrosis. Hypertension. 2012;60(5):1192‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Telmisartan improves morphologic and functional changes in both left ventricular myocardium and carotid arterial wall in patients with hypertension: assessment by tissue Doppler imaging and carotid ultrasonography. Echocardiography. 2010;27(7):864‐872. [DOI] [PubMed] [Google Scholar]
- 6. Simon A, Gariépy Jérome, Moyse D, Levenson J. Differential effects of nifedipine and co‐amilozide on the progression of early carotid wall changes. Circulation. 2001;103(24):2949‐2954. [DOI] [PubMed] [Google Scholar]
- 7. Zanchetti A, Bond MG, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double‐blind, long‐term trial. Circulation. 2002;106(19):2422‐2427. [DOI] [PubMed] [Google Scholar]
- 8. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236‐1241. [DOI] [PubMed] [Google Scholar]
- 9. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55(13):1318‐1327. [DOI] [PubMed] [Google Scholar]
- 10. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588‐2605. [DOI] [PubMed] [Google Scholar]
- 11. Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension. 2012;60(2):260‐268. [DOI] [PubMed] [Google Scholar]
- 12. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kovacs KR, Szekeres CC, Bajkó Z, et al. Cerebro‐ and cardiovascular reactivity and neuropsychological performance in hypertensive patients. J Neurol Sci. 2010;299(1–2):120‐125. [DOI] [PubMed] [Google Scholar]
- 14. Kovács KR, Bajkó Z, Szekeres CC, et al. Elevated LDL‐C combined with hypertension worsens subclinical vascular impairment and cognitive function. J Am Soc Hypertens. 2014;8(8):550‐560. [DOI] [PubMed] [Google Scholar]
- 15. Horváth IG, Németh Á, Lenkey Z, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28(10):2068‐2075. [DOI] [PubMed] [Google Scholar]
- 16. Baulmann J, Schillings U, Rickert S, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo‐electronic methods. J Hypertens. 2008;26(3):523‐528. [DOI] [PubMed] [Google Scholar]
- 17. Jatoi NA, Mahmud A, Bennett K, Feely J. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens. 2009;27(11):2186‐2191. [DOI] [PubMed] [Google Scholar]
- 18. Puato M, Boschetti G, Rattazzi M, et al. Intima‐media thickness remodelling in hypertensive subjects with long‐term well‐controlled blood pressure levels. Blood Press. 2017;26(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 19. Ohta Y, Kawano Y, Iwashima Y, et al. Control of home blood pressure with an amlodipine‐ or losartan‐based regimen and progression of carotid artery intima‐media thickness in hypertensive patients: the HOSP substudy. Clin Exp Hypertens. 2013;35(4):279‐284. [DOI] [PubMed] [Google Scholar]
- 20. Liu Z, Zhao Y, Wei F, et al. Treatment with telmisartan/rosuvastatin combination has a beneficial synergistic effect on ameliorating Th17/Treg functional imbalance in hypertensive patients with carotid atherosclerosis. Atherosclerosis. 2014;233(1):291‐299. [DOI] [PubMed] [Google Scholar]
- 21. Zhao XX, Liu J, Zhao H, Zhou Y, Li L, Wang H. The effect of cardiovascular risk factors on the carotid intima‐media thickness in an old‐aged cohort with hypertension: a longitudinal evolution with 4‐year follow‐up of a random clinical trial. Clin Exp Hypertens. 2018;41:658‐9. [DOI] [PubMed] [Google Scholar]
- 22. Yu JS,Choi YS, Kim JY et al. Carotid intima‐media thickness is not related with clinical outcomes in young hypertensives. Clin Hypertens. 2015;21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Townsend RR. Arterial stiffness: recommendations and standardization. Pulse (Basel). 2017;4(suppl 1):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andreadis EA, Angelopoulos ET, Kolyvas GN, Agaliotis GD, Mousoulis CG, Mousoulis GP. The effect of aliskiren versus ramipril‐based treatment on the ambulatory arterial stiffness index in hypertensive patients. Int Angiol. 2014;33(1):78‐83. [PubMed] [Google Scholar]
