Abstract
We conducted a cross‐sectional study among school/college students in Tanzania and Uganda to determine the prevalence of high blood pressure (BP) and associated factors. Participants were classified to have high BP if they had pre‐hypertension or hypertension. Interviews were done using the WHO STEPS instrument. Using data from both countries (n = 1596), the overall prevalence of high BP was 40% (95% CI: 37‐42). The prevalence of pre‐hypertension was 29% (95% CI: 26‐31) and that of hypertension was 11% (95% CI: 10‐13). High BP was independently associated with obesity (aOR = 6.7, 95% CI: 2.2‐20.0), male sex (aOR = 3.2, 95% CI: 2.4‐4.4), and among males aged above 20 years (aOR = 5.5, 95% CI: 2.9−10.5). Consumption of fruits/vegetables was associated with decreased odds for high BP (aOR = 0.7, 95% CI: 0.50‐0.98). The increasing burden of pre‐hypertension across age groups could explain the early onset of hypertension and cardiovascular diseases (CVDs) among young African adults. There is a need for longitudinal studies to explore the drivers of pre‐hypertension in East African adolescents.
Keywords: adolescents, hypertension, pre‐hypertension, schools, young adults
1. INTRODUCTION
Approximately 80% of all cardiovascular disease (CVD) deaths occur in low‐ and middle‐income countries including those in Africa.1 Moreover, CVDs occur at a younger age with significant impact on national productivity.1, 2, 3 The World Health Organization estimates that Africa has the highest age‐adjusted rates of CVDs in the world. 4
High blood pressure (BP) includes those with pre‐hypertension or hypertension. Among those aged below 18 years, pre‐hypertension is defined as mean systolic and/or diastolic BP at ≥90th to <95th percentile or ≥120/80 mm Hg to <95th percentile and hypertension is defined as mean systolic and/or diastolic BP ≥95th percentile (using age, sex, and height‐specific percentile tables). For those aged 18 years and above, pre‐hypertension is defined as mean blood pressure in the range of (120‐139) mm Hg for systolic BP and (80‐89) mm Hg for diastolic BP, while hypertension is defined as mean blood pressure above or equal to 140/90 mm Hg.5, 6
Pre‐hypertension is an important and distinct phase in the pathophysiology of high BP and an independent CVD's risk factor.7, 8, 9 The category was introduced to the US pediatric BP guidelines in 2004 to promote early diagnosis and intervention for high BP to prevent later CVDs.5 Adolescents with pre‐hypertension are known to be two to three times more likely to develop hypertension as young adults, and this risk may be higher among those of African ancestry.9, 10 Pre‐hypertension during adolescence is associated with end‐organ damage including left ventricular hypertrophy, diastolic dysfunction, and kidney disease.8, 11, 12, 13, 14, 15 Pre‐hypertension in African adolescents could therefore explain the onset of hypertension and CVDs in this region at a younger age.3, 4
Past studies on high BP in adolescents in sub‐Saharan Africa have been limited by use of non‐standardized methods of BP measurement, by use of BP definitions that do not take into account key variables such as sex, age, and height, and by involving small sample sizes or populations from a single location.16, 17
In order to contribute more accurate prevalence estimates of high BP among adolescents and young adults in sub‐Saharan Africa, we conducted a cross‐sectional study among secondary school/college students to determine the prevalence of pre‐hypertension and hypertension and to assess associated factors.
2. MATERIALS AND METHODS
2.1. Study participants and sampling
We conducted a cross‐sectional study among secondary school and college/university students in Mwanza (Tanzania) and Kampala (Uganda) cities in East Africa. Mwanza is the second largest city of Tanzania, situated on the southern shores of Lake Victoria, and Kampala is the capital city of Uganda situated on the northern shores of the lake.
In Mwanza, a list of all registered public and private‐owned secondary schools and colleges/universities with their respective number of students was obtained. From the list, we retained schools/colleges which enrolled students of both sexes. From this list, four schools (two from private and two from public) and four private colleges/universities were randomly selected on probability proportional to size. Since age and class/year of study were expected to be highly correlated, each participating institution was restricted to contribute one class/year of study which was randomly selected. All students aged between 15 and 24 years in the selected class/year of study were invited to join the study.
In Kampala, similar procedures were used to randomly select five schools (four public and one private). In each school, we invited all students aged between 12 and 24 years from each of the six classes to participate. We did not enroll from colleges/universities as they were closed during the survey.
