Abstract
Numerous researchers have investigated the associations among methylenetetrahydrofolate reductase gene (MTHFR) C677T polymorphism, homocysteine (Hcy) concentration, and hypertension. However, the results are controversial. Thus, a meta‐analysis implementing Mendelian randomization approach was conducted to examine the hypothesis that elevated Hcy concentration plausibly contributes to increased risk of hypertension. Based on several inclusion and exclusion criteria, eligible studies were selected to explore the correlation between MTHFR C677T and hypertension risk, MTHFR C677T and Hcy concentration in hypertension, and Hcy concentration and hypertension, and they were evaluated by odds ratios (ORs), effect size (ES), and standard mean difference with their corresponding 95% confidence intervals (95% CIs), respectively. Moreover, Mendelian randomization was implemented to evaluate the relationship between Hcy and hypertension. Consequently, 14 378 cases and 25 795 controls were involved in this study and the results showed that MTHFR C677T led to an elevated risk of hypertension (for T vs C: OR = 1.27, 95% CI = 1.17‐1.37; for TT vs CC: OR = 1.53, 95% CI = 1.30‐1.79). Additionally, in hypertensive subjects, the pooled Hcy concentration in individuals of TT genotype was 7.74 μmol/L (95% CI: 5.25‐10.23) greater than that in individuals of CC genotype. Moreover, the pooled Hcy concentration in hypertensive was 0.69 μmol/L (95% CI: 0.50‐0.87) greater than that in controls. The estimated causal OR associated with hypertension was 1.32 for 5 μmol/L Hcy increment. Via MTHFR C677T polymorphism, the findings in the present study demonstrated that there exists evidence on causal link between Hcy concentration and the risk of hypertension.
Keywords: homocysteine, hypertension, MTHFR, polymorphism
1. INTRODUCTION
According to the previous research reports, hypertension is influencing more than 1 billion people worldwide, and over 29.2% of adults are estimated to be recognized as hypertensive in 2025.1 Hypertension is a crucial risk factor for cardiovascular disease and the pivotal source of global health burden, which has contributed to 9.4 million deaths each year.2 Although the etiology of this disease has not been fully elucidated and numerous lifestyle risk factors lead to the development of hypertension, genes, environmental factors, and their interactions are indicated to have a substantial effect on the pathological process of hypertension.3, 4 Therefore, identifying genetic factors which led to the presence of hypertension and understanding the process affecting this disease could be of great importance not only in comprehending its pathogenesis, but also in giving more choices to take effective intervention for reducing the incidence of hypertension.
Over the past several decades, the candidate gene approach has been extensively applied to detect the deleterious genes efficiently in complex human disease.5 Methylenetetrahydrofolate reductase (MTHFR) serves as an important rate‐limiting enzyme playing a role in the altering of 5,10 methylenetetrahydrofolate to 5‐methylenetetrahydrofolate, which is regarded as a pivotal remethylated process of homocysteine (Hcy) to methionine.6 MTHFR C677T indicates the change in cysteine to thymine nucleotide, which signifies the substitution of alanine to valine. As a result, the subjects with TT genotype express a heat‐sensitive enzyme with reduced activity, which results in the elevated plasma Hcy concentration eventually.6, 7 The increasing level of plasma Hcy may contribute to certain pathological changes, such as vascular endothelial injury, nitric oxide production inhibition, and vascular smooth muscle proliferation,8 which might result in the development of hypertension.9
Recently, a large number of studies investigated the association between MTHFR C677T polymorphism and hypertension. For example, a study suggested that MTHFR C677T is a protective predictor of hypertension in a Maya‐Mestizo women population.10 However, another study announced an opposite result completely.11 Because of the contradicted results, it is difficult to come to an incontestable conclusion to date. The relationship of MTHFR C677T polymorphism, Hcy, and hypertension is still elusive.
In order to provide more solid evidence for this issue, a meta‐analysis of the published studies related to the risk of hypertension associated with an elevated Hcy level and the MTHFR C677T polymorphism was conducted. Here, we integrated the previous published studies with regard to the associations among MTHFR C677T, Hcy concentration, and hypertension risk to obtain pooled results of their reported associations. Furthermore, Mendelian randomization approach, which is recognized as an epidemiological method based on the fact that individuals randomly inherit their genetic variation from their parents, was carried out to test the hypothesis that elevated Hcy level is plausibly associated with the enhanced risk of hypertension.
2. MATERIALS AND METHODS
2.1. Selection of studies
Researches that estimated the association between the MTHFR gene 677 C>T polymorphism and Hcy level with the development of hypertension were encompassed in this study. To identify potentially relevant researches, a comprehensive literature retrieval was performed independently by two investigators from PubMed, Embase, and Web of Science with the following terms: “MTHFR,” “rs1801133,” “MTHFR C677T,” “homocysteine,” “Hcy,” “hypertension” and “blood pressure,” up to August 1, 2019.
The following criteria were proposed for the inclusion of eligible researches: (a) case‐control, cross‐sectional, or case‐cohort designed researches; and (b) descripting the distribution of the MTHFR C677T genotypes or sufficient data for calculating it in cases with hypertension and in controls free of hypertension. Reviews or letters, duplicated studies, abstracts, and editorials were excluded. The languages were limited to English and Chinese.
2.2. Data extraction
Two investigators extracted the following information from each study independently: (a) first author's surname, year of publication, subjects’ country, and ethnicity; (b) number of cases and controls, the diagnostic standard of cases and controls, and genotype distribution in both of groups. Data were extracted, respectively, for studies involving more than one population. Discrepancies were resolved after discussion with a third reviewer.
2.3. Statistical analysis
Chi‐square test was performed to estimate whether Hardy‐Weinberg equilibrium (HWE) was significantly deviated in controls of each study. Moreover, sensitivity analysis by removing one study with controls not in HWE was carried out to evaluate the stability of the results.
Under five genetic models including homozygous codominant model (TT vs CC), heterozygous codominant model (TT vs CT), allelic model (T vs C), dominant model (TT + TC vs CC), and recessive model (TT vs TC + CC), this meta‐analysis estimated the overall association of MTHFR C677T with the risk of hypertension. All associations for these five genetic models were represented as odds ratios (OR) with their corresponding 95% confidence interval (CI). Then, a pooled OR was estimated based on the ORs of separate study for each of those five models. Metan command in Stata, version 12.0 (StataCorp LP), was used to estimate the mean difference between MTHFR 677TT group and MTHFR 677CC group in hypertensive subjects. We also pooled the Hcy levels to identify the standardized mean difference (SMD) and its corresponding 95% CI for comparisons between hypertensive and healthy subjects. Between‐study heterogeneity testing was performed by Cochrane's Q test (significance at I 2 > 50.0% and P < .10).12, 13 Once the effects were assessed to be absent from heterogeneity, the fixed effects model was fitted; otherwise, the random effects model was employed.14 In this meta‐analysis, subgroup analyses by ethnicity and age range (children/adults) were also carried out. The publication bias was measured by Begg's funnel plot and Egger's regression test (P < .05 was considered as statistically significant) if too many studies were included.15 Extracted data for all statistical analyses were conducted by using the Stata.
Mendelian randomization analysis is a method to make unbiased intermediated phenotype‐disease plausible association by integrating the information of genotype‐intermediate phenotype and genotype‐disease association into an analytical framework. A valid instrumental variable in the Mendelian randomization needs to satisfy three conditions:16 (a) associated with Hcy strongly; (b) not associated with confounders, which could bias the correlation between Hcy and hypertension; and (c) affects hypertension only through its effect on the specific intermediate Hcy. MTHFR C677T may satisfy all these conditions well based on the existing studies.9, 17, 18, 19 Thus, the Mendelian randomization coefficient estimated by applying MTHFR C677T as an instrument would provide the causal link free of bias between Hcy and hypertension. Compared with MTHFR 677 CC, the genotype of MTHFR 677 TT elevates the hypertension risk and the effect is evaluated by the odds ratio (ORTT vs CC). Compared with CC, TT is associated with the mean difference (Δ) of Hcy concentration. Further OR1 = (ORTT vs CC)1/Δ would be an unconfounded evaluation of hypertension effect caused by one unit change in the Hcy. In this study, we employed the formula ORk = (ORTT vs CC)k/Δ for an increment of k units,20 and we analyzed 5 μmol/L increase in Hcy to evaluate the corresponding OR.21
3. RESULTS
3.1. Characteristics of the studies
Figure 1 describes the comprehensive information of screening progress in this meta‐analysis. As a result, fifty‐seven studies evaluated the association between MTHFR C677T and hypertension, which provided 14 378 cases and 25 795 controls.9, 10, 11, 17, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 In this pooled data, the frequencies of the CC genotype were the highest in pooled cases and in pooled controls, respectively, while that for genotype TT was the lowest. Ten studies only described the association between MTHFR C677T and Hcy level in hypertensive patients.66, 74, 75, 76, 77, 78, 79, 80, 81, 82 However, in eight studies,30, 44, 47, 54, 56, 66, 71, 72 the genotype distribution in the control subjects was not in line with HWE (P < .05; Table 1).
Figure 1.
