Abstract
Arterial hypertension is a well‐established cardiovascular risk factor, and blood pressure (BP) control has largely improved the prognosis of hypertensive patients. A number of studies have assessed the role of BP levels in the prognosis of patients with acute coronary syndromes. Pathophysiologic links of hypertension to acute myocardial infarction (MI) include endothelial dysfunction, autonomic nervous system dysregulation, impaired vasoreactivity, and a genetic substrate. A history of hypertension is highly prevalent among patients presenting with MI, and some, but not all, studies have associated it with a worse prognosis. Some data support that low levels of admission and in‐hospital BP may indicate an increased risk for subsequent events. Risk scores used in patients with MI have, therefore, included BP levels and a history of hypertension in their variables. Of note, good long‐term BP control, ideally initiated prior to discharge, should be pursued in order to improve secondary prevention.
Keywords: blood pressure, hospitalization, myocardial infarction
1. INTRODUCTION
Arterial hypertension is a leading cardiovascular risk factor worldwide, with a well‐established association with coronary artery disease.1 In turn, risk stratification is a crucial step in both ST and non–ST‐elevation acute coronary syndromes (ACS) and various risk scores are suggested by clinical guidelines in order to optimize patient care in the acute and chronic setting. By no surprise, a number of studies have considered the presence of hypertension as a possible event modifier in patients who are admitted in emergency departments due to ACS.2 However, optimal blood pressure (BP) levels in this acute setting are ill‐defined. The objective of the present review is to present current knowledge on the pathophysiologic and clinical associations of BP levels and a history of hypertension in patients with an ACS and their implementation in risk characterization.
2. PATHOPHYSIOLOGICAL LINKS BETWEEN HYPERTENSION AND ACUTE CORONARY SYNDROMES
Atherosclerotic disease and increased BP share certain common mechanisms, with the multiple effects of vasoactive molecules having been widely researched. The angiotensin‐converting enzyme (ACE) is the key enzyme in the production of angiotensin II and the catabolism of bradykinin, two peptides involved in the modulation of vascular tone and the proliferation of smooth muscle cells. Angiotensin II promotes the expression of adhesion molecules, tissue factor, and plasminogen activator inhibitor‐1. It further favors a reduction in smooth muscle cell proliferation, intraplaque inflammatory infiltration, and intraplaque neovascularization.3 The potent vasoconstrictor endothelin‐1, in the setting of acute myocardial infarction (MI), may facilitate myocardial necrosis and arrhythmogenesis but seems to exert a favorable effect on subsequent infarct healing and early ventricular remodeling. In the chronic post‐infarction phase, endothelin‐1 increases left ventricular afterload and is actively involved in the myocardial fibrotic process.4
Sympathetic overactivity has been proposed to participate in the atherosclerotic process in hypertensive patients through a number of pathways involving G protein–coupled adrenergic receptors,5 which promote atherogenesis and eventually may trigger an ACS episode: Sympathetic activation is associated per se with endothelial dysfunction; sympathetic vasoconstriction interferes with glucose extraction in skeletal muscle leading to beta‐adrenoreceptor–mediated insulin resistance; and induced vascular wall hypertrophy leads to small vessel crushing and vascular rarefaction. On the other hand, following an ACS there is an increased sympathetic overdrive as an adaptive mechanism to maintain BP and cardiac output. Although being useful in the short term, these effects may induce deleterious long‐term consequences. The direct sympathetic effect on the renin‐aldosterone axis results in development of left ventricular hypertrophy along with a simultaneous increase in cardiac output, oxygen consumption by myocardial cells, and eventually an increased risk of ischemia and arrhythmic events.6
A significant amount of evidence supports the presence of a prethrombotic state among hypertensive individuals. Hypertension‐related organ damage may be the repercussion of paradoxical activation of clotting factors such as fibrinogen.7 Hypertensive patients have a higher mean platelet volume and mean platelet mass and lower mean platelet granularity compared to controls.8 Furthermore, platelets produce more reactive oxygen species, which enhance platelet activity by causing a reduction in the bioavailability of nitric oxide and enhancing [Ca2+] cellular effect among others. Notwithstanding, catecholamines and renin‐angiotensin activation in hypertensives trigger platelet aggregation and activation.