2.2. Ethical considerations
The study was approved by the ethics committee of the National Institute for Medical Research in Tanzania, by the Uganda Virus Research Institute Research and Ethics committee as well as the National Council of Science and Technology in Uganda.
Before enrollment, all parents were informed about the study by using an information sheet which was sent to them through students. Parents/guardians were given an opportunity to opt out if they did not want their children to participate. Participants aged 12‐17 years signed an informed assent, and those aged 18‐24 years signed an informed consent. In addition, the head teachers signed student's informed assent/consent forms in their capacity as legal guardians to students. Obtained data were handled in a strict confidentiality manner.
2.3. Data collection
Between May and November 2015, we collected data in the selected institutions in both countries. Trained research assistants interviewed students in private using a structured questionnaire adapted from the WHO stepwise approach to non‐communicable disease risk factor surveillance (STEPS instrument).19 Data were captured electronically using pre‐programmed tablets. We collected data on demographic characteristics and potential risk factors for high BP, including alcohol and cigarette use, physical activity and consumption of fruits, vegetables, salt, and sugar.
Weight and height measurements were then performed using standardized procedures. Weight was measured to the nearest 0.1 kg using digital Seca® 813 weight scales (Seca, Hamburg, Germany) in minimal clothing and shoes removed. Height was measured to the nearest 0.1 centimeter using Seca® 213 stadiometer (Seca). These measurements were used to calculate body mass index (BMI) which was further categorized into four groups (underweight, normal weight, overweight, and obesity) using WHO's BMI for age Z‐scores for participants below 18 years.20 Adult BMI cutoff points were used for those aged 18 years and above.
Blood pressure was measured in an upright seated position after 15 minutes of rest, using the Omron digital monitor model M6 (Omron Health care, Binh Du'o'ng Province, Vietnam). Cuff size (small, medium, or large) was chosen based on the mid‐upper‐arm circumference. BP was measured on the right upper arm, then left upper arm, and again on the right upper arm at a spacing of 2 minutes. The average of the last two readings was used as final BP of the participant. We adapted this approach of BP measurements due to a high likelihood of clinical and sub‐clinical congenital heart diseases in this population including coarctation of aorta.21 For participants aged 12‐17 years of age, the final BP was categorized into normal, pre‐hypertension, and hypertension using percentile scores (taking into account participant's sex, age, and height).5 Similar BP categories using adult cutoff points were used in participants aged 18‐24 years.6 All study staff involved in measuring BP received standardized training.
2.4. Statistical analysis
Statistical analysis was performed using Stata IC version 13 (StataCorp, College Station, TX, USA). The primary outcome was high BP, defined as either having pre‐hypertension or hypertension.
In each country, we computed summary statistics, including median and interquartile range for systolic and diastolic BP stratified by age and sex. We also calculated the proportion of students with pre‐hypertension and hypertension stratified by age and sex. The resulting proportions were reported with their corresponding 95% confidence intervals (CI).
A hierarchical logistic regression model was used to determine independent factors associated with high BP, using a multilevel conceptual framework.22 We first calculated unadjusted odds ratios (ORs) for each potential risk factor and corresponding 95% CI. We then estimated adjusted odds ratios (aORs), adjusted in a stepwise approach starting with all demographic characteristics, followed by socioeconomic variables and lastly BMI and behavioral variables. Variables with a P‐value <0.05 in the final model were regarded as independently associated with high BP.
To assess potential interactions between age and sex, we examined the associations between high BP and age, stratified by sex. P‐value <0.05 for interaction was considered to be significant.
3. RESULTS
3.1. Sampled participants and background characteristics
In Mwanza, out of 1159 eligible students who were invited for enrollment, 908 (78%) provided consent/assent and were enrolled. In Kampala, out of 1098 eligible students were invited, 759 (69%) provided consent/assent and were enrolled. The main reason for non‐participation across sites was being absent at school/college on the enrollment day and during two subsequent follow‐up visits conducted within 7 days of enrollment.
In this analysis, we excluded 71 (4.3%) participants with incomplete data from missing physical examination. From Table 1, 1596 participants (891 from Mwanza and 705 from Kampala), who were included in the analysis, had significant differences in their demographic characteristics. Overall, the majority were male (54%) and from secondary schools (55%), and 72% reported to receive less than 20 USD as monthly pocket money (usually given by parents/guardians to cover for transport, snacks at school, stationeries, and other small personal items). The majority (56%) of participants spent their time resting or watching television after school sessions and/or during weekend (as opposed to engagement in sports or manual work). Overall, the majority of the participants had normal BMI (79%), with more females (21%) than males (5%) being either overweight or obese.