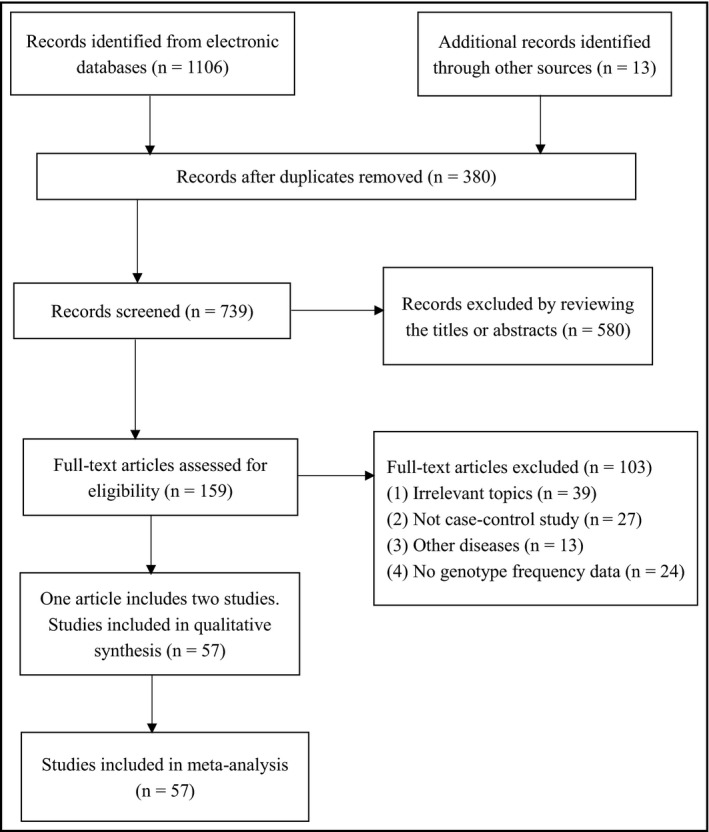
PRISMA flow diagram for selection of studies in the meta‐analysis
Table 1.
The genotypic and allelic distribution of MTHFR C677T for case and control
| First author | Year | Country | Ethnicity | Children/adults | Diagnose | Genotype distribution | Allele frequency | P‐value HWE | Number of cases/controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||||||||||||
| SBP | DBP | CC | CT | TT | CC | CT | TT | C | T | C | T | |||||||
| Nishio22 | 1996 | Japan | Asian | Adults | >140 mm Hg | >90 mm Hg | 29 | 44 | 9 | 16 | 26 | 5 | 102 | 62 | 58 | 36 | .24 | 82/47 |
| Nakata23 | 1998 | Japan | Asian | Adults | >160 mm Hg | >95 mm Hg | 63 | 91 | 19 | 65 | 83 | 36 | 217 | 129 | 213 | 155 | .31 | 173/184 |
| Powers24 | 1999 | United States | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 46 | 58 | 18 | 54 | 46 | 14 | 150 | 94 | 154 | 74 | .39 | 122/114 |
| Zusterzeel29 | 2000 | Netherlands | White | Adults | — | ≥90 mm Hg | 32 | 33 | 11 | 205 | 162 | 36 | 97 | 55 | 572 | 234 | .62 | 76/403 |
| Zhan28 | 2000 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 44 | 68 | 15 | 62 | 84 | 24 | 156 | 98 | 208 | 132 | .60 | 127/170 |
| Rajkovic27 | 2000 | United States | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 142 | 28 | 1 | 151 | 32 | 0 | 312 | 30 | 334 | 32 | .19 | 171/183 |
| Li26 | 2000 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 14 | 31 | 17 | 46 | 32 | 12 | 59 | 65 | 124 | 56 | .11 | 62/90 |
| Kobashi25 | 2000 | Japan | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 64 | 98 | 22 | 83 | 99 | 33 | 226 | 142 | 265 | 165 | .70 | 184/215 |
| Benes30 | 2001 | Czech | White | Adults | ≥140 mm Hg | ≥90 mm Hg | 73 | 93 | 27 | 86 | 106 | 17 | 239 | 147 | 278 | 140 | .05 | 193/209 |
| Kahleova31 | 2002 | Czech | White | Children and Adolescents | ≥90a | — | 82 | 55 | 27 | 86 | 69 | 18 | 219 | 109 | 241 | 105 | .46 | 164/173 |
| Wang32 | 2002 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 17 | 51 | 37 | 14 | 23 | 9 | 85 | 125 | 51 | 41 | .94 | 105/46 |
| Rodriguez‐Esparragon33 | 2003 | Spain | White | Adults | >140 mm Hg | >90 mm Hg | 83 | 115 | 34 | 95 | 100 | 20 | 281 | 183 | 290 | 140 | .39 | 232/215 |
| Sun34 | 2003 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 6 | 22 | 27 | 14 | 23 | 9 | 34 | 76 | 51 | 41 | .94 | 55/46 |
| Yilmaz35 | 2004 | Turkey | White | Adults | >140 mm Hg | >110 mm Hg | 29 | 28 | 7 | 24 | 17 | 6 | 86 | 42 | 65 | 29 | .30 | 64/47 |
| Heux9 | 2004 | Australia | White | Adults | >140 mm Hg | >95 mm Hg | 87 | 125 | 35 | 105 | 119 | 25 | 299 | 195 | 329 | 169 | .30 | 247/249 |
| Frederiksen17 | 2004 | Denmark | White | Adults | — | — | 576 | 555 | 136 | 3853 | 3406 | 712 | 1707 | 827 | 11 112 | 4830 | .30 | 1267/7971 |
| Cesari36 | 2005 | Italy | White | Adults | — | — | 40 | 39 | 11 | 42 | 38 | 10 | 119 | 61 | 122 | 58 | .75 | 90/90 |
| Liu37 | 2005 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 29 | 45 | 26 | 31 | 50 | 19 | 103 | 97 | 112 | 88 | .88 | 100/100 |
| Lwin42 | 2006 | Japan | Asian | Adults | >140 mm Hg | >90 mm Hg | 39 | 58 | 18 | 64 | 117 | 38 | 136 | 94 | 245 | 193 | .22 | 115/219 |
| Li41 | 2006 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 18 | 6 | 2 | 21 | 7 | 2 | 42 | 10 | 49 | 11 | .23 | 26/30 |
| Hu39 | 2006 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 55 | 39 | 16 | 61 | 42 | 12 | 149 | 71 | 164 | 66 | .25 | 110/115 |
| Kalita40 | 2006 | India | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 18 | 7 | 3 | 21 | 9 | 2 | 43 | 13 | 51 | 13 | .46 | 28/32 |
| Demir38 | 2006 | Turkey | White | Adults | >140 mm Hg | >90 mm Hg | 33 | 53 | 14 | 43 | 47 | 12 | 119 | 81 | 133 | 71 | .88 | 100/102 |
| Nagy46 | 2007 | Hungary | White | Adults | >160 mm Hg | >90 mm Hg | 49 | 43 | 9 | 32 | 35 | 6 | 141 | 61 | 99 | 47 | .40 | 101/73 |
| Markan45 | 2007 | India | Asian | Adults | >140 mm Hg | >90 mm Hg | 105 | 40 | 8 | 105 | 28 | 0 | 250 | 56 | 238 | 28 | .17 | 153/133 |
| Marinho44 | 2007 | Portugal | White | Adults | — | — | 15 | 49 | 0 | 57 | 67 | 4 | 79 | 49 | 181 | 75 | 2.87 × 10—3 | 64/128 |
| Hui43 | 2007 | Japan | Asian | Adults | >160 mm Hg | >100 mm Hg | 83 | 129 | 49 | 104 | 123 | 44 | 295 | 227 | 331 | 211 | .45 | 261/271 |
| Xing47 | 2007 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 202 | 300 | 184 | 182 | 222 | 105 | 704 | 668 | 586 | 432 | .02 | 686/509 |
| Soares51 | 2008 | Brazil | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 13 | 15 | 2 | 9 | 5 | 2 | 41 | 19 | 23 | 9 | .36 | 30/16 |
| Lin50 | 2008 | China | Asian | Adults | >140 mm Hg | >90 mm Hg | 19 | 27 | 4 | 73 | 44 | 6 | 65 | 35 | 190 | 56 | .85 | 50/123 |
| Ilhan49 | 2008 | Turkey | White | Adults | >140 mm Hg | >90 mm Hg | 36 | 32 | 10 | 72 | 26 | 2 | 104 | 52 | 170 | 30 | .84 | 78/100 |
| Fridman48 | 2008 | Argentina | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 15 | 21 | 4 | 39 | 38 | 9 | 51 | 29 | 116 | 56 | .95 | 40/86 |
| Canto10 | 2008 | Mexico | White | Adults | ≥140 mm Hg | ≥90 mm Hg | 36 | 66 | 23 | 61 | 131 | 82 | 138 | 112 | 253 | 295 | .53 | 125/274 |
| Ng11 | 2009 | Australia | White | Adults | ≥140 mm Hg | ≥90 mm Hg | 14 | 14 | 10 | 40 | 32 | 8 | 42 | 34 | 112 | 48 | .67 | 38/80 |
| Fakhrzadeh53 | 2009 | Iran | Asian | Adults | ≥160 mm Hg | ≥95 mm Hg | 99 | 44 | 17 | 36 | 31 | 9 | 242 | 78 | 103 | 49 | .56 | 160/76 |
| Deshmukh52 | 2009 | United States | American | Adults | >140 mm Hg | >90 mm Hg | 22 | 16 | 4 | 52 | 48 | 18 | 60 | 24 | 152 | 84 | .22 | 42/118 |
| Liu54 | 2011 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 58 | 70 | 27 | 74 | 47 | 19 | 186 | 124 | 195 | 85 | .01 | 155/140 |