Mechanical factors may also explain the association between increased BP and ACS. High BP denotes increased mechanical stress on blood vessels that contributes to endothelial dysfunction, atherosclerosis progression, and eventually plaque rupture. Shear stress has also been correlated with endothelial dysfunction, thrombotic events, and the formation of the vulnerable atherosclerotic plaque.9 It is this complex puzzle of local rheological disorders combined with endothelial dysfunction, changes in arterial wall substrates, and a varying degree of local inflammation of atherosclerotic plaques which contributes to an ACS.10 Similarly, left ventricular hypertrophy, a major target organ damage of hypertension, makes the myocardium prone to ischemia. The rise in wall tension along with the higher oxygen requirements leads to the development of collaterals that sustain myocardial susceptibility to ischemia and infarction.11
Resistance to insulin is strongly associated with BP levels and hypertension, as evident from the various definitions of the metabolic syndrome. Studies on patients treated in intensive care units have demonstrated that external insulin administration in order to improve blood glucose regulation may contribute to endothelial and anticoagulant protection.12 Several prospective studies have concluded that hyperinsulinemia is independently associated with MI, thus supporting the hypothesis that insulin dysregulation contributes to the steps preceding this event.13
From a genetics perspective, a number of gene polymorphisms and specific genotypes have been suggested to link the development of hypertension and occurrence of ACS. Genetic variations in angiotensin II and bradykinin B2 (BK‐B2) receptors have been shown to be associated not only with the development of idiopathic hypertension but also with MI.14 A deletion polymorphism of ACE, namely the ACE/ID, has been strongly associated with the level of circulating ACE.15 The DD genotype, which is correlated with higher levels of circulating ACE, is notably more frequent in patients suffering from MI.16 With respect to ion channel polymorphisms, in a population‐based genome study on 4786 participants, sequencing of the Ca++‐dependent potassium channel alpha1 subunit (KCNMA1) revealed two genetic variants (polymorphisms C864T and IVS17) and identified four C864T/IVS17 haplotypes. Haplotype 4 was related to increased severity of systolic hypertension along with increased risk of MI (Figure 1).17
Figure 1.
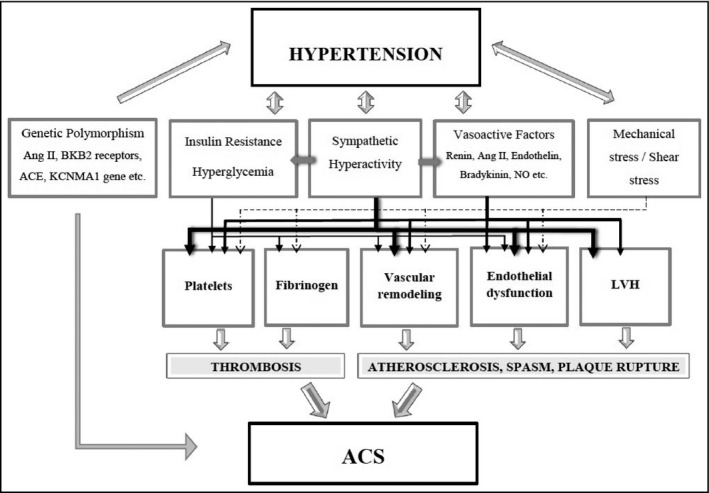
Pathophysiological factors that link hypertension with acute coronary syndromes. A complex interplay of genetic predisposition, abnormal vasoreactivity, and vessel wall shear stress coupled with neurohormonal activation triggers endothelial dysfunction, vessel wall remodeling, and development of atherosclerotic lesions. Coronary plaque rupture in the setting of a hypercoagulant state eventually leads to an ACS. ACE, angiotensin‐converting enzyme; ACS, acute coronary syndrome; Ang II, angiotensin II; BKB2, bradykinin B2; KCNMA1, Ca++‐dependent potassium channel alpha1 subunit; LVH, left ventricular hypertrophy; NO, nitric oxide
3. HYPERTENSION HISTORY IN PATIENTS WITH ACUTE CORONARY SYNDROMES
Epidemiological data with respect to hypertension history and associated prognosis in patients with ACS are rather scarce (Table 1).18 Prevalence of hypertension is reportedly 30%‐40% among patients with an ST‐elevation MI (STEMI) and rises up to 70% in patients with a non–ST‐elevation MI (NSTEMI).19, 20 Analyses from the Get With The Guidelines‐Coronary Artery Disease national registry data of the American Heart Association have shown that out of a population of 100 889 patients diagnosed with acute MI, 68% of patients in the 2002‐2003 group with NSTEMI had a history of hypertension. This percentage is reduced to 63.1% in the population studied in the 2007‐2008 period. The corresponding percentages of patients with STEMI were 62.2% and 52.1%, respectively.21 This difference can be attributed to the dissimilar characteristics among ACS patients, with NSTEMI patients being older and more susceptible to comorbidities such as diabetes mellitus and renal dysfunction.22
Table 1.