Table 1.
Background characteristics of the study participants by country (N = 1596)
| Variable (N = 1596) |
Tanzania, n (%) 891 (100) |
Uganda, n (%) 705 (100) |
Χ 2 (P‐value) |
|---|---|---|---|
| Sex | |||
| Male | 518 (58) | 347 (49) | <0.001 |
| Female | 373 (42) | 358 (51) | |
| Age, mean ( ± SD) | 19.2 (±3.1) | 16.3 (±2.8) | <0.001a |
| Religion | |||
| Muslims | 216 (24) | 101 (14) | <0.001 |
| Christians | 675 (76) | 604 (86) | |
| Level of study | |||
| Form 1‐4 | 377 (42) | 444 (63) | <0.001 |
| Form 5‐6 | 123 (14) | 261 (37) | |
| College/university | 391 (44) | 0 (0) | |
| Monthly pocket money given (USD) | |||
| 0 (None) | 121 (14) | 45 (6) | <0.001 |
| <20 | 447 (50) | 539 (77) | |
| ≥20 | 318 (36) | 121 (17) | |
| Household items owned | |||
| No car/refrigerator/house for rent | 231 (26) | 144 (20) | <0.001 |
| Car and either refrigerator or house for rent | 353 (40) | 354 (50) | |
| Refrigerator and house for rent only | 241 (27) | 160 (23) | |
| House for rent only | 66 (7) | 47 (7) | |
| Body Mass Index categories (BMI)b | |||
| Normal | 692 (78) | 570 (81) | 0.139 |
| Underweight | 90 (10) | 48 (7) | |
| Overweight | 87 (10) | 71 (10) | |
| Obese | 21 (2) | 16 (2) | |
t test.
Missing observation.
3.2. Blood pressure distribution
In Figure 1, we present the distribution of BP by age and sex across the two countries. In males, the median systolic and diastolic BP increased across age groups, with a nearly identical trend observed in both Uganda and Tanzania (Kruskal‐Wallis test P‐value = 0.001). Among females, median systolic BP decreased across age groups in both countries, while median diastolic BP increased across age groups in a similar trend as observed among males.
Figure 1.
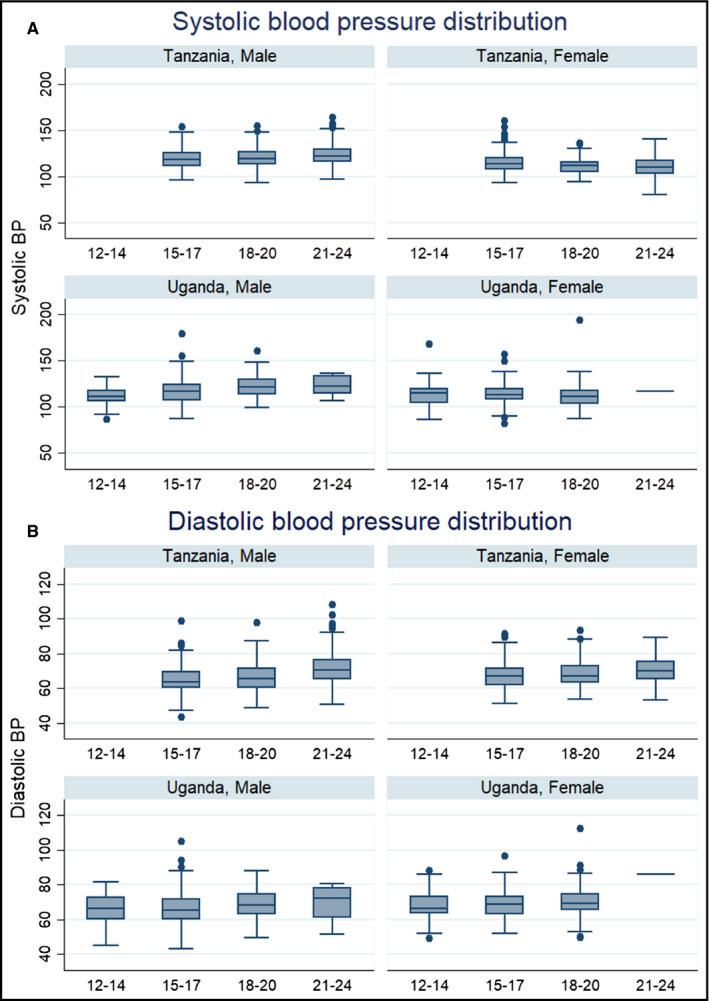
Box plots to show the distribution of blood pressure by age, sex, and country. Panel A for systolic BP and panel B for diastolic BP
3.3. Prevalence of pre‐hypertension and hypertension
Using combined data from both countries, the overall prevalence of high BP was 40% (95% CI: 37‐42). The prevalence of high BP increased across age groups among males (Figure 2). The overall prevalence of pre‐hypertension was 29% (95% CI: 26‐31) and that of hypertension was 11% (95% CI: 10‐13). The prevalence of pre‐hypertension among adolescents (12‐19 years old) was 24% (95% CI: 22‐27) and that of young adults (20‐24 years old) was 38% (95% CI: 34‐42). Similarly, the prevalence of hypertension in adolescents was 12% (95% CI: 10‐14) and that of young adults was 10% (95% CI: 7‐13).