| Fowdar55 | 2012 | Australia | White | Adults | ≥140 mm Hg | ≥90 mm Hg | 170 | 174 | 33 | 175 | 183 | 35 | 514 | 240 | 533 | 253 | .19 | 377/393 |
| Zhang57 | 2012 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 128 | 53 | 8 | 117 | 41 | 7 | 309 | 69 | 275 | 55 | .18 | 189/165 |
| Yin56 | 2012 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 244 | 358 | 68 | 322 | 309 | 51 | 846 | 494 | 953 | 411 | .05 | 670/682 |
| Fridman58 | 2013 | Argentina | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 29 | 40 | 6 | 71 | 64 | 15 | 98 | 52 | 206 | 94 | .92 | 75/150 |
| Yao59 | 2013 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 32 | 69 | 49 | 61 | 67 | 22 | 133 | 167 | 189 | 111 | .61 | 150/150 |
| Cai60 | 2014 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 39 | 99 | 62 | 61 | 89 | 50 | 177 | 223 | 211 | 189 | .13 | 200/200 |
| Husemoen61 | 2014 | Denmark | White | Adults | ≥140 mm Hg | ≥90 mm Hg | 2231 | 2012 | 451 | 3790 | 3208 | 699 | 6474 | 2914 | 10 788 | 4606 | .59 | 4694/7697 |
| Vazquez‐Alaniz62 | 2014 | Mexico | American | Adults | >140 mm Hg | >90 mm Hg | 62 | 93 | 39 | 54 | 97 | 43 | 217 | 171 | 205 | 183 | .96 | 194/194 |
| Wen68 | 2015 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 45 | 53 | 76 | 258 | 291 | 85 | 143 | 205 | 807 | 461 | .84 | 174/634 |
| Perez‐Razo(Adults)65 | 2015 | Mexico | American | Adults | ≥140 mm Hg | ≥90 mm Hg | 112 | 174 | 87 | 90 | 200 | 101 | 398 | 348 | 380 | 402 | .64 | 373/391 |
| Perez‐Razo(Children)65 | 2015 | Mexico | American | Children and Adolescents | ≥95thb | — | 34 | 98 | 67 | 35 | 108 | 56 | 166 | 232 | 178 | 220 | .17 | 199/199 |
| Wei67 | 2015 | Malaysia | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 143 | 82 | 21 | 260 | 78 | 10 | 368 | 124 | 598 | 98 | .17 | 246/348 |
| Nassereddine64 | 2015 | Morocco | African | Adults | ≥140 mm Hg | ≥90 mm Hg | 47 | 40 | 14 | 54 | 45 | 3 | 134 | 68 | 153 | 51 | .07 | 101/102 |
| Bayramoglu63 | 2015 | Turkey | White | Adults | >140 mm Hg | >90 mm Hg | 65 | 38 | 22 | 56 | 38 | 5 | 168 | 82 | 150 | 48 | .65 | 125/99 |
| Wang66 | 2015 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 96 | 60 | 34 | 144 | 91 | 52 | 252 | 128 | 379 | 195 | 6.78 × 10−7 | 190/287 |
| Fan70 | 2016 | China | Asian | Adults | ≥140 mm Hg | ≥90 mm Hg | 37 | 102 | 75 | 119 | 234 | 141 | 176 | 252 | 472 | 516 | .26 | 214/494 |
| Amrani‐Midoun69 | 2016 | Algerian | African | Adults | ≥140 mm Hg | ≥90 mm Hg | 37 | 36 | 9 | 44 | 25 | 3 | 110 | 54 | 113 | 31 | .81 | 82/72 |
| Dwivedi71 | 2017 | India | Asian | Adults | — | — | 71 | 24 | 5 | 184 | 34 | 5 | 166 | 34 | 402 | 44 | .03 | 100/223 |
| Rios72 | 2017 | Brazil | American | Adults | ≥160 mm Hg | ≥110 mm Hg | 13 | 29 | 54 | 20 | 20 | 45 | 55 | 137 | 60 | 110 | 7.82 × 10−6 | 96/85 |
| Arina73 | 2019 | Indonesia | Asian | Adults | — | — | 32 | 16 | 5 | 43 | 10 | 0 | 80 | 26 | 96 | 10 | .45 | 53/53 |
The P‐value for HWE testing for controls is shown.
Abbreviations: DBP, diastolic blood pressure; HWE, Hardy‐Weinberg equilibrium test; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure.
According to developmental distribution tables and curves: “Anonymous: Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents.”
According to the criteria: “The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents.”
3.2. The mean Hcy concentration difference between MTHFR genotypes in hypertensive
According to the inclusion criteria, nineteen studies (9931 hypertensive patients) 26, 32, 35, 36, 45, 49, 53, 58, 66, 71, 74, 75, 76, 77, 78, 79, 80, 81, 82 were selected to report the plasma Hcy concentration of different genotypic groups with the arithmetic mean and the matching standard deviation in hypertensive patients. The mean Hcy level was higher in MTHFR 677TT subjects than that in the other genotypes. The pooled mean Hcy level in MTHFR 677TT subjects was 7.74 μmol/L (95% CI: 5.25‐10.23) greater than that in MTHFR 677CC subjects (P < .001; Figure 2). Moreover, the MTHFR 677TT subjects had 5.58 μmol/L (95% CI: 4.15‐7.01) higher Hcy level than MTHFR 677CT subjects (P < .001; Figure 3).
Figure 2.
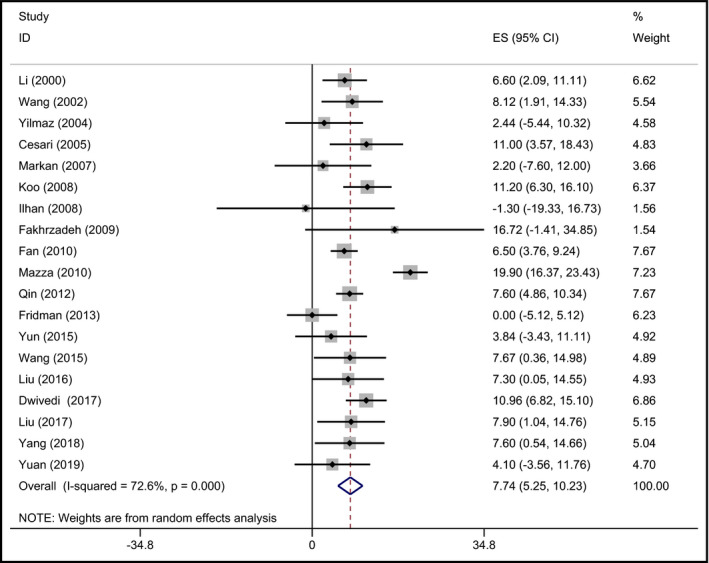
Forest plot of the evaluation for the effect size (ES) in Hcy level between the MTHFR genotypes (TT vs CC) in hypertensive patients
Figure 3.
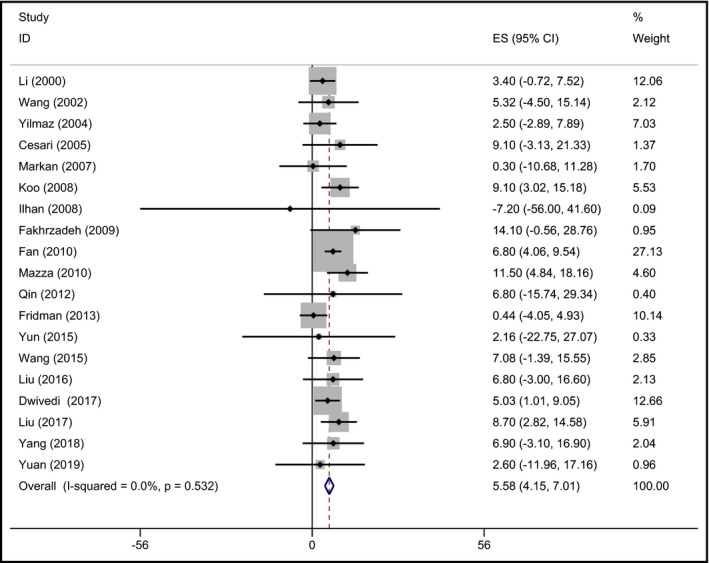
Forest plot of the evaluation for the effect size (ES) in Hcy level between the MTHFR genotypes (TT vs CT) in hypertensive patients
3.3. The association between MTHFR C677T and the risk of hypertension
The prime outcome for investigating the association of MTHFR 677 TT and hypertension risk compared with MTHFR 677 CC showed significant heterogeneity (I 2 = 69.5%, P < .001) among the involved studies; the pooled OR was statistically significant: OR = 1.53 (95% CI: 1.30‐1.79; P < .001; Figure 4). Similarly, the T allele in the MTHFR C677T conferred a higher risk of hypertension (OR = 1.27 [95% CI: 1.17‐1.37; P < .001], I 2 = 73.4%, P < .001; Figure 5). Moreover, compared with MTHFR 677 CC, MTHFR 677 CT indicated a significantly higher hypertension risk (OR = 1.22 [95% CI: 1.12‐1.32; P < .001], I 2 = 42.2%, P = .001). MTHFR 677 TT showed a significantly greater risk of hypertension compared with CC + CT genotype (OR = 1.37 [95% CI: 1.19‐1.58; P < .001], I 2 = 67.7%, P < .001). MTHFR 677 TT + CT also revealed a significantly higher risk of hypertension compared with CC genotype (OR = 1.31 [95% CI: 1.19‐1.43; P < .001], I 2 = 58.2%, P < .001). Stratification by ethnicity implicated the correlations under all the genetic models in Asian and European. What's more, subgroup of the age range stratification showed the associations under all studied genetic models in adults with the risk of developing hypertension (Table 2).