Clinical studies examining the prognostic value of a history of HTN in patients with ACS
| Study | Patients (N) | Short‐term prognosis | Long‐term prognosis |
|---|---|---|---|
| Studies supporting an unfavorable association | |||
| GISSI‐2 Investigators23 | 10 712 with MI treated with thrombolysis (3306 history of treated HTN) | Patients with HTN had a significantly higher in‐hospital mortality, left ventricular failure, and recurrent ischemic events | Patients with HTN had a significantly higher mortality, left ventricular failure, and recurrent ischemic events after 6 mo of follow‐up |
| Dumaine et al24 | 15 414 with ACS (10 998 history of HTN) | HTN was associated with the composite end point of death/MI at 30 d (OR = 1.61, 95% CI: 1.30‐1.99, P < 0.001), 30‐d mortality (OR = 1.72, 95% CI: 1.21‐2.42, P < 0.001), MI (OR = 1.61, 95% CI: 1.25‐2.06, P < 0.001), recurrent ischemia (OR = 1.26, 95% CI: 1.10‐1.44, P < 0.001), and major bleeding (OR = 1.45, 95% CI: 1.03‐2.06, P = 0.036) | HTN was associated with the composite end point of death/MI at 1 y (OR = 1.54, 95% CI: 1.31‐1.81, P < 0.001), mortality (OR = 1.70, 95% CI: 1.34‐2.16, P < 0.001), MI (OR = 1.50, 95% CI: 1.23‐1.82, P < 0.001), and recurrent ischemia (OR = 1.24, 95% CI: 1.11‐1.38, P < 0.001) |
| Lingman et al25 | 2329 with ACS (974 hypertensives and 446 diabetic) undergoing revascularization | HTN was weakly associated with impaired long‐term prognosis (HR = 1.18, 95% CI: 1.02‐1.37, P = 0.02) compared with DM, but the combination was even additive (HR = 2.10, 95% CI: 1.71‐2.57, P < 0.001) | |
| De Luca et al26 | 6298 STEMI patients (2764 with HTN) undergoing primary angioplasty | HTN was associated with impaired postprocedural TIMI 0‐2 flow (adjusted OR = 1.22, 95% CI: 1.01‐1.47, P = 0.034) | HTN was associated with higher mortality (adjusted HR = 1.24, 95% CI: 1.01‐1.54, P = 0.048) and reinfarction (adjusted HR = 1.31, 95% CI: 1.03‐1.66, P = 0.027) |
| Studies showing a favorable or non‐significant association | |||
| De Luca et al27 | 830 STEMI patients (362 with HTN) undergoing primary PCI | HTN did not affect the rate of postprocedural TIMI 3 flow and infarct size [12.5% (4.1%‐23.8%) vs 12.8% (4.3%‐24.7%), P = 0.38]. Similar results were observed in subanalyses in major high‐risk subgroups | |
| Majahalme et al22 | 979 ACS patient (630 with HTN) | No differences in rehospitalization (adjusted OR = 1.3, 95% CI: 0.9‐1.9, P = 0.12) and the composite of death, rehospitalization for cardiac reasons, MI, and stroke at 6 mo (OR = 1.2, 95% CI: 0.9‐1.7, P = 0.19) | |
| Lazzeri et al28 | 560 STEMI patients (300 with HTN) undergoing primary PCI | No difference in in‐hospital mortality rates | No differences in mortality after a median of 32.5‐mo follow‐up (log rank χ 2 = 0.38, P = 0.538) |
| Cecchi et al29 | 1031 STEMI patients (551 with HTN) and 437 non‐STEMI patients (322 with HTN) undergoing PCI | HTN was not associated with in‐hospital mortality in either group | HTN was not associated with long‐term mortality in either group after a mean of 40.2‐mo follow‐up |
| Abrignani et al30 | 1830 first MI patients (915 with HTN) from a data of 4994 MI patients | Hypertensive patients less frequently presented with cardiogenic shock (4.0% vs 11.6%, P < 0 0.01), atrioventricular block (4.9% vs 7.4%, P = 0.02), ventricular fibrillation (2.2% vs 3.7%, P = 0.04), and cardiac rupture (0.1% vs 0.9%, P = 0.02) | Mortality was higher in normotensives than in hypertensives (17.8% v 6.2%, P < 0.001), regardless of infarction site |
| Erne et al31 | 41 771 ACS patients (24 916 with HTN) | HTN associated with a more favorable in‐hospital prognosis (OR = 0.82, 95% CI: 0.73‐0.93, P = 0.022) | HTN was not an independent predictor of 1‐y mortality in a subgroup of 7801 patients followed (OR = 1.07, 95% CI: 0.78‐1.47, P = 0.68) |
Abbreviations: ACS, acute coronary syndrome; DM, diabetes mellitus; HTN, hypertension; MI, acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Prognosis of patients with ACS may be affected by hypertension history independent of treatment modality. An older retrospective analysis of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI‐2) study on 11 483 patients suffering from STEMI and treated with thrombolysis documented that hypertension history was associated with greater in‐hospital and 6‐month mortality. Additionally, left heart failure and recurrent ischemia were more frequent among hypertensives during the entire study period.23 Data from 15 414 patients included in six randomized Thrombolysis in Myocardial Infarction (TIMI) trials showed that hypertension history was associated with a 54% greater risk for the composite cardiovascular end point independently of confounders. Significant associations were also separately observed with the risk of mortality, MI, recurrent ischemia, and major bleeding.24
Presence of hypertension may be associated with softer end points such as disease severity and infarct size. Lingman et al studied the relative risk profile of hypertension and diabetes among 2329 coronary patients with unstable angina or acute MI. Patients with either preexisting hypertension or diabetes or both displayed more often multivessel disease as well as a higher age‐adjusted mortality rate over a median follow‐up period of 8 years.25 An analysis of the Drug Eluting Stents in Primary Angioplasty database on more than 6000 patients with STEMI treated with percutaneous coronary intervention (PCI) revealed that hypertension was associated with reduced TIMI flow post‐PCI and with greater mortality, reinfarction, and target vessel revascularization rates during a more than three‐year follow‐up.26 Yet, in another study, no significant association of hypertension with scintigraphic infarct size in 830 patients with STEMI subjected to primary PCI was documented.27