Figure 2.
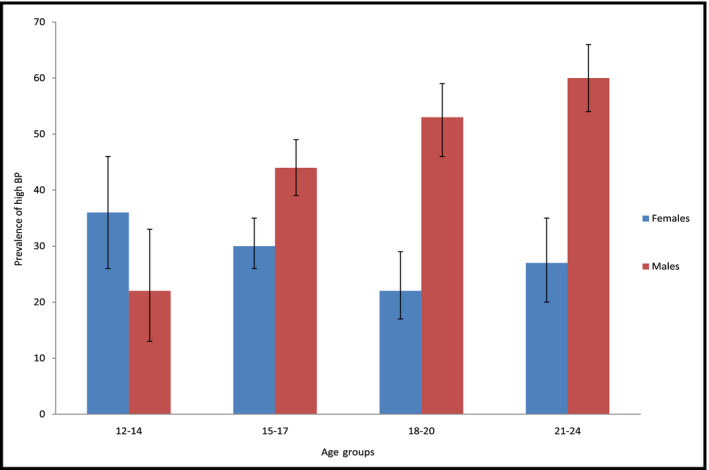
Bar graph showing the prevalence of high blood pressure by age groups and sex
In Table 2, we present the prevalence of pre‐hypertension and hypertension by age and sex using data combined from both countries. Overall, the sex combined prevalence of pre‐hypertension significantly increased from 16% (95% CI: 11‐23) in participants aged 12‐14 years to 39% (95% CI: 34 −44) in those aged 21‐24 years, while that of hypertension decreased from 15% (95% CI: 10‐21) in 12‐14 years to 9% (95% CI: 7‐13) in 21‐24 years.
Table 2.
Prevalence of pre‐hypertension (Pre‐HTN) and hypertension (HTN) by sex and age groups, N = 1596
| Age groups | Males and Females (combined) | Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N |
Normal BP % (95% CI) |
Pre‐hypertension % (95% CI) |
Hypertension % (95% CI) |
N |
Normal BP % (95% CI) |
Pre‐hypertension % (95% CI) |
Hypertension % (95% CI) |
N |
Normal BP % (95% CI) |
Pre‐hypertension % (95% CI) |
Hypertension % (95% CI) |
|
| 12‐14 | 155 | 69 (61, 76) | 16 (11, 23) | 15 (10, 21) | 65 | 77 (65, 86) | 17 (9, 28) | 6 (2, 15) | 90 | 63 (53, 73) | 16 (9, 25) | 21 (14, 31) |
| 15‐17 | 672 | 62 (58, 66) | 23 (20, 26) | 15 (12, 18) | 345 | 56(50, 61) | 28 (24, 33) | 16 (12, 20) | 327 | 69 (64, 74) | 17 (13, 22) | 14 (10, 18) |
| 18‐20 | 393 | 61 (56, 66) | 33 (29, 38) | 6 (4, 8) | 219 | 47 (41, 54) | 46 (39, 52) | 7 (4, 11) | 174 | 78 (71, 83) | 18 (13, 25) | 4 (2, 8) |
| 21‐24 | 376 | 52 (47, 57) | 39 (34, 44) | 9 (7, 13) | 236 | 40 (34, 46) | 47 (41, 54) | 13 (9, 18) | 140 | 73 (65, 80) | 24 (17, 31) | 3 (1, 8) |
On stratification by sex, the prevalence of pre‐hypertension among males increased sharply from 17% (95% CI: 9‐28) in 12‐14 years to 47% (95% CI: 41‐54) in 21‐24 years, whereas among females in same‐age groups, the increment was modest, from 16% (95% CI: 9‐25) to 24% (95% CI: 17‐31). In contrast, the prevalence of hypertension did not show a clear trend among males and a decreasing trend among females.