- 25. Guo JQ, Wang HY, Sun NL. Effect of aliskiren on arterial stiffness, compared with ramipril in patients with mild to moderate essential hypertension. Chin Med J (Engl). 2013;126(7):1242‐1246. [PubMed] [Google Scholar]
- 26. Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta‐analysis and meta‐regression of randomised controlled trials. Atherosclerosis. 2012;221(1):18‐33. [DOI] [PubMed] [Google Scholar]
- 27. Sasamura H, Kitamura Y, Nakamura M, Ryuzaki M, Saruta T. Effects of the angiotensin receptor blocker candesartan on arterial stiffness and markers of extracellular matrix metabolism in patients with essential hypertension. Clin Exp Hypertens. 2006;28(5):511‐520. [DOI] [PubMed] [Google Scholar]
- 28. Sumbria M, Negi PC, Sahai AK, Kaundal PK. To compare the effect of telmisartan with metoprolol on arterial stiffness in hypertension: prospective randomized parallel group trial. Indian Heart J. 2014;66(4):415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koumaras C, Tziomalos K, Stavrinou E, et al. Effects of renin‐angiotensin‐aldosterone system inhibitors and beta‐blockers on markers of arterial stiffness. J Am Soc Hypertens. 2014;8(2):74‐82. [DOI] [PubMed] [Google Scholar]
- 30. Takami T, Saito Y. Azelnidipine plus olmesartan versus amlodipine plus olmesartan on arterial stiffness and cardiac function in hypertensive patients: a randomized trial. Drug Des Devel Ther. 2013;7:175‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Werner TJ, Boutagy NE, Osterberg KL, Rivero JM, Davy KP. Singular and combined effects of nebivolol and lifestyle modification on large artery stiffness in hypertensive adults. Ther Adv Cardiovasc Dis. 2013;7(6):285‐292. [DOI] [PubMed] [Google Scholar]
- 32. Wu CF, Liu PY, Wu TJ, Hung Y, Yang SP, Lin GM. Therapeutic modification of arterial stiffness: an update and comprehensive review. World J Cardiol. 2015;7(11):742‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jekell A, Kahan T. The usefulness of a single arm cuff oscillometric method (Arteriograph) to assess changes in central aortic blood pressure and arterial stiffness by antihypertensive treatment: results from the Doxazosin‐Ramipril Study. Blood Press. 2018;27(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 34. Rajzer M, Wojciechowska W, Kameczura T, et al. The effect of antihypertensive treatment on arterial stiffness and serum concentration of selected matrix metalloproteinases. Arch Med Sci. 2017;13(4):760‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30(3):445‐448. [DOI] [PubMed] [Google Scholar]
- 36. Hanon O, Berrou J‐P, Negre‐Pages L, et al. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the observational study on cognitive function and systolic blood pressure reduction open‐label study. J Hypertens. 2008;26(8):1642‐1650. [DOI] [PubMed] [Google Scholar]
- 37. Hajjar I, Hart M, Chen Y‐L, et al. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. J Am Geriatr Soc. 2013;61(2):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muldoon MF, Waldstein SR, Ryan CM, et al. Effects of six anti‐hypertensive medications on cognitive performance. J Hypertens. 2002;20(8):1643‐1652. [DOI] [PubMed] [Google Scholar]
- 39. Levi Marpillat N, Macquin‐Mavier I, Tropeano A‐I, Bachoud‐Levi A‐C, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta‐analysis. J Hypertens. 2013;31(6):1073‐1082. [DOI] [PubMed] [Google Scholar]
- 40. Rouch L, Cestac P, Hanon O, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta‐analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29(2):113‐130. [DOI] [PubMed] [Google Scholar]
- 41. Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord. 2016;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pan Y, Cai W, Cheng Q, Dong W, An T, Yan J. Association between anxiety and hypertension: a systematic review and meta‐analysis of epidemiological studies. Neuropsychiatr Dis Treat. 2015;11:1121‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]