Figure 4.
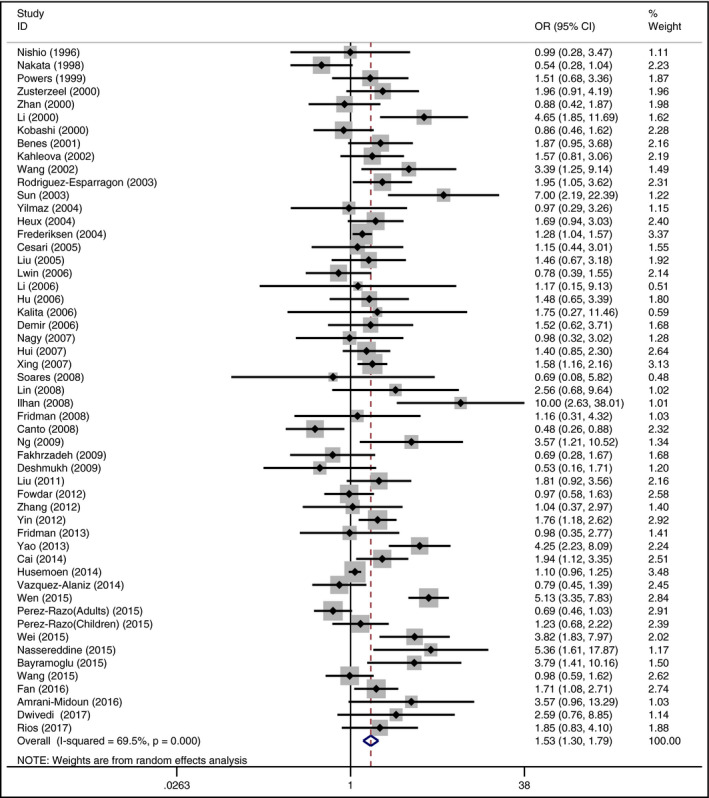
Forest plot of the MTHFR C677T associated with hypertension risk (under homozygous codominant model: TT vs CC)
Figure 5.
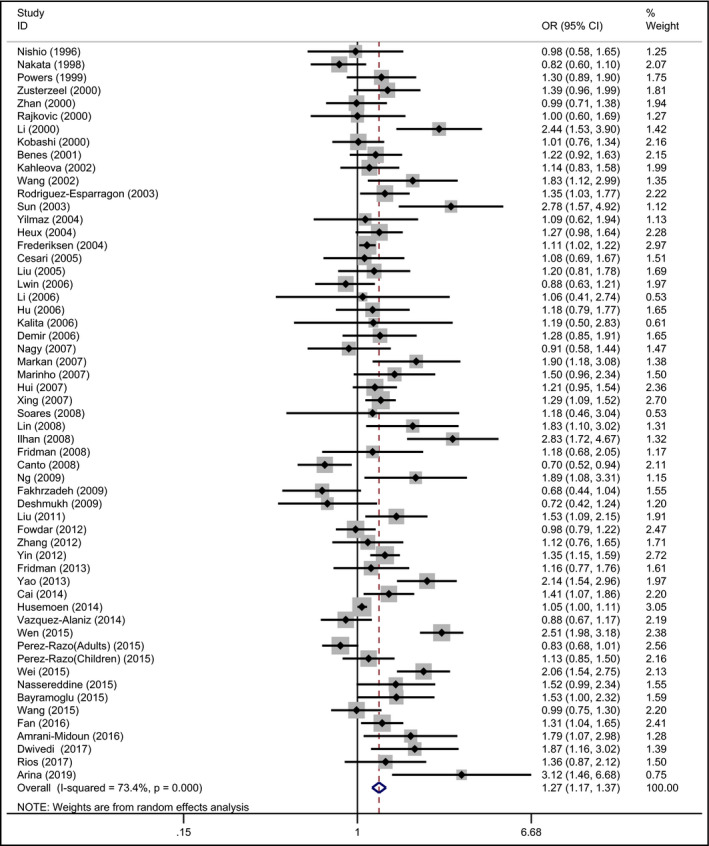
Forest plot of the MTHFR C677T associated with hypertension risk (under allelic model: T vs C)
Table 2.
Stratified analysis of associations of MTHFR C677T polymorphisms with hypertension
| Subgroup | Dominant | Recessive | Homozygous codominant | Heterozygous codominant | Allelic model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P h | OR (95% CI) | P h | OR (95% CI) | P h | OR (95% CI) | P h | OR (95% CI) | P h | |
| Overall | 1.31 (1.19‐1.43) | <.001 | 1.37 (1.19‐1.58) | <.001 | 1.53 (1.30‐1.79) | <.001 | 1.22 (1.12‐1.32) | .001 | 1.27 (1.17‐1.37) | <.001 |
| Ethnicity | ||||||||||
| Asian | 1.45 (1.26‐1.67) | <.001 | 1.47 (1.16‐1.85) | <.001 | 1.70 (1.32‐2.19) | <.001 | 1.34 (1.18‐1.52) | .021 | 1.39 (1.22‐1.58) | <.001 |
| European | 1.18 (1.06‐1.33) | .025 | 1.34 (1.09‐1.65) | .002 | 1.43 (1.14‐1.79) | .001 | 1.12 (1.01‐1.23) | .158 | 1.18 (1.07‐1.30) | .001 |
| American | 1.05 (0.84‐1.32) | .101 | 0.98 (0.81‐1.20) | .825 | 0.95 (0.73‐1.22) | .373 | 1.06 (0.84‐1.35) | .111 | 1.01 (0.89‐1.15) | .281 |
| African | 1.53 (1.01‐2.32) | .364 | 4.01 (1.69‐9.56) | .481 | 4.45 (1.83‐10.82) | .654 | 1.29 (0.78‐2.13) | .252 | 1.63 (1.17‐2.26) | .632 |
| Age range | ||||||||||
| Adults | 1.32 (1.20‐1.45) | <.001 | 1.37 (1.18‐1.58) | <.001 | 1.54 (1.30‐1.81) | <.001 | 1.23 (1.14‐1.34) | <.001 | 1.27 (1.18‐1.38) | <.001 |
| Children and Adolescents | 1.01 (0.72‐1.40) | .892 | 1.41 (0.99‐2.01) | .489 | 1.37 (0.88‐2.13) | .59 | 0.88 (0.62‐1.25) | .762 | 1.13 (0.92‐1.40) | .963 |
Abbreviations: CI, confidence interval; MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; P h, P‐value for heterogeneity test.
3.4. The associations of plasma Hcy level with hypertension
The SMD of Hcy between hypertensive group and control group indicated that Hcy has an effect on hypertension. A forest plot revealed the SMD outcomes in all the included studies, which was shown in Figure 6. In this meta‐analysis, there existed significant heterogeneity (I 2 = 97.8%, P < .001) among the included studies. In these 42 studies,11, 26, 31, 32, 33, 34, 35, 49, 50, 51, 53, 57, 58, 59, 60, 68, 71, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 the pooled mean Hcy level in hypertension group was 0.69 μmol/L (95% CI: 0.50‐0.87) greater than that in control group in random effect model (P < .001). We further performed a subgroup analysis for ethnicity, and all the results from European, Asian, and others indicated significant difference in Hcy level between hypertensive subjects and control subjects. Additionally, both Begg's and Egger's tests were conducted to assess the potential publication bias. There was no indication of any substantial publication bias for the Hcy‐hypertension association (data not shown).
Figure 6.
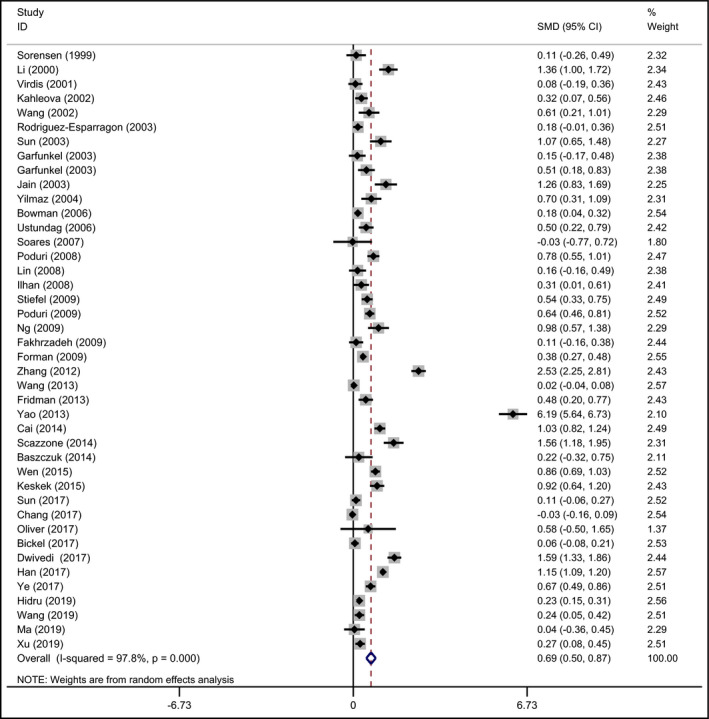
Forest plot of standardized mean difference (SMD) in Hcy levels between hypertensive patients and control subjects in included studies
3.5. Plausible relationship between Hcy level and hypertension via Mendelian randomization
By virtue of MTHFR C677T as an instrument variable for Hcy level, the Hcy per unit increase associated with the predicted OR of hypertension by direct or indirect measurement was shown in Figure 7. The Hcy concentrations were positively associated with the risk of developing hypertension. The estimated plausible OR was 1.32 (95% CI: 1.22‐1.49) for 5 μmol/L Hcy increment.
