Abstract
This was a post hoc analysis of Systolic Blood Pressure Intervention Trial (SPRINT), aimed to investigate whether intensive blood pressure treatment has differential therapeutic outcomes on patients with different baseline Framingham risk score (FRS). The 9298 SPRINT participants were categorized into low‐risk (baseline FRS < 10%), intermediate‐risk (FRS = 10%‐20%), or high‐risk (FRS > 20%) arms. The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes. Serious adverse events were defined as hypotension, syncope, and bradycardia. Multiple Cox regression was used to calculate hazard ratios for study outcomes with intensive compared with standard SBP treatment between these three groups. After a median follow‐up time of 3.26 years, the primary outcome hazard ratio (HR) for intensive versus standard treatment was 0.73 (95% CI: 0.61‐0.88, P = .0044) in the high‐risk arm. And, for all‐cause mortality, the hazard ratio with intensive SBP treatment was 1.58 (95% CI: 0.55‐1.06), 0.9 (95% CI: 0.26‐9.50), and 0.53 (95% CI: 0.34‐0.82) in three arms (all P values for interaction > 0.05). Effects of intensive versus standard SBP control on serious adverse events were similar among patients with different FRS. Our results suggested that regardless of the FRS level, the intensive blood pressure control was beneficial.
Keywords: cardiovascular disease, Framingham risk score, intensive blood pressure treatment, Systolic Blood Pressure Intervention Trial (SPRINT)
1. INTRODUCTION
Hypertension is a leading but preventable risk factor for stroke, cardiovascular disease (CVD), renal disease, and a significant cause of morbidity and mortality,1 affecting more than one billion adults worldwide.2 Inadequate control of hypertension increases risks for acute coronary syndrome (ACS), heart failure (HF), and chronic kidney disease (CKD).3 Studies have shown that systolic blood pressure (SBP) is a more important independent risk factor for cardiovascular and renal disease than diastolic blood pressure (DBP).4 It has been shown that adequate control of hypertension can effectively reduce the risk of CVD outcomes, including stroke, myocardial infarction, and HF.5, 6, 7
Most of the current guidelines recommend a target SBP lower than 140 mm Hg to reduce cardiovascular events.8, 9 Nevertheless, some studies have suggested that intensive treatment of hypertension could provide beneficial effects in some specific populations.10, 11, 12 Current guidelines suggest that antihypertensive treatment should be determined by blood pressure level together with other major CVD risk factors (such as sex, age, diabetes, smoking, and cholesterol).13, 14, 15, 16 The beneficial effect of using the predicted CVD risk model to guide the antihypertensive treatment has been documented.17, 18 A meta‐analysis including 47 872 participants by Karmali et al. showed that the blood pressure–lowering treatment strategy was more effective based on the predicted CVD risk than based on blood pressure levels alone.18 However, in clinical practice, physicians typically make a therapeutic decision based on blood pressure level.19 However, more studies are needed to confirm the benefit of predicted CVD risk model in determining antihypertensive treatment strategy.
The Systolic Blood Pressure Intervention Trial (SPRINT) is a large study with the purpose to compare the effect of intensive SBP treatment (SBP target < 120) and standard SBP treatment (SBP target < 140) on the mortality, cardiovascular, and kidney diseases of the patients, which enrolled 9361 non‐diabetic participants aged 50 years from approximate 100 medical centers in the United States and Puerto Rico between 2009 and 2013.12 The results suggest that in the non‐diabetic high‐risk patients (such as those patients companied with CKD, CVD, or a Framingham risk score (FRS) more than 15%), intensive SBP treatment results in lower rate of the composite primary outcome, including myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular cause compared with standard SBP treatment.12 Several post hoc analyses of SPRINT have been conducted to investigate the effect of intensive SBP treatment on several specific populations, such as patients with high‐normal fasting glucose,21, 22 patients aged older than 75 years.20
The purpose of the current study was to compare the effect of intensive blood pressure control among the patients with different baseline FRS, using data from the SPRINT.