3.4. Factors associated with high BP
In Table 3, we present factors associated with high BP. Males had more than threefold increased odds of high BP compared to females, which remained statistically significant after adjusting for sociodemographic, economic, BMI, and behavioral factors (aOR = 3.2, 95% CI: 2.4‐4.4). We also observed a positive association between high BP and age (P‐value, test for linear trend = 0.001). However, high BP remained significantly associated with age only in males, with those aged above 20 years having more than fivefold increased odds of high BP when compared to males aged 12‐14 years (aOR = 5.5, 95% CI: 2.9‐10.5; P‐value, test for interaction <0.001).
Table 3.
Factors associated with high blood pressure among adolescents and young people
| Factors |
High BP N (%) |
Crude OR (95% CI) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|---|---|---|---|---|---|
| Overall prevalence | 1596 (40) | ||||
| Demographic factors | |||||
| Country | |||||
| Uganda | 705 (37) | 1 | 1 | 1 | 1 |
| Tanzania | 891 (41) | 1.19 (0.97, 1.46) | 0.83 (0.64, 1.07) | 0.80 (0.62, 1.04) | 1.04 (0.69, 1.56) |
| Sex | |||||
| Female | 731 (28) | 1 | 1 | 1 | 1 |
| Male | 865 (49) | 2.41 (1.95, 2.96) | 2.29 (1.86, 2.83) | 2.30 (1.89, 2.85) | 3.22 (2.35, 4.41) |
| Age in years | |||||
| 12‐14 | 155 (30) | 1 | 1 | 1 | 1 |
| 15‐17 | 672 (37) | 1.41 (0.97, 2.06) | 1.36 (0.91, 2.03) | 1.32 (0.88, 1.98) | 1.97 (1.13, 3.33) |
| 18‐20 | 393 (39) | 1.53 (1.02, 2.28) | 1.42 (0.93, 2.16) | 1.44 (0.95, 2.20) | 2.22 (1.22, 4.02) |
| 21‐24 | 376 (48) | 2.18 (1.46, 3.25) | 2.16 (1.36, 3.48) | 2.24 (1.36, 3.68) | 2.64 (1.30, 5.35) |
| Religion | |||||
| Muslim | 317 (44) | 1 | 1 | 1 | 1 |
| Christians | 1279 (38) | 0.79 (0.61, 1.01) | 0.75 (0.58, 0.98) | 0.74 (0.57, 0.97) | 0.63 (0.43, 0.92) |
| Socioeconomic factors | |||||
| Monthly income given (USD) | |||||
| 0 (None) | 166 (46) | 1 | 1 | 1 | |
| <20 | 986 (37) | 0.69 (0.50, 0.97) | 0.76 (0.53, 1.08) | 0.68 (0.39, 1.18) | |
| ≥20 | 439 (42) | 0.83 (0.58, 1.18) | 0.72 (0.49, 1.06) | 0.74 (0.42, 1.32) | |
| Economic items owned | |||||
| No car/refrigerator/house for rent | 375 (39) | 1 | 1 | 1 | |
| Car and either refrigerator/house for rent | 707 (40) | 1.04 (0.80, 1.34) | 0.93 (0.70, 1.23) | 1.34 (0.89, 2.03) | |
| Refrigerator and house for rent only | 401 (39) | 1.03 (0.77, 1.38) | 0.99 (0.73, 1.34) | 1.36 (0.89, 2.09) | |
| House for rent only | 113 (42) | 1.17 (0.76, 1.79) | 1.24 (0.80, 1.93) | 1.50 (0.81, 2.80) | |
| Behavioral factors | |||||
| Work‐related activity after study hours | |||||
| Manual work | 704 (38) | 1 | 1 | ||
| Relaxing/watching TV | 887 (41) | 1.13 (0.92, 1.38) | 0.76 (0.54, 1.06) | ||
| Eating at least 1 serving of fruits/vegetables day | |||||
| No | 497 (41) | 1 | 1 | ||
| Yes | 537 (36) | 0.79 (0.61, 1.01) | 0.73 (0.54, 0.98) | ||
| Addition of more salt to food | |||||
| No | 785 (42) | 1 | 1 | ||
| Yes | 810 (38) | 0.84 (0.69, 1.04) | 1.10 (0.82, 1.49) | ||
| Number of sugar teaspoon added to your cup of tea or drinks/day | |||||
| 0 | 57 (37) | 1 | 1 | ||
| ≤2 | 700 (41) | 0.67, 2.06) | 1.01 (0.49, 2.08) | ||
| >2 | 605 (39) | 1.07 (0.61, 1.88) | 0.88 (0.41, 1.88) | ||
| Alcohol drinking | |||||
| Never drinkers | 1201 (39) | 1 | 1 | ||
| Current drinkers | 204 (39) | 1.01 (0.75, 1.37) | 0.78 (0.47, 1.28) | ||
| Past drinkers | 191 (44) | 1.23 (0.91, 1.68) | 1.24 (0.78, 1.99) | ||
| Anthropometric | |||||
| Body Mass Index (BMI)a | |||||
| Normal | 1262 (39) | 1 | 1 | ||
| Underweight | 138 (33) | 0.77 (0.53, 1.12) | 0.91 (0.55, 1.51) | ||
| Overweight | 158 (42) | 1.11 (0.79, 1.55) | 1.70 (1.02, 2.83) | ||
| Obese | 37 (62) | 2.54 (1.29, 4.98) | 6.66 (2.21, 20.02) | ||