Few data propose that a hypertension history may exert a neutral or even a protective effect in the acute and subacute setting of an ACS. Lazzeri et al did not find a significant association of a history of hypertension with mortality among 560 STEMI patients who underwent PCI.28 Similarly, Cecchi et al studied the differential effect of hypertension on prognosis among 1031 patients with STEMI and 437 patients with NSTEMI treated with PCI. Hypertension was not associated with in‐hospital or long‐term mortality in either groups after adjustment for other risk factors.29 On the other hand, Abrigagni et al studied 4994 patients with MI and documented that hypertensive patients presented less frequently with cardiogenic shock, atrioventricular block, ventricular fibrillation, cardiac rupture, and ventricular thrombosis. In‐hospital mortality was significantly higher in normotensive than in hypertensive patients, regardless of infarct site.30 Finally, Erne et al using data of ACS patients enrolled in the Acute Myocardial Infarction in Switzerland Plus Registry from 1997 to 2013 (N = 41 771) observed that preexisting hypertension (N = 24 916) was a factor for a more favorable in‐hospital outcome after adjustment for high baseline risk parameters, but not an independent predictor of 1‐year mortality. Treatment‐wise, angiotensin‐converting enzyme inhibitors or angiotensin II receptor antagonists and statins prescribed at discharge improved the outcome.31
4. ADMISSION BLOOD PRESSURE AND PATIENT OUTCOME IN ACUTE CORONARY SYNDROMES
It is well recognized that BP levels at presentation affect outcome in patients with ACS.32 In the presence of cardiogenic shock, systolic BP is a main prognostic determinant and this is evident in the inclusion of BP criteria in widely used risk scores, with a reversed linear correlation for values under 80 mm Hg on admission.33, 34 Fewer data are available on the predictive value of diastolic BP. Data from a small retrospective study on patients with cardiogenic shock concluded that among several hemodynamic parameters, only diastolic BP, and particularly diastolic BP < 40 mm Hg during the first 24 hours in the intensive care unit, was independently associated with 28‐day mortality in cardiogenic shock patients.35
Only few studies have examined whether admission BP levels per se may influence hard outcomes as well as cardiac function in such patients (Table 2). The Acute Coronary Syndrome Israel Survey (ACSIS) studied 7645 patients diagnosed with MI and delved into the link between admission systolic BP and cardiovascular events as well as total mortality. In contrast to patients with normal admission systolic BP (defined as 110‐140 mm Hg), those with low systolic BP (<110 mm Hg) displayed significantly increased hazard ratios (HR) for 7‐day and 1‐year mortality as well as major adverse cardiovascular events (MACEs). Conversely, patients with high admission systolic BP (>140 mm Hg) presented with a lower risk for the same end points.36 Accordingly, in another study on 3943 patients with acute MI treated at a tertiary hospital, a systolic BP greater than 160 mm Hg was associated with the best outcome compared to normal admission BP defined as 121‐140 mm Hg. A 70% relative risk reduction for mortality in the highest vs the lowest BP category was documented. Regarding diastolic BP, levels <60 mm Hg were associated with a worse outcome.37
Table 2.
Clinical studies showing the prognostic value of admission blood pressure due to acute coronary syndromes
| Study | Patients (N) | Index | Type of ACS | Short‐term prognosis | Long‐term prognosis | Limitations |
|---|---|---|---|---|---|---|
| ACSIS study36 | 7645 | Admission SBP < 110 mm Hg vs admission SBP = 110‐140 mm Hg | ACS (STEMI‐NSTEMI‐UA) |
7‐d all‐cause mortality HR = 2.37, 95% CI: 1.66‐3.38, P < 0.001 MACE at 30 d OR = 1.51, 95% CI: 1.23‐1.86, P < 0.001 |
1‐y all‐cause mortality HR = 1.92, 95% CI: 1.57‐2.35, P < 0.001 |
Observational study Patients with cardiogenic shock were not excluded at entry No adjustment for medication prior to presentation |
| Roth et al37 | 3943 (1786 Hypertensives) | Admission SBP < 120 mm Hg vs admission SBP = 121‐140 mm Hg | ACS (STEMI‐NSTEMI‐UA) | – |
Overall 1‐y mortality adjusted RR = 0.65, 95% CI: 0.54‐0.8, P < 0.01 Cardiovascular mortality adjusted RR = 0.65, 95% CI: 0.47‐0.9, P < 0.01 |
Based on data from a registry Cardiovascular risk factors were analyzed as categorical variables Treatment data were limited to acute care only Patients with cardiogenic shock were not excluded at entry No adjustment for medication prior to presentation |
| Admission DBP < 60 mm Hg vs admission DBP = 61‐80 mm Hg |
Overall 1‐y mortality adjusted RR = 0.45, 95% CI: 0.36‐0.56, P < 0.01 Cardiovascular mortality adjusted RR = 0.33, 95% CI: 0.23‐0.48, P < 0.01 |
|||||
| Lee et al38 | 10 337 (prior hypertension = 6605) | Admission SBP | NSTACS |
Higher in‐hospital mortality among patients with lower SBP: adjusted OR = 1.21 per 10 mm Hg lower, 95% CI: 1.15‐1.27, P < 0.001 No differences between patients with and without hypertension |