2. METHODS
2.1. Study design and population
This was a post hoc analysis of SPRINT. The SPRINT data were obtained from the National Heart, Lung, and Blood Institute (NHLBI) Data Repository (https://biolincc.nhlbi.nih.gov/studies/sprint_pop). SPRINT was a randomized, single‐blinded treatment trial.12
According to the original paper of SPRINT,12 a total of 9361 participants from the United States and Puerto Rico were recruited and randomized into the intensive treatment group (SBP target < 120 mm Hg) and the standard treatment group (SBP target < 140 mm Hg).12 The inclusion criteria in SPRINT were as follows: 1) ≥50 years old; 2) with an SBP 130‐180 mm Hg; 3) with an increased risk of CVD, which was defined as one or more of the following: clinical or subclinical CVD other than stroke; age ≥ 75 years; or a FRS for 10‐year CVD risk ≥ 15%; or CKD, estimated glomerular filtration rate (eGFR) 20 to <60 mL/min/1.73 m2). The exclusion criteria included diabetes mellitus, prior stroke, advanced CKD (eGFR < 20 ml/min/1.73 m2), proteinuria >1 g/day, polycystic kidney disease, HF.12
The FRS is a continuous score, ranging from 0% to 100%. Clinical guidelines recommend categorizing asymptomatic individuals into low (FRS < 10%)‐, intermediate (10‐20%)‐, and high‐risk arms (>20%) for risk management purpose.23, 24 In this study, we used the same cut‐offs to categorize the participants of SPRINT into three categories. We excluded those participants without FRS levels (n = 63), leaving 9298 patients in the final analysis.
2.2. Interventions
Participants were followed up monthly for the first 3 months and then every 3 months thereafter. All major antihypertensive drug classes were included in the medication formulary. Medications were adjusted to target a SBP <120 mm Hg in the intensive treatment group and 135‐139 mm Hg in the standard treatment group, respectively.
2.3. Data collection
Clinical and laboratory data were collected at baseline and every 3 months thereafter and quarterly thereafter for measurement of serum creatinine. An automated measurement system (Model 907, Omron Healthcare, USA) was used to record BP at the clinic visit after the participant had seated for 5 minutes of quiet rest. The mean of three office BP measurements was used to estimate BP. A structured interview was undertaken every 3 months to obtain the CVD outcomes by patient self‐report.25
2.4. Clinical outcomes
The primary CVD outcome was a composite of nonfatal myocardial infarction (MI), ACS not resulting in an MI, nonfatal stroke, nonfatal acute decompensated HF, and death from cardiovascular causes. The secondary outcome included the individual components of the primary outcome and all‐cause death.12, 25
2.5. Serious adverse events
Serious adverse events (SAEs) were defined as fatal or life‐threatening events causing significant or persistent disability, requiring hospitalization or prolonged hospitalization or significant hazard or harm medical events requiring medical or surgical intervention. The following conditions were reported as adverse events if they were evaluated in an emergency department: hypotension, syncope, bradycardia, and acute renal failure.12, 25
2.6. Statistical analysis
Baseline characteristics were compared between the intensive treatment and the standard treatment groups two treatment groups stratified by FRS (FRS < 10%; 10% ≤ FRS ≤ 20%; FRS > 20%) using ANOVA for continuous variables and chi‐square tests for categorical variables.
Cox proportional hazards regression was performed to calculate the hazard ratio (HR) and 95% confidence interval (CI) of clinical outcomes and severe adverse events between intensive treatment group and standard group, according to baseline FRS levels. Multivariate model adjusted for age, race, sex, body mass index (BMI), eGFR, SBP, DBP, albumin‐to‐creatinine ratio, high‐density lipoprotein cholesterol (HDL‐C), total cholesterol (TC), and history of CKD and CVD, number of antihypertensive agents, and use of statin. We also modeled FRS as restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles of its distribution. To test for differences within the various subgroups, P values for interaction were derived from multivariable Cox regression models.26, 27 All tests were two‐sided, and the significance level was set at P < .05. All statistical analyses were performed using R 3.3.0 software (http://www.R-project.org).