Model 1‐Adjusted for sociodemographic factors
Model 2‐Adjusted for sociodemographic and economic factors
Model 3‐Adjusted for sociodemographic, economic factors, behavioral factors, and anthropometric (BMI)
BMI—Participants aged below 18 years, the World Health Organization's BMI for age Z‐scores was used whereas for participants aged 18 years and above, adult cutoff points were used.
Daily consumption of at least one serving of fruits and/or vegetables was protective to high BP (aOR 0.73, 95% CI: 0.54, 0.98). Other economic or behavioral factors (salt and sugar consumption, alcohol drinking) did not show independent association with high BP.
We observed a positive association between high BP and BMI categories (P‐value, test for linear trend = 0.005). Obese student had nearly sevenfold increased odds of high BP compared to those with normal BMI (aOR = 6.7, 95% CI: 2.2‐20.0). Overweight males had relatively higher odds of high BP than overweight females, but this did not reach statistical significance in the adjusted model (aOR = 3.0, 95% CI: 1.2‐7.7 vs 1.1, 95% CI: 0.6‐1.9; P‐value, test for interaction = 0.07). Similarly, obesity remained significantly associated with high BP in both males and females. However, obesity and overweight contributed to only 2.5% of the burden of high BP (population attributable fraction).
4. DISCUSSION
In our study, about 40% of participants had high BP. High prevalence of high BP in adolescents and young adults have previously been reported in other African countries. 17, 23, 24 Interestingly, these previous estimates (5%‐40%) are considerably higher than the estimates observed in similar populations living in high‐income countries including the United States (nearly 15%).25
We found that 75% of participants with high BP had pre‐hypertension. This is one of the few studies to examine the prevalence of pre‐hypertension using standard definitions among adolescents and young people in sub‐Saharan Africa. Evidence has shown that adolescents with pre‐hypertension have a two to three times higher risk of developing hypertension as young adults and that this risk is even higher in those with African ancestry.9, 10 Studies from the United States and Europe have demonstrated that pre‐hypertension in adolescents is associated with end‐organ damage.8, 12, 13 Therefore, pre‐hypertension in African adolescents could partly explain the high prevalence of hypertension and CVDs seen among young and middle‐aged adults in Africa.
In both Uganda and Tanzania, the prevalence of pre‐hypertension increased dramatically with age among males. Overall, 47% of males between the ages of 21 and 24 years had pre‐hypertension compared to 15% of males between the ages of 12 and 14 years. This finding can partly be explained by normal physiological changes during puberty driven by increase in testosterone and muscle mass. However, the physiological annual increase in BP with age usually remains modest up to 20 years when it predicts adult healthy BP level.26 Factors such as high salt consumption have earlier been reported to exaggerate BP increase with age13 and could be an important factor in a salt‐sensitive population. Interestingly, our findings differ from surveys from West and Southern Africa where the increase in BP with age was more pronounced among females.13, 23 Higher proportion of overweight and obese females in those studies can partly explain the difference. In a 20‐year follow‐up study in South Africa, the prevalence of pre‐hypertension remained fairly constant up to the age of 16 before it decreased.27 This contrasting finding may highlight the role of socioeconomic factors of the post‐independence South Africa and those prevailing in East Africa on high BP.