Single SBP measurement Potential selection bias (severely sick may have not been enrolled) Potential underdiagnosis or underreporting of prior hypertension Doses of antihypertensive drugs and patient adherence before admission were not recorded |
|
| Park et al39 | 11 292 | Admission SBP = 100‐139 mm Hg vs admission SBP > 140 mm Hg | STEMI patients undergoing PCI | Normal SBP → higher in‐hospital mortality (1.5% vs 3.7%), P < 0.001. Adjusted HR = 2.268, 95% CI: 1.144‐4.498, P < 0.019 | No differences at 1‐y follow‐up |
Observational study Incidence and effect of right ventricular infarction were not evaluated Lack of information regarding antihypertensive drugs Lack of follow‐up BP levels |
| Ma et al40 | 7033 |
Admission SBP > 140 mm Hg vs SBP < 110 mm Hg |
STEMI |
30‐d all‐cause mortality HR = 0.70, 95% CI :0.55‐0.87, P = 0.003 |
Retrospective observational analysis Not all patients received reperfusion therapy Small sample size on PCI group |
|
| DBP > 90 mm Hg vs DBP < 70 mm Hg | No difference | |||||
| PP > 60 mm Hg vs PP < 40 mm Hg | HR = 0.60, 95% CI :0.47‐0.75, P < 0.001) | |||||
| MAP > 106.7 mm Hg vs MAP < 83.3 mm Hg | No difference |
Abbreviations: ACS, acute coronary syndrome; DBP, diastolic blood pressure; MACE, major adverse cardiac events; MAP, mean arterial pressure; NSTACS, non–ST‐elevation acute coronary syndromes; NSTEMI, non–ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; PP, pulse pressure; SBP, systolic blood pressure; STEMI, ST‐elevation myocardial infarction; UA, unstable angina.
The prognostic value of admission BP seems to exhibit slight differences with respect to ACS type. Using data from more than 10 000 patients with non–ST‐elevation ACS participating in large registries, Lee et al found an independent correlation between lower systolic BP and in‐hospital mortality. History of hypertension or use of antihypertensive medication did not affect these associations.38 On the other hand, in a prospective multicenter observational study on 11 292 Korean patients with STEMI, those with normal systolic BP (≥100 mm Hg and ≤139 mm Hg) had a higher risk for in‐hospital mortality compared to those with high BP (≥140 mm Hg) but not for all‐cause death or MACE, through a median of 330 days of follow‐up. In the same study, a history of hypertension was not linked to a worse outcome, presumably due to the use of cardioprotective therapy.39
Limited data exist regarding the potential predictive importance of other bp indices in patients with ACS. In a recent retrospective analysis on 7033 STEMI patients, comparison of admission systolic BP, diastolic BP, pulse pressure, and mean BP showed that only systolic BP and pulse pressure were associated with 30‐day all‐cause mortality.40
5. BLOOD PRESSURE DURING AND AFTER HOSPITALIZATION AND PATIENT OUTCOME
There are limited data pertaining to the relation between BP during hospitalization and outcomes following ACS. Wong CK et al explored the prognostic role of the last BP value prior to discharge in 1053 patients hospitalized for ACS. A reverse J‐shaped correlation was actually displayed between diastolic BP and mortality rate during a 5‐year follow‐up, even after accounting for the use of cardiac medication, in‐hospital revascularization, and risk scoring. However, no more benefit was observed when diastolic BP exceeded 90 mm Hg.41 The significance of diastolic BP is typically demonstrated in a study by Rabkin et al where it was found that among patients with coronary artery disease, a diastolic BP < 70 mm Hg was associated with more patients with coronary blood flow in the left anterior descending artery approaching zero. This finding was strongly more evident in the presence of increased left ventricular mass.42 In a study on 3311 placebo patients derived from five trials, who had an acute MI and left ventricular systolic dysfunction, Yap et al inferred that during a 2‐year follow‐up, lower systolic BP measured during hospitalization was associated with an elevated risk of all‐cause mortality and arrhythmic mortality. Similar results were obtained for low diastolic BP.43
According to recent guidelines, target BP in coronary artery disease is systolic BP < 130 mm Hg if tolerated but not <120 mm Hg, and diastolic BP < 80 mm Hg but not <70 mm Hg.44 Nevertheless, this goal is not usually achieved by the majority of patients as reported in numerous studies. In fact, in both EUROASPIRE I and II surveys an estimated 50% of patients hospitalized for coronary artery disease did not manage to reach the desired BP levels at six months postdischarge, in spite of receiving antihypertensive treatment.45, 46 In the PREVENIR study, a multicenter retrospective cohort study that assessed hypertension control rates during hospitalization for ACS and the associated prognosis, out of 1247 patients, 33% had uncontrolled hypertension at hospital discharge. Isolated systolic hypertension was associated with the composite outcome of cardiovascular death and non‐fatal infarction.47 Data from the PROVE IT‐TIMI 22 trial (Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis In Myocardial Infarction) documented the existence of a J‐ or U‐shaped association between BP levels and cardiovascular risk. In 4162 high‐risk, post‐ACS patients, a BP nadir of 136/85 mm Hg was associated with the lowest risk. The risk curve was relatively flat for systolic BP levels of 110‐130 mm Hg and diastolic BP of 70‐90 mm Hg, indicating that a BP < 110/70 mm Hg should be avoided.48 Similar data in coronary artery disease patients have been reported in some but not all studies.