3. RESULTS
3.1. Baseline characteristics of participants
There were 1473, 3937, and 3887 cases in the low‐, intermediate‐, and high‐risk arms, respectively. The median follow‐up time was 3.26 years (interquartile range 2.79‐3.79 years). The demographic and clinical characteristics of the SPRINT study population are summarized in Table 1. Participants in the high‐risk group were older, fatter, and with higher SBP and DBP. There was a significant difference in the mean age, female proportion, SBP, DBP, BMI, lipid levels, and medication use compared with those in lower FRS arms (all P < .001). A trend could be observed that the ranked order of the FRS was consistent with that of the mean age and SBP among the three arms. However, there were no significant differences in these parameters between the intensive group and standard group among the three FRS‐stratified analyses, indicating the two groups were mainly comparable.
Table 1.
Baseline demographic and clinical characteristics of participants
| Characteristics | Baseline FRS, % | P value | |||||
|---|---|---|---|---|---|---|---|
| Low risk (FRS < 10%) | Intermediate risk (10% ≤ FRS ≤ 20%) | High risk (FRS > 20%) | |||||
| Intensive | Standard | Intensive | Standard | Intensive | Standard | ||
| N | 740 | 733 | 1988 | 1949 | 1923 | 1964 | |
| FRS, % | 7.8 ± 1.6 | 7.6 ± 1.7 | 14.8 ± 2.8 | 14.7 ± 2.9 | 30.2 ± 9.3 | 30.1 ± 9.0 | <.001 |
| Age, y | 62.6 ± 7.9 | 62.6 ± 8.2 | 66.1 ± 8.6 | 66.1 ± 8.6 | 71.9 ± 9.0 | 71.7 ± 9.2 | <.001 |
| Female | 586 (79.2%) | 576 (78.6%) | 864 (43.5%) | 827 (42.4%) | 217 (11.3%) | 232 (11.8%) | <.001 |
| Smoking status | |||||||
| Never | 434 (58.6%) | 413 (56.3%) | 932 (46.9%) | 926 (47.5%) | 679 (35.3%) | 725 (36.9%) | .272 |
| Former | 250 (33.8%) | 276 (37.7%) | 856 (43.1%) | 846 (43.4%) | 864 (44.9%) | 860 (43.8%) | |
| Current | 56 (7.6%) | 44 (6.0%) | 200 (10.1%) | 177 (9.1%) | 380 (19.8%) | 379 (19.3%) | |
| SBP, mm Hg | 128.1 ± 12.3 | 128.2 ± 12.4 | 138.0 ± 13.9 | 137.9 ± 13.4 | 145.9 ± 15.8 | 145.7 ± 15.4 | <.001 |
| DBP, mm Hg | 75.8 ± 11.0 | 76.0 ± 11.3 | 78.6 ± 11.4 | 78.2 ± 11.6 | 78.7 ± 12.6 | 78.7 ± 12.6 | <.001 |
| BMI, kg/m2 | 31.6 ± 6.8 | 31.2 ± 6.8 | 30.0 ± 5.8 | 30.2 ± 5.9 | 29.1 ± 5.2 | 28.8 ± 4.9 | <.001 |
| TC, mg/dL | 185.5 ± 37.3 | 185.4 ± 39.4 | 190.0 ± 41.0 | 188.7 ± 40.1 | 192.2 ± 43.2 | 193.3 ± 42.1 | <.001 |
| HDL‐C, mg/dL | 58.5 ± 16.0 | 59.7 ± 16.5 | 54.4 ± 14.1 | 54.3 ± 14.8 | 49.2 ± 12.9 | 48.9 ± 12.3 | <.001 |
| TG, mg/dL | 104.2 ± 50.7 | 104.9 ± 62.4 | 118.3 ± 62.6 | 120.3 ± 69.1 | 139.7 ± 111.2 | 142.0 ± 121.3 | .910 |
| Glucose, mg/dL | 97.6 ± 14.7 | 96.9 ± 14.6 | 98.7 ± 13.6 | 99.1 ± 13.8 | 99.5 ± 13.4 | 99.2 ± 12.4 | .002 |
| ALCR, mg/g | 31.7 ± 117.6 | 51.0 ± 238.5 | 34.0 ± 126.0 | 31.3 ± 104.4 | 58.9 ± 234.2 | 47.1 ± 153.2 | <.001 |
| CKD history | 203 (27.4%) | 205 (28.0%) | 514 (25.9%) | 481 (24.7%) | 611 (31.8%) | 624 (31.8%) | .184 |
| CVD history | 144 (19.5%) | 163 (22.2%) | 350 (17.6%) | 358 (18.4%) | 444 (23.1%) | 353 (18.0%) | .031 |
| N_AGENTS | |||||||
| 0 | 10 (1.4%) | 10 (1.4%) | 112 (5.6%) | 100 (5.1%) | 307 (16.0%) | 337 (17.2%) | <.001 |
| 1 | 228 (30.8%) | 213 (29.1%) | 577 (29.0%) | 598 (30.7%) | 550 (28.6%) | 564 (28.7%) | |