We found that obesity was associated with approximately sevenfold increased odds of high BP. Obesity is a well‐documented predictor of high BP.28 Sadly, the global burden of obesity among adolescents has tripled over the past three decades, from 5.0% to 18.8%.29In females, obesity is known to overcome the protective effects of estrogen and can reverse the downward trend of BP.23 Additionally, our observation is of particular importance in sub‐Saharan Africa, where obesity is traditionally perceived as a sign of beauty and health.13, 23 Hence, BP interventions among adolescents and young people must address the problem of obesity.
Daily consumption of at least one serving of fruits/vegetables was associated with decreased odds for high BP. This finding is in agreement with other studies.30, 31 However, we did not find associations with economic indicators and most of other behavioral factors, despite being established risk factors to high BP. Some of these factors may have been underreported due to potential stigma or social disapproval (eg, alcohol use or cigarette smoking). Additionally, other diet‐related factors (excessive salt and sugar consumption) may have been underreported because of difficulties to recall past diet practices in absence of diet diaries. Also, it is acknowledged that some of these factors (alcohol consumption and cigarette use) may need longer exposure to impact on BP.
Our study had some strengths and limitations. A strength was that we enrolled participants from two countries in East Africa and found consistent results in the study sites. Another strength was that we used standard definitions of pre‐hypertension and hypertension in adolescents while these had not been applied in most similar surveys in Africa.
The main limitation was the cross‐sectional design of our study, which does not allow causal relationships to be established. In addition, behavioral and socioeconomic factors were self‐reported and may have been biased by recall and social desirability. Moreover, despite taking three BP measurements and final BP obtained by averaging the last two readings with the aim of reducing measurement errors and the potential effect of “white coat hypertension,”5 we acknowledge that there could be some residual “white coat hypertension” and hence overestimation of the disease burden.32 Our approach of averaging two readings from different arms might limit comparability of our results from other studies. Also, despite the sampling procedures used, our findings may not be representative to a wider population of school/college attending adolescents/young people in sub‐Saharan Africa.
In conclusion, the prevalence of high BP among school/college attending adolescents in Mwanza and Kampala cities (East Africa) is high. Pre‐hypertension is the major contributor to high BP particularly in males across age groups. The increasing burden of high BP across age groups could explain the early onset of hypertension and CVDs in young African adults. Obesity was associated with high BP but could only explain a small fraction of the high BP we observed. Prevention of high BP in African adolescents might be an important target for interventions to combat the epidemic of CVDs in Africa. There is a need for longitudinal studies to explore on incidence and drivers of pre‐hypertension in East African adolescents.
CONFLICT OF INTEREST
Authors have no conflict of interest to disclose on the content of the present manuscript.
AUTHOR CONTRIBUTIONS
All authors were involved in developing the study concept and design, data acquisition, data management, and interpretation of results. Data analysis was performed by NM and CH. This submission was drafted by MKN and SK; all other authors were involved in editing and review. All authors have approved of the final version of this submission.
ACKNOWLEDGMENT
This work was supported by the Training Health Researchers into Vocational Excellence in East Africa (THRiVE) consortium, grant number 087540/Z/08/Z funded by the Wellcome Trust. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the THRiVE consortium or the Wellcome Trust. Authors are grateful to Robert Peck (Tanzania) and Samuel Biraro (Uganda) for their contributions in the study design and study implementation.