With respect to antihypertensive drugs, there is a relative paucity of trial data in patients undergoing PCI and ACS, even though earlier data had shown an apparent benefit for certain drug classes. Beta‐blockers reduce oxygen demand, infarct size, and arrhythmogenesis.49 Angiotensin‐converting enzyme inhibitors limit infarct expansion, unfavorable chamber remodeling, and 30‐day mortality.49, 50 Similarly, aldosterone receptor antagonists provide a mortality benefit in post‐MI patients with a reduced ejection fraction.51 Calcium channel blockers do not seem to be of clear benefit in the early post‐MI period and may be of harm, considering their potential negative inotropy.32
6. RISK STRATIFICATION: THE ROLE OF BLOOD PRESSURE
Blood pressure–related variables have been included in many risk stratification scores in patients with ACS. A set of at least three risk factors out of hypertension, hypercholesterolemia, diabetes, smoking, and family history receives one point in the TIMI risk score, with an odds ratio of 1.70 (1.30‐2.21) in the NSTEMI multiple regression model.52 In the Global Registry of Acute Coronary Events (GRACE) score, history of hypertension has an HR of 1.2 (1.05‐1.33) in the regression model for death. In this model, emphasis is also placed on admission systolic BP and a value <100 mm Hg provides 58 points, with a total of ≥118 points indicating a post‐MI six‐month mortality >8%.53 In the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) risk model, the combination of diabetes, smoking, and history of hypertension accounted for only 2.5% of the risk of 30‐day mortality.54 Of note, hypertension is included in other scores potentially used in the management of ACS patients such as the CHA2DS2‐VASc and HAS‐BLED scores that indirectly associate hypertension with both thrombotic and hemorrhagic risks.55, 56
7. CONCLUSIONS
Even though hypertension is a principal risk factor for cardiovascular end points, evidence regarding its place in the evaluation of patients with ACS has not provided clear‐cut results. It appears that hypertension may act in a cardioprotective way in some cases, such as the acute phase of an MI when hypertensive patients appear to have better in‐hospital prognosis. It may be speculated that an admission systolic BP over 110 mm Hg and a diastolic BP not <70 mm Hg prior to discharge are associated with better long‐term prognosis of these patients. In any case, it should be pointed out that good long‐term BP control, ideally initiated prior to discharge, is needed to reduce subsequent events.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Konstantinos Konstantinou contributed toward data collection, reference search, and article writing; Tsioufis Costas conceived the idea of this review and contributed toward data collection; Areti Koumelli contributed toward critically reviewing the manuscript and proofreading; Manos Mantzouranis contributed toward critically reviewing the manuscript and proofreading; Alexandros Kasiakogias contributed toward data collection, article writing, and proofreading; Michalis Doumas contributed toward critically reviewing the manuscript and proofreading; and Dimitrios Tousoulis contributed toward critically reviewing the manuscript and proofreading.