| 2 | 276 (37.3%) | 287 (39.2%) | 758 (38.1%) | 721 (37.0%) | 621 (32.3%) | 606 (30.9%) | |
| 3 | 172 (23.2%) | 177 (24.1%) | 429 (21.6%) | 413 (21.2%) | 353 (18.4%) | 367 (18.7%) | |
| 4 | 52 (7.0%) | 44 (6.0%) | 109 (5.5%) | 113 (5.8%) | 90 (4.7%) | 86 (4.4%) | |
| 5 | 1 (0.1%) | 2 (0.3%) | 3 (0.2%) | 4 (0.2%) | 2 (0.1%) | 4 (0.2%) | |
| 6 | 1 (0.1%) | 0 (0.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Statin | 307 (41.5%) | 332 (45.8%) | 879 (44.5%) | 906 (46.7%) | 791 (41.4%) | 823 (42.3%) | <.001 |
Values are mean ± SD or number (%).
Abbreviations: ALCR, albumin‐to‐creatinine ratio; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; FRS, Framingham risk score; HDL‐C, high‐density lipoprotein cholesterol; N_AGENTS, number of antihypertensive agents; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
3.2. FRS‐stratified comparison in the clinical outcomes between the intensive and the standard groups
As shown in Table 2, in participants with high‐risk levels, intensive treatment could obviously reduce the risk of primary outcome compared with the standard treatment after multiple adjustment (HR = 0.73, 95% CI: 0.61‐0.88, P = .0044). The between‐group differences in the secondary outcome were consistent with primary outcome. The HRs in the total population were 0.73 (95% CI: 0.62‐0.87, P = .0004) and 0.77 (95% CI: 0.66‐0.89, P < .001) in the primary and secondary outcomes (Table 2, Figure 1). For all‐cause mortality, the hazard ratios for patients in three groups were 1.58 (95% CI: 0.26‐9.50), 0.9 (95% CI: 0.23‐3.60), and 0.53 (95% CI: 0.34‐0.82), respectively. However, no significant differences were found in the primary and secondary outcomes in patients with low‐ and intermediate‐risk levels. We further did P for interaction to investigate the association between baseline FRS and intensive SBP control (P value for interaction = .84; Figure 2), suggesting that intensive blood pressure control was beneficial in total population regardless of FRS levels.
Table 2.
Cox regression analysis for primary and secondary outcomes
| Subgroup | Primary outcome | Secondary outcome | ||
|---|---|---|---|---|
| Univariate (HR [95% CI] P value) | Multivariate (HR [95% CI] P value) | Univariate (HR [95% CI] P value) | Multivariate (HR [95% CI] P value) | |
| Low risk (FRS < 10%) | ||||
| Standard | ref | ref | ref | ref |
| Intensive | 1.10 (0.66‐1.82) .7224 | 1.11 (0.57‐2.16) .4726 | 1.45 (0.41‐5.14) .5646 | 1.58 (0.26‐9.50) .4138 |
| Intermediate risk (10% ≤ FRS < 20%) | ||||
| Standard | ref | ref | ref | ref |
| Intensive | 0.68 (0.50‐0.92) .0128* | 0.83 (0.44‐1.56) .069 | 0.97 (0.21‐1.03) .0606 | 0.90 (0.23‐3.60) .0569 |
| High risk (FRS > 20%) | ||||
| Standard | ref | ref | ref | ref |
| Intensive | 0.75 (0.61‐0.94) .0120* | 0.73 (0.61‐0.88) .0044** | 0.56 (0.33‐0.94) .0286* | 0.53 (0.34‐0.82) .0109* |
| Total population | ||||
| Standard | ref | ref | ref | ref |
| Intensive | 0.76 (0.64‐0.90) .0014** | 0.73 (0.62‐0.87) .0004*** | 0.59 (0.39‐0.88) .0106* | 0.56 (0.37‐0.85) .0068** |
Multivariate model adjusted for: age; sex; body mass index; chronic kidney disease; cardiovascular disease; glomerular filtration rate; systolic blood pressure; diastolic blood pressure; number of antihypertensive agents; albumin‐to‐creatinine ratio; statin.