Nsanya MK, Kavishe BB, Katende D, et al. Prevalence of high blood pressure and associated factors among adolescents and young people in Tanzania and Uganda. J Clin Hypertens. 2019;21:470–478. 10.1111/jch.13502
REFERENCES
- 1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):470‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falkner B, Lurbe E, Schaefer F. High blood pressure in children: clinical and health policy implications. J Clin Hypertens. 2010;12:261‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31(6):642‐648. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organisation . A Global Brief on Hypertension. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 5. Falkner B. Fourth Report on Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. Bethesda, MD: National Institute of Health; 2005. 05–5267. [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206‐1252. [DOI] [PubMed] [Google Scholar]
- 7. Habib GB, Viran SS, Jneid H. Is 2015 the primetime year for prehypertension? Prehypertension: a cardiovascular risk factor or simply a risk marker? J Am Heart Assoc. 2015;4(2):1792‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y, Wang S, Cai X, et al. Prehypertension and incidence of cardiovascular disease: a meta‐analysis. BMC Med. 2013;11(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redwine KM, Daniels SR. Pre‐hypertension in adolescents: risk and progression. J Clin Hypertens. 2012;14(6):360‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8(7):657‐665. [DOI] [PubMed] [Google Scholar]
- 11. Falkner B. Recent clinical and translational advances in pediatrics hypertension. J Hypertens. 2015;65(1):926‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahajan AM, Negi PC. Hypertension and prehypertension among adolescents in Shimla, Northern India‐time to awaken. Nig J Cardiol. 2015;12(2):71‐76. [Google Scholar]
- 13. Odunaiya NA, Louw QA, Grimmer KA. Are lifestyle cardiovascular disease risk factors associated with pre‐hypertension in 15‐18 years rural Nigerian youth? A cross sectional study. BMC Cardiovasc Disord. 2015;15(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redwine KM, Acosta AA, Poffenbarger T, Portman RJ, Samuels J. Development of hypertension in adolescents with pre‐hypertension. J Pediatr. 2012;160(1):98‐103. [DOI] [PubMed] [Google Scholar]
- 15. Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58(4):579‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kavishe B, Biraro S, Baisley K, et al. High prevalence of hypertension and of risk factors for non‐communicable diseases (NCDs): a population based cross‐sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med. 2015;13(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidy F, Rutebarika D, Lule SA, et al. Blood pressure in primary school children in Uganda: a cross‐sectional survey. BMC Public Health. 2014;14(1):1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta‐analysis. Lancet Public health. 2017;2(8):e375‐e386. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organisation . Stepwise Approach to Non Communicable Diseases (NCDs) Risk Factor Surveillance (STEPS Instrument). Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 20. World Health Organisation . Growth reference for 5‐19 years. 2007; http://www.who.int/growthref/who2007_bmi_for_age/en/. Accessed February 20, 2018.
- 21. Zuhlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart. 2013;99(21):1554‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Victoria CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 26(1):224‐227. [DOI] [PubMed] [Google Scholar]
- 23. Nkeh‐Chungang BN, Sekokotla AM, Sewani‐Rusike C, Namugowa A, Iputo JE. Prevalence of hypertension and prehypertension in 13‐17 year old adolescents living in Mthatha – South Africa; a cross‐sectional study. Cent Eur J Public Health. 2015;23(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 24. Ujunwa FA, Ikefuna AN, Nwokocha A, Chinawa JM. Hypertension and pre‐hypertension among adolescents in secondary schools in Enugu, South East Nigeria. Ital J Pediatr. 2013;39(1):70‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson SL, Zhang Z, Wiltz JL, et al. Hypertension among youths‐United States, 2001‐2016. MMWR. 2018;67(27):758‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shankar RR, Eckert GJ, Saha C, Wanzhu T, Pratt JH. The change in blood pressure during pubertal growth. J Clin Endocrinol Metab. 2005;90(1):163‐167. [DOI] [PubMed] [Google Scholar]
- 27. Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA. Blood pressure tracking in urban black South African children, birth to twenty cohort. BMC Pediatr. 2015;15(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mushengezi B, Chillo P. Association between body fat composition and blood pressure level among secondary school adolescents in Dar es Salaam, Tanzania. Pan Afr Med J. 2014;19(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pangani IN, Kiplamai FK, Kamau JW, Onywera VO. Prevalence of overweight and obesity among primary school children aged 8‐13 years in Dar es Salaam city, Tanzania. Adv Prev Med. 2016;2016(1):1345017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Y, Dong B, Zou Z, et al. Association between Vegetable Consumption and Blood Pressure, Stratified by BMI, among Chinese Adolescents Aged 13‐17 Years: a national cross‐sectional study. Nutrients. 2018;10(4):451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li B, Li F, Wang L, Zhang D. Fruit and vegetables consumption and risk of hypertension: a meta‐analysis. J Clin Hypertens (Greenwich). 2016;18(5):468‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. deOliveira L, daSilva AO, Diniz P, et al. The number of visits and blood pressure measurements influence the prevalence of high blood pressure in adolescents. J Am Soc Hypertens. 2017;11(6):343‐349. [DOI] [PubMed] [Google Scholar]