Figure 7.
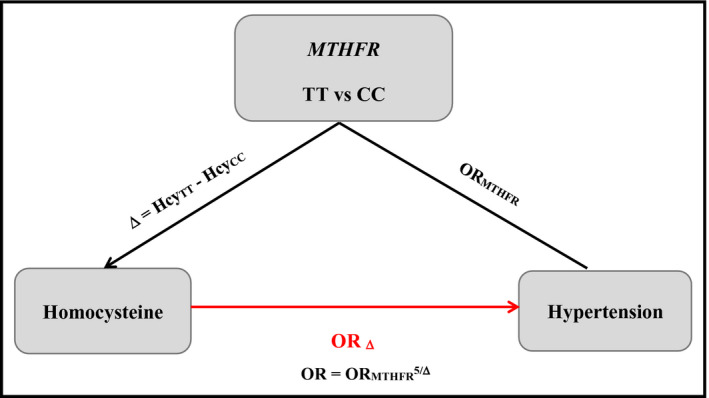
The potential causal association between Hcy and hypertension risk was assessed via Mendelian randomization
4. DISCUSSION
Overall, this study suggested that MTHFR 677T allele was positively associated with plasma Hcy concentration. Furthermore, the mean Hcy concentration in hypertensive patients was higher than those without hypertension. The findings via Mendelian randomization method reinforced the assumption that elevated Hcy level had a plausible effect on the elevated risk of hypertension.
MTHFR C677T signifies the change in cysteine into thymine nucleotide at its corresponding position, which leads to the transition of alanine to valine in the MTHFR enzyme.107 Furthermore, the thermostability of the enzyme would reduce due to the variant in the MTHFR gene with the decreasing MTHFR enzyme activity, especially when the temperature is 37 ℃ or higher. The MTHFR enzyme activity in homozygous individuals is lower approximately 50%‐60% at 37°C and 65% at 46°C compared with the normal non‐mutated controls.108, 109 The decrease in the enzyme activity accelerates the conversion of 5,10‐methylenetetrahydrofolate to 5‐methylenetetrahydrofolate, which results in the increase in Hcy concentration in the mutated subjects. Therefore, the Hcy levels of homozygous subjects are greater than those of heterozygous mutated subjects, and the heterozygous subjects have slightly increased Hcy concentrations compared with the non‐mutated controls.109 The findings of our study demonstrated the assumption that the MTHFR C677T was strongly associated with Hcy concentrations in hypertensive patients. The homozygous subjects confer significantly higher Hcy levels than the heterozygous subjects in hypertensive group, which are in consistent with previous report in hypertensive patients.110
Recently, many studies have reported inconclusive results concerning the altering Hcy levels in hypertensive compared with normal subjects.97, 98, 100, 101 However, other studies suggested that the levels of Hcy in hypertensive were higher than in subjects without hypertension.71, 99, 102 This study analyzed the weighted mean difference of Hcy concentrations between hypertensive cases and normal controls in all included studies, which showed significant heterogeneity among different studies. Therefore, a random effects model was employed to estimate the pooled mean Hcy. Subsequently, the absolute pooled mean Hcy level in hypertensive was significantly higher than that in controls under the random effects model. The presence of the heterogeneity in these studies might be explained by the ethnicity of different regions and the coexistence of hypertension‐related disease.71, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 According to the fact that plasma Hcy could not only induce arteriolar constriction, increase sodium reabsorption, but also increase arterial stiffness,58, 111 Hcy may be involved in the pathogenesis of hypertension. Our previous findings have demonstrated that the MTHFR C677T is significantly associated with childhood obesity, and a strong relationship between Hcy level and obesity was emphasized by virtue of MTHFR C677T polymorphism.112, 113 However, the underlying role by MTHFR C677T polymorphism in leading to hypertension remains unclear, and further researches on genetics and animal experiments are needed to elucidate this hypothesis.
To the best of our knowledge, MTHFR C677T was first identified as a significant variant associated with hypertension in cardiovascular disease subjects in a Japanese population.23 Subsequently, another study indicated that MTHFR 677TT homozygosity is a predictor of essential hypertension in a Spanish male population.33 Although a large number of studies have investigated the association between MTHFR C677T and hypertension, the results are inconsistent in different population.10, 11, 54, 65 A recent meta‐analysis comprising thirty studies with 5207 patients and 5383 control subjects examined the association between MTHFR C677T polymorphism and hypertension, which revealed that carriers of the T allele and TT genotype significantly elevated the hypertension risk in all genetic models.114 Compared with that study, the present meta‐analysis had several advantages in the following aspects: First, the present study investigated 57 eligible studies with a total of 14 378 hypertensive cases and 25 795 controls, which provides more reliable conclusions. Second, we assessed not only the association between MTHFR C677T and hypertension, but also the relationship between MTHFR C677T and plasma Hcy concentration, which could provide more extra information about the effect of MTHFR C677T on hypertensive risk. Finally, by means of Mendelian randomization method, the effect size of MTHFR C677T on hypertension may be estimated more accurately. The reasons why inconsistent results exist in studies regarding MTHFR C677T and hypertension are still unclear, but a major reason for equivocal results might be the racial difference in the included studies. The frequency distribution of the 677TT genotype was highest in the Italian and Hispanics.115 Nevertheless, the homozygous frequency was very low for Black in Brazil and American.116, 117, 118 Furthermore, the study design flaws, small sample size, or other biases seem to be more common factors for the discrepancies comprising in different studies with regard to genetic studies.119, 120 Based on case‐control, cross‐sectional, or case‐cohort designed studies, the overall results of the present meta‐analysis indicated that MTHFR C677T is associated with hypertension and MTHFR TT genotype has a great effect on increasing the risk of hypertension. Additionally, sensitivity analysis indicated that an omission of studies that departure from HWE did not alter the magnitude of present effect, suggesting that the results were generally reliable and robust.
5. STRENGTHS AND LIMITATIONS
To date, this is the first meta‐analysis aggregating all the data available for evaluating the effect of MTHFR‐linked Hcy on hypertension. Therefore, it has provided the most sufficient data on this issue. However, several limitations should be mentioned: First, although applying Mendelian randomization approach could obtain an unbiased result because of avoiding the advent of confounding factors, the gene‐environment and gene‐gene interactions have affected the development of hypertension unavoidably. The result would be more precise if these factors are taken into account. Second, by virtual of different high‐performance liquid chromatography methods, the Hcy measurement showed discrepancies among different laboratories.121 Thus, Hcy measurement seems to contribute to variation among various studies applying different approaches. Third, the method of genotyping was used by different approaches, which could influence the results. Finally, only English and Chinese articles were included, which might result in publication bias, although Egger's and Begg's tests revealed no sign of the existence of pivotal publication bias with respect to the small sample size.
6. CONCLUSIONS
In summary, our results provided sufficient data supporting the assumption that the TT genotype in MTHFR C677T contributes to the susceptibility of hypertension. By means of Mendelian randomization approach, the findings are in support of the hypothesis that enhanced Hcy level is plausibly linked to increase the risk of hypertension. To some extent, the presence of gene‐environment interactions may result in the discordance of results comprising in the genetic association studies. Thus, prospective studies that investigate gene‐environment with large sample size are needed to further elucidate the genetic pathogenesis of hypertension.
DISCLOSURES
The authors declare there are no conflicts of interests.
AUTHOR CONTRIBUTIONS
LF and YQH conceptualized and designed the study. LF, YQH, and YL acquired the data. LF and YL analyzed and interpreted the data and drafted the manuscript. LF, YL, DL, SD, BW, and YQH involved in critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of manuscript.
Fu L, Li Y‐N, Luo D, Deng S, Wu B, Hu Y‐Q. Evidence on the causal link between homocysteine and hypertension from a meta‐analysis of 40 173 individuals implementing Mendelian randomization. J Clin Hypertens. 2019;21:1879–1894. 10.1111/jch.13737
Fu and Li contributed equally.
Funding information
This study was supported by grants to YQH from the National Natural Science Foundation of China (grants no. 11571082, 11971117), Key Research Project of the Ministry of Science and Technology of China (grant no. 2016YFC0904400), and Scientific Research Foundation of Fudan University.