The primary CVD outcome was a composite of nonfatal myocardial infarction (MI), acute coronary syndrome (ACS) not resulting in a MI, nonfatal stroke, nonfatal acute decompensated HF, and death from cardiovascular causes.
The secondary outcomes included the individual components of the primary outcome and all‐cause death.
P < .05.
P < .01.
P < .001.
Figure 1.
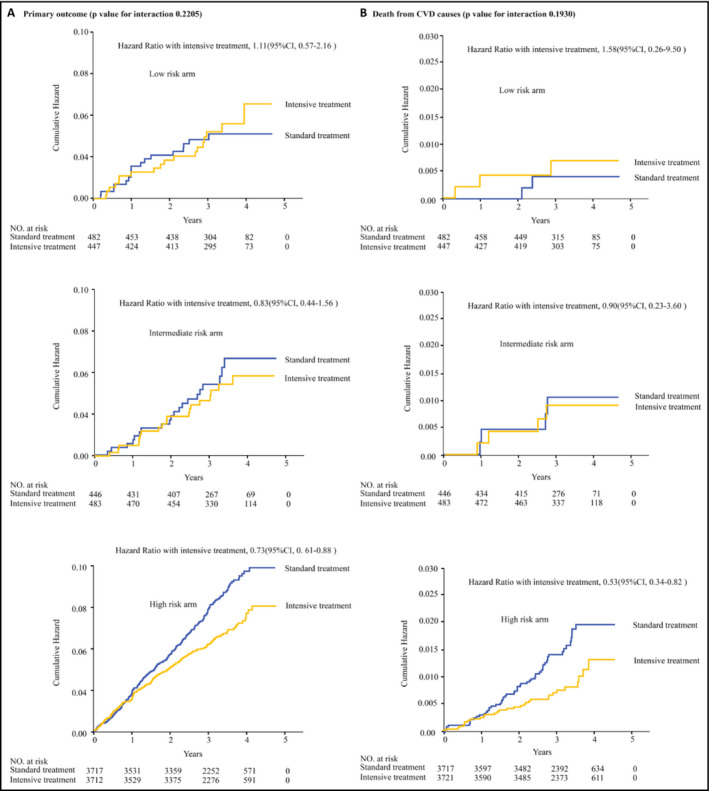
FRS‐stratified Kaplan‐Meier curve analysis with log‐rank test was performed to compare the primary outcome (A) or the secondary outcome (B) between the intensive treatment and the standard treatment groups within low‐, intermediate‐, and high‐risk arms. Low‐risk arm: baseline FRS < 10%; intermediate‐risk arm: 10% ≤baseline FRS < 20%; high‐risk arm: baseline FRS > 20%
Figure 2.