Konstantinou K, Tsioufis C, Koumelli A, et al. Hypertension and patients with acute coronary syndrome: Putting blood pressure levels into perspective. J Clin Hypertens. 2019;21:1135–1143. 10.1111/jch.13622
REFERENCES
- 1. Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. J Hypertens. 2018;36:1953‐2041. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW. An epidemiologic perspective of systemic hypertension, ischemic heart disease, and heart failure (the Framingham heart study). Am J Cardiol. 1997;13(80):3J‐8J. [DOI] [PubMed] [Google Scholar]
- 3. da Silva AR, Fraga‐Silva RA, Stergiopulos N, Montecucco F, Mach F. Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest. 2015;45:274‐287. [DOI] [PubMed] [Google Scholar]
- 4. Kolettis TM, Barton M, Langleben D, Matsumura Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev. 2013;21:249‐256. [DOI] [PubMed] [Google Scholar]
- 5. Santulli G, Campanile A, Spinelli L, et al. G protein‐coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1125‐1130. [DOI] [PubMed] [Google Scholar]
- 6. Julius S. Sympathetic hyperactivity and coronary risk in hypertension. Hypertension. 1993;21:886‐893. [DOI] [PubMed] [Google Scholar]
- 7. Catena C, Colussi G, Brosolo G, Sechi LA. A prothrombotic state is associated with early arterial damage in hypertensive patients. J Atheroscler Thromb. 2012;19:471‐478. [DOI] [PubMed] [Google Scholar]
- 8. El Haouari M, Rosado JA. Platelet function in hypertension. Blood Cells Mol Dis. 2009;42:38‐43. [DOI] [PubMed] [Google Scholar]
- 9. Vergallo R, Papafaklis MI, Yonetsu T, et al. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency‐domain optical coherence tomography and computational fluid dynamics study. Circ Cardiovasc Imaging. 2014;7:905‐911. [DOI] [PubMed] [Google Scholar]
- 10. Cimmino G, Conte S, Morello A, et al. The complex puzzle underlying the pathophysiology of acute coronary syndromes: from molecular basis to clinical manifestations. Expert Rev Cardiovasc Ther. 2012;10:1533‐1543. [DOI] [PubMed] [Google Scholar]
- 11. Rakugi H, Yu H, Kamitani A, et al. Links between hypertension and myocardial infarction. Am Heart J. 1996;132:213‐221. [PubMed] [Google Scholar]
- 12. Langouche L, Vanhorebeek I, Vlasselaers D, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Després J‐P, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952‐957. [DOI] [PubMed] [Google Scholar]
- 14. Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin‐converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641‐644. [DOI] [PubMed] [Google Scholar]
- 15. Danser A, Schalekamp MA, Bax WA, et al. Angiotensin‐converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387‐1388. [DOI] [PubMed] [Google Scholar]
- 16. Aoki S, Mukae S, Itoh S, et al. The genetic factor in acute myocardial infarction with hypertension. Jpn Circ J. 2001;65:621‐626. [DOI] [PubMed] [Google Scholar]
- 17. Tomás M, Vázquez E, Fernández‐Fernández JM, et al. Genetic variation in the KCNMA1 potassium channel alpha subunit as risk factor for severe essential hypertension and myocardial infarction. J Hypertens. 2008;26:2147‐2153. [DOI] [PubMed] [Google Scholar]
- 18. Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183‐1192. [DOI] [PubMed] [Google Scholar]
- 19. Reinstadler SJ, Eitel C, Thieme M, et al. Comparison of characteristics of patients aged ≤45 years versus >45 years with ST‐elevation myocardial infarction (from the AIDA STEMI CMR substudy). Am J Cardiol. 2016;117:1411‐1416. [DOI] [PubMed] [Google Scholar]
- 20. Shah B, Bangalore S, Gianos E, et al. Temporal trends in clinical characteristics of patients without known cardiovascular disease with a first episode of myocardial infarction. Am Heart J. 2014;167:480‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tickoo S, Bhardwaj A, Fonarow GC, Liang LI, Bhatt DL, Cannon CP. Relation between hospital length of stay and quality of care in patients with acute coronary syndromes (from the American Heart association's get with the guidelines‐coronary artery disease data set). Am J Cardiol. 2016;117:201‐205. [DOI] [PubMed] [Google Scholar]
- 22. Majahalme SK, Smith DE, Cooper JV, et al. Comparison of patients with acute coronary syndrome with and without systemic hypertension. Am J Cardiol. 2003;92:258‐263. [DOI] [PubMed] [Google Scholar]
- 23. Fresco C, Avanzini F, Bosi S, et al. Prognostic value of a history of hypertension in 11,483 patients with acute myocardial infarction treated with thrombolysis. GISSI‐2 Investigators. J Hypertens. 1996;14:743‐750. [DOI] [PubMed] [Google Scholar]
- 24. Dumaine R, Gibson CM, Murphy SA, et al. Thrombolysis in Myocardial Infarction (TIMI) Study Group. Association of a history of systemic hypertension with mortality, thrombotic, and bleeding complications following non‐ST‐segment elevation acute coronary syndrome. J Clin Hypertens. 2006;8:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lingman M, Herlitz J, Bergfeldt L, Karlsson T, Caidahl K, Hartford M. Acute coronary syndromes–the prognostic impact of hypertension, diabetes and its combination on long‐term outcome. Int J Cardiol. 2009;137:29‐36. [DOI] [PubMed] [Google Scholar]
- 26. De Luca G, Dirksen MT, Spaulding C, et al. Impact of hypertension on clinical outcome in STEMI patients undergoing primary angioplasty with BMS or DES: insights from the DESERT cooperation. Int J Cardiol. 2014;175:50‐54. [DOI] [PubMed] [Google Scholar]