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison M, Maresso K, Broeckel U. Genetic determinants of hypertension: an update. Curr Hypertens Rep. 2008;10(6):488‐495. [DOI] [PubMed] [Google Scholar]
- 4. Liu X, Zhang D, Liu YU, et al. Dose‐response association between physical activity and incident hypertension: a systematic review and meta‐analysis of cohort studies. Hypertension. 2017;69(5):813‐820. [DOI] [PubMed] [Google Scholar]
- 5. Lander ES. The new genomics: global views of biology. Science. 1996;274(5287):536‐539. [DOI] [PubMed] [Google Scholar]
- 6. Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111‐113. [DOI] [PubMed] [Google Scholar]
- 7. Pereira AC, Schettert IT, Morandini Filho AA, Guerra‐Shinohara EM, Krieger JE. Methylenetetrahydrofolate reductase (MTHFR) c677t gene variant modulates the homocysteine folate correlation in a mild folate‐deficient population. Clin Chim Acta. 2004;340(1–2):99‐105. [DOI] [PubMed] [Google Scholar]
- 8. Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium‐dependent vasodilation in humans. Circulation. 1997;95(5):1119‐1121. [DOI] [PubMed] [Google Scholar]
- 9. Heux S, Morin F, Lea RA, Ovcaric M, Tajouri L, Griffiths LR. The methylentetrahydrofolate reductase gene variant (C677T) as a risk factor for essential hypertension in Caucasians. Hypertens Res. 2004;27(9):663‐667. [DOI] [PubMed] [Google Scholar]
- 10. Canto P, Canto‐cetina T, Juárez‐velázquez R, et al. Methylenetetrahydrofolate reductase C677T and glutathione S‐transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya‐Mestizo women. Hypertens Res. 2008;31(5):1015‐1019. [DOI] [PubMed] [Google Scholar]
- 11. Ng X, Boyd L, Dufficy L, et al. Folate nutritional genetics and risk for hypertension in an elderly population sample. J Nutrigenet Nutrigenomics. 2009;2(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 13. Zintzaras E, Ioannidis JP. Heterogeneity testing in meta‐analysis of genome searches. Genet Epidemiol. 2005;28(2):123‐137. [DOI] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glymour MM, Tchetgen Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175(4):332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frederiksen J, Juul K, Grande P, et al. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and case‐control studies from the Copenhagen City Heart Study. Blood. 2004;104(10):3046‐3051. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka T, Scheet P, Giusti B, et al. Genome‐wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84(4):477‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang T, Tucker KL, Lee Y‐C, et al. Methylenetetrahydrofolate reductase variants associated with hypertension and cardiovascular disease interact with dietary polyunsaturated fatty acids to modulate plasma homocysteine in puerto rican adults. J Nutr. 2011;141(4):654‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson JR, Minelli C, Abrams KR, Tobin MD, Riley RD. Meta‐analysis of genetic studies using Mendelian randomization–a multivariate approach. Stat Med. 2005;24(14):2241‐2254. [DOI] [PubMed] [Google Scholar]
- 21. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ. 2002;325(7374):1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishio H, Lee MJ, Fujii M, et al. A common mutation in methylenetetrahydrofolate reductase gene among the Japanese population. Jpn J Hum Genet. 1996;41(2):247‐251. [DOI] [PubMed] [Google Scholar]
- 23. Nakata Y, Katsuya T, Takami S, et al. Methylenetetrahydrofolate reductase gene polymorphism: relation to blood pressure and cerebrovascular disease. Am J Hypertens. 1998;11(8 Pt 1):1019‐1023. [DOI] [PubMed] [Google Scholar]
- 24. Powers RW, Minich LA, Lykins DL, Ness RB, Crombleholme WR, Roberts JM. Methylenetetrahydrofolate reductase polymorphism, folate, and susceptibility to preeclampsia. J Soc Gynecol Investig. 1999;6(2):74‐79. [DOI] [PubMed] [Google Scholar]
- 25. Kobashi G, Yamada H, Asano T, et al. Absence of association between a common mutation in the methylenetetrahydrofolate reductase gene and preeclampsia in Japanese women. Am J Med Genet. 2000;93(2):122‐125. [DOI] [PubMed] [Google Scholar]
- 26. Li K, Sun Y, Chen L, Zhang G, Li P. Study on the relationship between methylenetetrahydrofolate reductase gene polymorphism and plasma homocysteine in pregnancy induced hypertension patients. Chin J Obstet Gynecol. 2000;35(04):205‐207. [PubMed] [Google Scholar]
- 27. Rajkovic A, Mahomed K, Rozen R, Malinow MR, King IB, Williams MA. Methylenetetrahydrofolate reductase 677 C –> T polymorphism, plasma folate, vitamin B(12) concentrations, and risk of preeclampsia among black African women from Zimbabwe. Mol Genet Metab. 2000;69(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 28. Zhan S, Gao Y, Yin X, Huang Y, Hu Y, Li L. A case‐control study on the relationship between abnormal homocysteine metabolism and essential hypertension. Chin J Epidemiol. 2000;21(03):194‐197. [PubMed] [Google Scholar]
- 29. Zusterzeel PLM, Visser W, Bolm HJ, et al. Methylenetetrahydrofolate reductase polymorphisms in preeclampsia and the HELLP syndrome. Hypertens Pregnancy. 2000;19(3):299‐307. [DOI] [PubMed] [Google Scholar]
- 30. Beneš P, Kaňková K, Mužík J, et al. Methylenetetrahydrofolate reductase polymorphism, type II diabetes mellitus, coronary artery disease, and essential hypertension in the Czech population. Mol Genet Metab. 2001;73(2):188‐195. [DOI] [PubMed] [Google Scholar]
- 31. Kahleova R, Palyzova D, Zvara K, et al. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens. 2002;15(10 Pt 1):857‐864. [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Guo H, Li Y. MTHFR gene C 677 T polymorphisms and variation of plasma homocysteine level in primary hypertension. Tianjin Med J. 2002;30(10):579‐582. [Google Scholar]
- 33. Rodríguez‐Esparragón F, Hernández‐Perera O, Rodríguez‐Pérez JC, et al. The effect of methylenetetrahydrofolate reductase C677T common variant on hypertensive risk is not solely explained by increased plasma homocysteine values. Clin Exp Hypertens. 2003;25(4):209‐220. [DOI] [PubMed] [Google Scholar]
- 34. Sun X, Li Y, Guo H. The gene polymorphisms of homocysteine metabolism‐related enzymes and the associated factors in isolated systolic hypertension. Chin J Cardiol. 2003;31(04):269‐273. [Google Scholar]
- 35. Yilmaz H, Unlucerci Y, Gurdol F, Isbilen E, Isbir T. Association of pre‐eclampsia with hyperhomocysteinaemia and methylenetetrahydrofolate reductase gene C677T polymorphism in a Turkish population. Aust N Z J Obstet Gynaecol. 2004;44(5):423‐427. [DOI] [PubMed] [Google Scholar]
- 36. Cesari M, Zanchetta M, Burlina A, et al. Hyperhomocysteinemia is inversely related with left ventricular ejection fraction and predicts cardiovascular mortality in high‐risk coronary artery disease hypertensives. Arterioscler Thromb Vasc Biol. 2005;25(1):115‐121. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Ye L, Liu J, Li X. Study on homocysteine metabolism‐related enzymes gene polymorphisms in elderly essential hypertension patients with peripheral arterial occlusive disease. Chin J Geriatr. 2005;24(05):332‐335. [Google Scholar]
- 38. Demir SC, Evruke C, Ozgunen T, Kadayifci O, Altintas U, Kokangul S. The relationship between pregnancy induced hypertension and congenital thrombophilia. Saudi Med J. 2006;27(8):1161‐1166. [PubMed] [Google Scholar]
- 39. Hu R, Niu G, Zhao S, et al. The association between gene polymorphisms of N5, 10 methylenetetrahydrofolate reductase and Mongolian patients with drimary hypertension. Chin J Hypertension. 2006;14(04):274‐276. [Google Scholar]
- 40. Kalita J, Srivastava R, Bansal V, Agarwal S, Misra UK. Methylenetetrahydrofolate reductase gene polymorphism in Indian stroke patients. Neurol India. 2006;54(3):260‐263. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Huang W. The analysis of MTHFR gene polymorphism in patients with renal damage caused by hypertension and patients with renal parenchymal hypertension. J Capital Univ Med Sci. 2006;27(04):497‐500. [Google Scholar]
- 42. Lwin H, Yokoyama T, Yoshiike N, et al. Polymorphism of methylenetetrahydrofolate reductase gene (C677T MTHFR) is not a confounding factor of the relationship between serum uric acid level and the prevalence of hypertension in Japanese men. Circ J. 2006;70(1):83‐87. [DOI] [PubMed] [Google Scholar]
- 43. Hui P, Nakayama T, Morita A, et al. Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens Res. 2007;30(7):585‐592. [DOI] [PubMed] [Google Scholar]
- 44. Marinho C, Alho I, Arduino D, Falcao LM, Bras‐Nogueira J, Bicho M. GST M1/T1 and MTHFR polymorphisms as risk factors for hypertension. Biochem Biophys Res Commun. 2007;353(2):344‐350. [DOI] [PubMed] [Google Scholar]