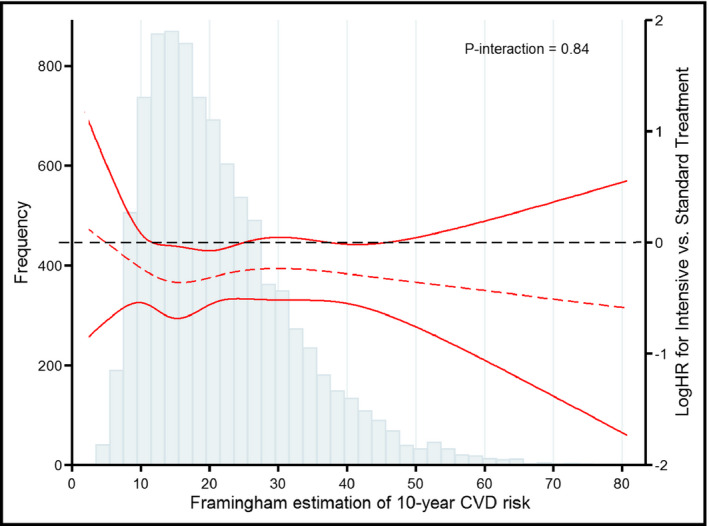
The treatment effect of intensive versus standard blood pressure control across the Framingham risk score spectrum for the primary outcome. The curves represent adjusted hazard ratios (solid line) and their 95% confidence intervals (dashed lines) or the primary outcome among different FRS levels. The histogram represents the frequency distribution of the Framingham risk score at baseline
3.3. FRS‐stratified subgroup analysis of the serious adverse events between intensive and standard groups
The incidence of treatment‐related SAEs was lower (3.16%, 157/4964) in the low‐SBP group compared with the high‐SBP group (4.12%, 181/4397). As shown in Table 3, the risk for treatment‐related SAEs was significantly increased in the total population (HR = 1.89, 95% CI: 1.50‐2.38, P < .0001), intermediate‐risk arm (HR = 1.98, 95% CI: 1.38‐2.85, P < .0002), and high‐risk arm (HR = 2.07, 95% CI: 1.46‐2.93, P < .0001). However, no significant interaction trend was detected between these three groups (all P values for interaction > .05).
Table 3.
Cox regression analysis for serious adverse events (SAEs)
| Subgroup analysis | Univariate (HR [95% CI] P value) | Multivariate (HR [95% CI] P value) |
|---|---|---|
| Low risk (FRS < 10%) | ||
| Standard | ref | ref |
| Intensive | 1.28 (0.70‐2.32) .4215 | 1.22 (0.65‐2.27) .5398 |
| Intermediate risk (10% ≤ FRS < 20%) | ||
| Standard | ref | ref |
| Intensive | 1.86 (1.32‐2.63) .0004*** | 1.98 (1.38‐2.85) .0002*** |
| High risk (FRS > 20%) | ||
| Standard | ref | ref |
| Intensive | 2.14 (1.52‐3.01) <.0001*** | 2.07 (1.46‐2.93) <.0001*** |
| Total population | ||
| Standard | ref | ref |
| Intensive | 1.88 (1.50‐2.35) <.0001*** | 1.89 (1.50‐2.38) <.0001*** |
| P for interaction | 0.312 | 0.356 |
Multivariate model adjusted for: age; sex; race; body mass index; chronic kidney disease; cardiovascular disease; glomerular filtration rate; systolic blood pressure; diastolic blood pressure; number of antihypertensive agents; albumin‐to‐creatinine ratio; statin; serious adverse events (SAEs) were defined as fatal or life‐threatening events causing significant or persistent disability, requiring hospitalization or prolonged hospitalization or significant hazard or harm medical events requiring medical or surgical intervention. The following conditions were reported as adverse events if they were evaluated in an emergency department: hypotension, syncope, bradycardia, and acute renal failure.
P < .001.
4. DISCUSSION
In this study, we investigated whether intensive blood pressure treatment has differential therapeutic outcomes on patients with different baseline FRS using data from the SPRINT. Our results showed that intensive SBP treatment could reduce CVD events and all‐cause mortality in hypertensives regardless of baseline FRS. And the effect of intensive SBP control on safety outcomes was also not statistically different between those with different baseline FRS. Our study could remind clinicians paid more attention to baseline SBP when a proper SBP target to be determined for hypertensives.
The SPRINT suggests that in the non‐diabetic hypertensive patients with high risk for CVD, intensive SBP treatment reduces the rate of fatal and nonfatal cardiovascular events as well as all‐cause death.12 In SPRINT, the increased risk of CVD was defined as one or more following conditions: clinical or subclinical CVD other than stroke; age ≥ 75 years; FRS ≥ 15%; CKD.12 To standardize the definition of high risk for CVD, we conducted the post hoc subgroup analysis of the data from SPRINT stratified by the patients’ baseline FRS. FRS is a well‐accepted score to estimate the cardiovascular risk in the general population,28 considering age, sex, smoking, antihypertensive treatment, baseline SBP, and cholesterol levels and predicts the risk of coronary events by stratifying individuals into three risk categories: low (<10% risk of an event in 10 years), intermediate (10%‐20%), and high (>20%).29 As a result, our analysis showed that there were significant differences in the age, sex, baseline SBP, number of antihypertensive agents and cholesterol levels among the low‐, intermediate‐, and high‐risk arms. The SPRINT‐Senior subgroup analysis by Williamson et al demonstrated that the intensive treatment reduces the risk for the primary outcome by 34% and the risk for all‐cause mortality by 33% the participants older than 75,20 indicating that the intensive treatment more likely benefits the senior patients. Supporting this notion, our analysis showed that the intensive treatment reduced the risk for primary by 27% (HR = 0.73) and secondary outcomes by 47% (HR = 0.53) in the high‐risk arm with a mean age of 71.8 years.
There is still controversy about whether the blood pressure–lowering strategy should be determined based on the blood pressure. And, more studies are needed to clear a proper SBP target for hypertensive individuals with different diseases and baseline characteristics.30 Guidelines suggested that clinicians should include CVD risk into the consideration of therapeutic decision‐making.13, 14, 15, 16 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults provides recommendations for lower BP medication initiation thresholds (130/80 mm Hg) and BP target goals.31 However, current clinicians typically prescribe antihypertensive medications only based on blood pressure level.19 The ACCORD trial showed targeting a SBP of <120 mm Hg compared with <140 mm Hg did not reduce the rate of MACE in patients with type 2 diabetes.32 Our study suggested that the significant benefits of intensive blood pressure control could be observed, no matter what FRS categories of patients were. Our results were in line with a meta‐analysis of 67,475 individuals demonstrating that lowering blood pressure provides similar relative protection at all levels of baseline 5‐year major CVD risk. 33 These results suggested that although FRS would affect the cardiovascular outcomes of the patient, it does not affect the therapeutic benefits of intensive SBP control. Our finding further confirmed that antihypertensive treatment strategy could be determined based on a combination of the predicted cardiovascular risk and the blood pressure level. In clinical practice, it is reasonable to determine a proper blood pressure–lowering treatment strategy based on an individual's CVD risk. In SPRINT, the benefits of intensive treatment are accompanied by an elevated incidence of SAEs.12 Although treatment‐related SAEs increased in our study, no interaction P value was detected between patients with different CVD risks. The current analysis provided more evidence to the benefits of intensive SBP treatment in patients with different levels of CVD risks, and the beneficial effects of intensive treatment did not appear to be attenuated by lower FRS levels.
A few clinical implications should be addressed in our study. To start with, the current study investigated the association of baseline FRS and treatment target of SBP, contributing additional information on SBP management strategies in hypertensives without diabetes. Secondly, SPRINT is a well‐designed, randomized controlled study, allowing for large subgroups of those with different FRS at baseline.
There are still some limitations of this study. First, this was a post hoc study of SPRINT. In addition, our result is not applicable for the patients younger than 50 years, with diabetes, prior stroke, advanced CKD (eGFR < 20 mL/min/1.73 m2), congestive heart failure as those populations were excluded from SPRINT. A well‐designed prospective trial with a large sample size should be conducted to assess the effect of intensive treatments in these populations and validate the findings of this study.
In summary, the current study suggested that regardless of the FRS level, intensive blood pressure control (<120 mm Hg) could bring more benefits for patients compared with the standard blood pressure treatment (<140 mm Hg). However, the benefits were accompanied by an elevated risk for SAEs.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
We declare that all the listed authors have participated actively in the study and all meet the requirements of the authorship. Drs. Xiaodong Zhuang and Xinxue Liao had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Xinxue Liao, Xiaodong Zhuang, Ling Zhang, Xiuting Sun, and Lizhen Liao were responsible for the study concept and design. Drs. Xiuting Sun, Shaozhao Zhang, Huimin Zhou, and Xiangbin Zhong analyzed and interpreted the data. Drs. Shaozhao Zhang, Huimin Zhou, and Xiangbin Zhong performed the statistical analysis. Drs. Xiaodong Zhuang and Xinxue Liao obtained funding. Drs. Xiaodong Zhuang, Xinxue Liao, Ling Zhang, and Xiuting Sun drafted the manuscript. Drs. Xiaodong Zhuang and Xinxue Liao critically revised the manuscript. All authors approved the final version of the manuscript.