- 45. Markan S, Sachdeva M, Sehrawat BS, Kumari S, Jain S, Khullar M. MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem. 2007;302(1–2):125‐131. [DOI] [PubMed] [Google Scholar]
- 46. Nagy B, Hupuczi P, Papp Z. High frequency of methylenetetrahydrofolate reductase 677TT genotype in Hungarian HELLP syndrome patients determined by quantitative real‐time PCR. J Hum Hypertens. 2007;21(2):154‐158. [DOI] [PubMed] [Google Scholar]
- 47. Xing X, Hua Q. Relationships between the polymorphism of methylenetetrahydrofolate reductase gene C677T and hypertension, cardiac structure and function. Med J Chin PLA. 2007;32(07):741‐744. [Google Scholar]
- 48. Fridman O, Porcile R, Vanasco V, et al. Study on homocysteine levels and methylenetetrahydrofolate reductase gene variant (C677T) in a population of Buenos Aires City. Clin Exp Hypertens. 2008;30(7):574‐584. [DOI] [PubMed] [Google Scholar]
- 49. Ilhan N, Kucuksu M, Kaman D, Ilhan N, Ozbay Y. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch Med Res. 2008;39(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 50. Lin PT, Cheng CH, Wei JC, Huang YC. Low plasma pyridoxal 5'‐phosphate concentration and MTHFR 677C–>T genotypes are associated with increased risk of hypertension. Int J Vitam Nutr Res. 2008;78(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 51. Soares AL, Fernandes AP, Cardoso JE, et al. Plasma total homocysteine levels and methylenetetrahydrofolate reductase gene polymorphism in patients with type 2 diabetes mellitus. Pathophysiol Haemost Thromb. 2008;36(5):275‐281. [DOI] [PubMed] [Google Scholar]
- 52. Deshmukh A, Rodrigue KM, Kennedy KM, Land S, Jacobs BS, Raz N. Synergistic effects of the MTHFR C677T polymorphism and hypertension on spatial navigation. Biol Psychol. 2009;80(2):240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fakhrzadeh H, Mirarefin M, Sharifi F, et al. Association of methylenetetrahydrofolate reductase gene polymorphism (C677T) with metabolic syndrome in an Iranian Population: Tehran homocysteine survey. J Diabetes Metab Disord. 2009;8:37‐46. [Google Scholar]
- 54. Liu H, Ma P, Xu Q. The correlation between polymorphisms of N5,10 methylenetetrahydrofolate reductase and essential hypertension in Han population in Ningxia. Guangdong Med J. 2011;32(15):1977‐1980. [Google Scholar]
- 55. Fowdar JY, Lason MV, Szvetko AL, Lea RA, Griffiths LR. Investigation of homocysteine‐pathway‐related variants in essential hypertension. Int J Hypertens. 2012;2012:190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yin R‐X, Wu J‐Z, Liu W‐Y, et al. Association of several lipid‐related gene polymorphisms and blood pressure variation in the Bai Ku Yao population. Am J Hypertens. 2012;25(8):927‐936. [DOI] [PubMed] [Google Scholar]
- 57. Zhang Y, Wang H, Zhang X, Wang L, Wu G. Relationship between homocysteine, methylene tetrahydrofolate reductase C677T polymorphisms and essential hypertension in Kazak nationality in Xinjiang. J Clin Cardiol (China). 2012;28(08):570‐573. [Google Scholar]
- 58. Fridman O, Porcile R, Morales AV, Gariglio LO, Potenzoni MA, Turk Noceto PC. Association of methylenetetrahydrofolate reductase gene 677C>T polymorphism with hypertension in older women in a population of Buenos Aires City. Clin Exp Hypertens. 2013;35(3):159‐166. [DOI] [PubMed] [Google Scholar]
- 59. Yao R, Zhang H, Zhang J, Li D, Fan Y. Detection of C677T polymorphism of MTHFR gene in patients with primary hypertension of han nationality in henan province. Chin J Gerontol. 2013;33(05):1001‐1003. [Google Scholar]
- 60. Cai W, Yin L, Yang F, Zhang L, Cheng J. Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomed Rep. 2014;2(6):861‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Husemoen LLN, Skaaby T, Jørgensen T, et al. MTHFR C677T genotype and cardiovascular risk in a general population without mandatory folic acid fortification. Eur J Nutr. 2014;53(7):1549‐1559. [DOI] [PubMed] [Google Scholar]
- 62. Vazquez‐Alaniz F, Lumbreras‐Márquez MI, Sandoval‐Carrillo AA, et al. Association of COMT G675A and MTHFR C677T polymorphisms with hypertensive disorders of pregnancy in Mexican mestizo population. Pregnancy Hypertens. 2014;4(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 63. Bayramoglu A, Urhan Kucuk M, Guler HI, Abaci O, Kucukkaya Y, Colak E. Is there any genetic predisposition of MMP‐9 gene C1562T and MTHFR gene C677T polymorphisms with essential hypertension? Cytotechnology. 2015;67(1):115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nassereddine S, Kassogue Y, Korchi F, Habbal R, Nadifi S. Association of methylenetetrahydrofolate reductase gene (C677T) with the risk of hypertension in Morocco. BMC Res Notes. 2015;8:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pérez‐Razo JC, Cano‐Martínez LJ, Vargas Alarcón G, et al. Functional Polymorphism rs13306560 of the MTHFR Gene Is Associated With Essential Hypertension in a Mexican‐Mestizo Population. Circ Cardiovasc Genet. 2015;8(4):603‐609. [DOI] [PubMed] [Google Scholar]
- 66. Wang YU, Xu X, Huo Y, et al. Predicting hyperhomocysteinemia by methylenetetrahydrofolate reductase C677T polymorphism in Chinese patients with hypertension. Clin Appl Thromb Hemost. 2015;21(7):661‐666. [DOI] [PubMed] [Google Scholar]
- 67. Wei LK, Menon S, Griffiths LR, Gan SH. Signaling pathway genes for blood pressure, folate and cholesterol levels among hypertensives: an epistasis analysis. J Hum Hypertens. 2015;29(2):99‐104. [DOI] [PubMed] [Google Scholar]
- 68. Wen C, Lv JF, Wang L, Zhu WF, Wan FS, Wang XZ. Association of a methylene tetrahydrofolate reductase C677T polymorphism with several blood chemical levels in a Chinese population. Genet Test Mol Biomarkers. 2015;19(1):24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amrani‐Midoun A, Kiando SR, Treard C, Jeunemaitre X, Bouatia‐Naji N. The relationship between MTHFR C677T gene polymorphism and essential hypertension in a sample of an Algerian population of Oran city. Int J Cardiol. 2016;225:408‐411. [DOI] [PubMed] [Google Scholar]
- 70. Fan S, Yang B, Zhi X, et al. Interactions of methylenetetrahydrofolate reductase C677T polymorphism with environmental factors on hypertension susceptibility. Int J Environ Res Public Health. 2016;13(6):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dwivedi MK, Sinha D. Role of MTHFR 677 C>T polymorphism on blood homocysteine and susceptibility to hypertension. Int J Hum Genet. 2017;17(3):118‐125. [Google Scholar]
- 72. Rios DRA, Alpoim PN, Godoi LC, et al. Is there a link among thrombophilia factors and preeclampsia? J Thromb Thrombolysis. 2017;44(4):516‐518. [DOI] [PubMed] [Google Scholar]
- 73. Arina CA, Amir D, Siregar Y, Sembiring RJ. The role of polymorphism gen methylene tetra hydrofolate reductase (MTHFR) C677T in ischaemic stroke patients with and without hypertension. Open Access Maced J Med Sci. 2019;7(1):29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koo HS, Lee HS, Hong YM. Methylenetetrahydrofolate reductase TT genotype as a predictor of cardiovascular risk in hypertensive adolescents. Pediatr Cardiol. 2008;29(1):136‐141. [DOI] [PubMed] [Google Scholar]
- 75. Fan F‐F, Huo Y, Wang XU, et al. Effect of enalapril on plasma homocysteine levels in patients with essential hypertension. J Zhejiang Univ Sci B. 2010;11(8):583‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mazza A, Montemurro D, L'Erario R, et al. Could genetic analysis be useful in reducing cerebrovascular risk in hypertensive subjects with hyperhomocysteinemia and patent foramen ovale? A 2‐year follow‐up study. Microvasc Res. 2010;80(3):545‐548. [DOI] [PubMed] [Google Scholar]
- 77. Qin X, Li J, Cui Y, et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults. Nutr J. 2012;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yun L, Xu R, Li G, et al. Homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR are risk factors of early renal damage in hypertension in a Chinese Han Population. Medicine (Baltimore). 2015;94(52):e2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu X, Sun N, Yu T, et al. The independent and joint association of blood pressure, serum total homocysteine, and fasting serum glucose levels with brachial‐ankle pulse wave velocity in Chinese hypertensive adults. Int Heart J. 2016;57(5):627‐633. [DOI] [PubMed] [Google Scholar]
- 80. Liu Y, Li K, Venners SA, et al. Individual and joint associations of methylenetetrahydrofolate reductase C677T genotype and plasma homocysteine with dyslipidemia in a Chinese population with hypertension. Clin Appl Thromb Hemost. 2017;23(3):287‐293. [DOI] [PubMed] [Google Scholar]
- 81. Yang X, Zhang M, Song R, Liu C, Huo Y, Qian G. The modifying effect of the MTHFR genotype on the association between folic acid supplementation and pulse wave velocity: findings from the CSPPT. Cardiovasc Ther. 2018;36(6):e12473. [DOI] [PubMed] [Google Scholar]