Zhang L, Sun X, Liao L, et al. Effectiveness of blood pressure–lowering treatment by the levels of baseline Framingham risk score: A post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). J Clin Hypertens. 2019;21:1813–1820. 10.1111/jch.13720
Zhang and Sun contributed to this study equally.
Funding information
This study was supported by the National Natural Science Foundation of China (81600206; 81870195) and Natural Science Foundation of Guangdong Province (2016A030310140; 20160903). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Xiaodong Zhuang, Email: zhuangxd3@mail.sysu.edu.cn.
Xinxue Liao, Email: liaoxinx@mail.sysu.edu.cn.
REFERENCES
- 1. Bromfield S, Muntner P. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr Hypertens Rep. 2013;15:134‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480‐486. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143‐152. [DOI] [PubMed] [Google Scholar]
- 4. Sundström J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension. Ann Intern Med. 2015;162:184. [DOI] [PubMed] [Google Scholar]
- 5. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA. 1997;277:739‐745. [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of ACE inhibitors, calcium antagonists, and other blood‐pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet (London, England). 2000;356:1955‐1964. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 9. James PA, Oparil S, Carter BL, et al. 2014 Evidence‐Based Guideline for the Management of High Blood Pressure in Adults. JAMA. 2014;311:507. [DOI] [PubMed] [Google Scholar]
- 10. Wei Y, Jin Z, Shen G, et al. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens. 2013;15:420‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chrysant SG. Aggressive systolic blood pressure control in older subjects: benefits and risks. Postgrad Med. 2018;130:159‐165. [DOI] [PubMed] [Google Scholar]
- 12. SPRINT Research Group , Wright JT, Williamson JD, Whelton PK, et al. A Randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasgupta K, Quinn RR, Zarnke KB, et al. The 2014 Canadian hypertension education program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;2014(30):485‐501. [DOI] [PubMed] [Google Scholar]
- 14. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group . Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. [DOI] [PubMed] [Google Scholar]
- 15. Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;2016(37):2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2013;22:193‐278. [DOI] [PubMed] [Google Scholar]
- 17. Sussman J, Vijan S, Hayward R. Using benefit‐based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karmali KN, Lloyd‐Jones DM, van der Leeuw J, et al. Blood pressure‐lowering treatment strategies based on cardiovascular risk versus blood pressure: a meta‐analysis of individual participant data. PLoS Medicine. 2018;15:e1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weber AM, Schiffrin LE, White BW, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;16:14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong Y, Smith SM, Handberg EM, Pepine CJ, Cooper‐DeHoff RM. Intensive blood pressure lowering reduces adverse cardiovascular outcomes among patients with high‐normal glucose: an analysis from the Systolic Blood Pressure Intervention Trial database. J Clin Hypertens. 2018;20:620‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017;40:1401‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grundy SM, Cleeman JI, Merz C, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227‐239. [DOI] [PubMed] [Google Scholar]
- 24. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486‐2497. [DOI] [PubMed] [Google Scholar]
- 25. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med. 2004;23:2509‐2525. [DOI] [PubMed] [Google Scholar]
- 27. Royston P, Sauerbrei W. Interaction of treatment with a continuous variable: simulation study of significance level for several methods of analysis. Stat Med. 2013;32:3788‐3803. [DOI] [PubMed] [Google Scholar]
- 28. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Predict Coron Hear Dis. 1998;97:1837‐1847. [DOI] [PubMed] [Google Scholar]
- 29. Ford ES, Giles WH, Mokdad AH. The distribution of 10‐year risk for coronary heart disease among US adults ‐ Findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791‐1796. [DOI] [PubMed] [Google Scholar]
- 30. Kulenthiran S, Ewen S, Böhm M, et al. Hypertension up to date: SPRINT to SPYRAL. Clin Res Cardiol. 2017;106(7):475‐484. [DOI] [PubMed] [Google Scholar]
- 31. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 32. Group AS , Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blood Pressure Lowering Treatment Trialists’ Collaboration . Blood pressure‐lowering treatment based on cardiovascular risk: a meta‐analysis of individual patient data. Lancet. 2014;384:591‐598. [DOI] [PubMed] [Google Scholar]