- 82. Yuan X, Wang T, Gao J, et al. Associations of homocysteine status and homocysteine metabolism enzyme polymorphisms with hypertension and dyslipidemia in a Chinese hypertensive population. Clin Exp Hypertens. 2019;1‐9. [DOI] [PubMed] [Google Scholar]
- 83. Sorensen TK, Malinow MR, Williams MA, King IB, Luthy DA. Elevated second‐trimester serum homocyst(e)ine levels and subsequent risk of preeclampsia. Gynecol Obstet Invest. 1999;48(2):98‐103. [DOI] [PubMed] [Google Scholar]
- 84. Virdis A, Ghiadoni L, Cardinal H, et al. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. J Am Coll Cardiol. 2001;38(4):1106‐1115. [DOI] [PubMed] [Google Scholar]
- 85. Garfunkel VAM, Porto PI, Garcia SI, et al. Hyperhomocysteinemia but not MTHFR genotype is associated with young‐onset essential hypertension. J Hum Hypertens. 2003;17(5):361‐364. [DOI] [PubMed] [Google Scholar]
- 86. Jain S, Ram H, Kumari S, Khullar M. Plasma homocysteine levels in Indian patients with essential hypertension and their siblings. Ren Fail. 2003;25(2):195‐201. [DOI] [PubMed] [Google Scholar]
- 87. Bowman TS, Gaziano JM, Stampfer MJ, Sesso HD. Homocysteine and risk of developing hypertension in men. J Hum Hypertens. 2006;20(8):631‐634. [DOI] [PubMed] [Google Scholar]
- 88. Ustundag S, Arikan E, Sen S, Esgin H, Ciftci S. The relationship between the levels of plasma total homocysteine and insulin resistance in uncomplicated mild‐to‐moderate primary hypertension. J Hum Hypertens. 2006;20(5):379‐381. [DOI] [PubMed] [Google Scholar]
- 89. Poduri A, Kaur J, Thakur JS, Kumari S, Jain S, Khullar M. Effect of ACE inhibitors and beta‐blockers on homocysteine levels in essential hypertension. J Hum Hypertens. 2008;22(4):289‐294. [DOI] [PubMed] [Google Scholar]
- 90. Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med. 2009;169(2):155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Poduri A, Kumari S, Jain S, Khullar M. A case‐control study of the association between the MTHFR gene and essential hypertension in Asian Indians. J Hum Hypertens. 2009;23(2):140‐142. [DOI] [PubMed] [Google Scholar]
- 92. Stiefel P, Miranda ML, Bellido LM, et al. Genotype of the CYBA promoter ‐930A/G, polymorphism C677T of the MTHFR and APOE genotype in patients with hypertensive disorders of pregnancy: an observational study. Med Clin (Barc). 2009;133(17):657‐661. [DOI] [PubMed] [Google Scholar]
- 93. Wang YU, Li X, Qin X, et al. Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45–75 years. Br J Nutr. 2013;109(7):1284‐1293. [DOI] [PubMed] [Google Scholar]
- 94. Baszczuk A, Musialik K, Kopczyński J, et al. Hyperhomocysteinemia, lipid and lipoprotein disturbances in patients with primary hypertension. Adv Med Sci. 2014;59(1):68‐73. [DOI] [PubMed] [Google Scholar]
- 95. Scazzone C, Bono A, Tornese F, et al. Correlation between low folate levels and hyperhomocysteinemia, but not with vitamin B12 in hypertensive patients. Ann Clin Lab Sci. 2014;44(3):286‐290. [PubMed] [Google Scholar]
- 96. Keskek SO, Cinar Y, Kirim S, Saler T. High renal resistive index in hypertensive patients is also associated with serum homocysteine level. Clin Exp Nephrol. 2015;19(4):639‐645. [DOI] [PubMed] [Google Scholar]
- 97. Bickel C, Schnabel RB, Zengin E, et al. Homocysteine concentration in coronary artery disease: influence of three common single nucleotide polymorphisms. Nutr Metab Cardiovasc Dis. 2017;27(2):168‐175. [DOI] [PubMed] [Google Scholar]
- 98. Chang Y, Li Y, Guo X, Chen Y, Dai D, Sun Y. The prevalence of hypertension accompanied by high homocysteine and its risk factors in a rural population: a cross‐sectional study from Northeast China. Int J Environ Res Public Health. 2017;14(4):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Han L, Liu Y, Wang C, et al. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: A population‐based study and systematic review. Clin Nutr. 2017;36(5):1215‐1230. [DOI] [PubMed] [Google Scholar]
- 100. Oliver E, Monto F, Rovira E, Valldecabres C, Muedra V, D'Ocon P. Changes in the expression of alpha1B‐adrenoceptor in peripheral mononuclear cells correlates with blood pressure and plasmatic homocysteine. Biomed Pharmacother. 2017;88:721‐727. [DOI] [PubMed] [Google Scholar]
- 101. Sun F, Qian W, Zhang C, Fan JX, Huang HF. Correlation of maternal serum homocysteine in the first trimester with the development of gestational hypertension and preeclampsia. Med Sci Monit. 2017;23:5396‐5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ye Z, Wang C, Zhang Q, et al. Prevalence of homocysteine‐related hypertension in patients with chronic kidney disease. J Clin Hypertens (Greenwich). 2017;19(2):151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hidru TH, Yang X, Xia Y, Ma L, Li HH. The relationship between plasma markers and essential hypertension in middle‐aged and elderly Chinese population: a community based cross‐sectional study. Sci Rep. 2019;9(1):6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ma L, Li L, Han P, Meng F, Jiao C, Zhang H. Effect of the drug combination of magnesium sulfate and phentolamine on homocysteine and C‐reactive protein in the serum of patients with pregnancy‐induced hypertension syndrome. Exp Ther Med. 2019;17(5):3682‐3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang C, Xu G, Wen QI, et al. CBS promoter hypermethylation increases the risk of hypertension and stroke. Clinics. 2019;74:e630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xu M, Li J, Chen X, Han L, Li L, Liu Y. MTHFD1 promoter hypermethylation increases the risk of hypertension. Clin Exp Hypertens. 2019;41(5):422‐427. [DOI] [PubMed] [Google Scholar]
- 107. Rosenberg N, Murata M, Ikeda Y, et al. The frequent 5,10‐methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. Am J Hum Genet. 2002;70(3):758‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43(4):414‐421. [PMC free article] [PubMed] [Google Scholar]
- 109. Rozen R. Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost. 1997;78(1):523‐526. [PubMed] [Google Scholar]
- 110. Tavakkoly Bazzaz J, Shojapoor M, Nazem H, et al. Methylenetetrahydrofolate reductase gene polymorphism in diabetes and obesity. Mol Biol Rep. 2010;37(1):105‐109. [DOI] [PubMed] [Google Scholar]
- 111. Sen U, Tyagi SC. Homocysteine and hypertension in diabetes: does PPARgamma have a regulatory role? PPAR Res. 2010;2010:806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fu L, Zhang M, Hu Y‐Q, et al. Gene‐gene interactions and associations of six hypertension related single nucleotide polymorphisms with obesity risk in a Chinese children population. Gene. 2018;679:320‐327. [DOI] [PubMed] [Google Scholar]
- 113. Fu L, Li Y, Luo D, Deng S, Hu YQ. Plausible relationship between homocysteine and obesity risk via MTHFR gene: a meta‐analysis of 38,317 individuals implementing Mendelian randomization. Diabet Metab Syndr Obes. 2019;12:1201‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu Y‐L, Hu C‐Y, Lu S‐S, et al. Association between methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms and essential hypertension: a systematic review and meta‐analysis. Metabolism. 2014;63(12):1503‐1511. [DOI] [PubMed] [Google Scholar]
- 115. Botto LD, Yang Q. 5,10‐Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862‐877. [DOI] [PubMed] [Google Scholar]
- 116. Stevenson RE, Schwartz CE, Du YZ, Adams MJ Jr. Differences in methylenetetrahydrofolate reductase genotype frequencies, between Whites and Blacks. Am J Hum Genet. 1997;60(1):229‐230. [PMC free article] [PubMed] [Google Scholar]
- 117. Arruda VR, Siqueira LH, Goncalves MS, et al. Prevalence of the mutation C677 –> T in the methylene tetrahydrofolate reductase gene among distinct ethnic groups in Brazil. Am J Med Genet. 1998;78(4):332‐335. [DOI] [PubMed] [Google Scholar]
- 118. Dilley A, Austin H, Hooper WC, et al. Relation of three genetic traits to venous thrombosis in an African‐American population. Am J Epidemiol. 1998;147(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 119. Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos‐Ioannidis DG. Genetic associations in large versus small studies: an empirical assessment. Lancet. 2003;361(9357):567‐571. [DOI] [PubMed] [Google Scholar]
- 120. Ioannidis JP, Ntzani EE, Trikalinos TA. 'Racial' differences in genetic effects for complex diseases. Nat Genet. 2004;36(12):1312‐1318. [DOI] [PubMed] [Google Scholar]
- 121. Pfeiffer CM, Huff DL, Smith SJ, Miller DT, Gunter EW. Comparison of plasma total homocysteine measurements in 14 laboratories: an international study. Clin Chem. 1999;45(8 Pt 1):1261‐1268. [PubMed] [Google Scholar]