- 27. De Luca G, Parodi G, Sciagrà R, et al. Impact of hypertension on infarct size in ST elevation myocardial infarction patients undergoing primary angioplasty. J Hypertens. 2013;31(12):2433‐2437. [DOI] [PubMed] [Google Scholar]
- 28. Lazzeri C, Valente S, Chiostri M, Attanà P, Picariello C, Gensini GF. Impact of hypertension on short‐ and long‐term prognoses in patients with ST elevation myocardial infarction and without previously known diabetes. Heart Vessels. 2012;27(4):370‐376. [DOI] [PubMed] [Google Scholar]
- 29. Cecchi E, D'Alfonso MG, Chiostri M, et al. Impact of hypertension history on short and long‐term prognosis in patients with acute myocardial infarction treated with percutaneous angioplasty: comparison between STEMI and NSTEMI. High Blood Press Cardiovasc Prev. 2014;21(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 30. Abrignani M, Dominguez L, Biondo G, et al. In‐hospital complications of acute myocardial infarction in hypertensive subjects. Am J Hypertens. 2005;18:165‐170. [DOI] [PubMed] [Google Scholar]
- 31. Erne P, Radovanovic D, Schoenenberger AW, et al. Impact of hypertension on the outcome of patients admitted with acute coronary syndrome. J Hypertens. 2015;33:860‐867. [DOI] [PubMed] [Google Scholar]
- 32. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients with persistent elevations in ST segment: task force for the management of acute coronary syndromes in patients having a study ST‐exercise selection of the European Heart Association (ESC) . Eur Heart J. 2016;14:267‐315. [DOI] [PubMed] [Google Scholar]
- 33. Hasdai D, Califf RM, Thompson TD, et al. Predictors of cardiogenic shock after thrombolytic therapy for acute myocardial infarction. J Am Coll Cardiol. 2000;35:136‐143. [DOI] [PubMed] [Google Scholar]
- 34. Katz JN, Stebbins AL, Alexander JH, et al. Predictors of 30‐day mortality in patients with refractory cardiogenic shock following acute myocardial infarction despite a patent infarct artery. Am Heart J. 2009;158:680‐687. [DOI] [PubMed] [Google Scholar]
- 35. Rigamonti F, Graf G, Merlani P, et al. The short‐term prognosis of cardiogenic shock can be determined using hemodynamic variables: a retrospective cohort study. Crit Care Med. 2013;41:2484‐2491. [DOI] [PubMed] [Google Scholar]
- 36. Shlomai G, Kopel E, Goldenberg I, Grossman E. The association between elevated admission systolic blood pressure in patients with acute coronary syndrome and favorable early and late outcomes. J Am Soc Hypertens. 2015;9:97‐103. [DOI] [PubMed] [Google Scholar]
- 37. Roth D, Van Tulder R, Heidinger B, Herkner H, Schreiber W, Havel C. Admission blood pressure and 1‐year mortality in acute myocardial infarction. Int J Clin Pract. 2015;69:812‐819. [DOI] [PubMed] [Google Scholar]
- 38. Lee D, Goodman SG, Fox K, et al. Prognostic significance of presenting blood pressure in non‐ST‐segment elevation acute coronary syndrome in relation to prior history of hypertension. Am Heart J. 2013;166:716‐722. [DOI] [PubMed] [Google Scholar]
- 39. Park JS, Cha KS, Shin D, et al. Prognostic significance of presenting blood pressure in patients with ST‐elevation myocardial infarction undergoing percutaneous coronary intervention. Am J Hypertens. 2015;28:797‐805. [DOI] [PubMed] [Google Scholar]
- 40. Ma WF, Liang Y, Zhu J, et al. Comparison of 4 Admission blood pressure indexes for predicting 30‐day mortality in patients with ST‐segment elevation myocardial infarction. Am J Hypertens. 2016;29:332‐339. [DOI] [PubMed] [Google Scholar]
- 41. Wong CK, Herbison P, Tang EW. Relation between blood pressure at hospital discharge after an acute coronary syndrome and long‐term survival. Am J Cardiol. 2008;1(101):1239‐1241. [DOI] [PubMed] [Google Scholar]
- 42. Rabkin SW, Shiekh IA, Wood DA. The impact of left ventricular mass on diastolic blood pressure targets for patients with coronary artery disease. Am J Hypertens. 2016;29:1085‐1093. [DOI] [PubMed] [Google Scholar]
- 43. Yap YG, Duong T, Bland JM, et al. Prognostic value of blood pressure measured during hospitalization after acute myocardial infarction: an insight from survival trials. J Hypertens. 2007;25:307‐313. [DOI] [PubMed] [Google Scholar]
- 44. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953‐2041. [DOI] [PubMed] [Google Scholar]
- 45. EUROASPIRE . European Society of Cardiology survey of secondary prevention of coronary heart disease: principal results. EUROASPIRE Study Group. European action on secondary prevention through intervention to reduce events. Eur Heart J. 1997;18:1569‐1582. [DOI] [PubMed] [Google Scholar]
- 46. EUROASPIRE II Study Group . Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J. 2001;22:554‐572. [DOI] [PubMed] [Google Scholar]
- 47. Amar J, Chamontin B, Ferriéres J, et al. Hypertension control at hospital discharge after acute coronary event: influence on cardiovascular prognosis–the PREVENIR study. Heart. 2002;88:587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bangalore S, Qin J, Sloan S, Murphy SA, Cannon CP. What is the optimal blood pressure in patients after acute coronary syndromes?: Relationship of blood pressure and cardiovascular events in the PRavastatin OR atorVastatin Evaluation and Infection Therapy‐Thrombolysis In Myocardial Infarction (PROVE IT‐TIMI) 22 trial. Circulation. 2010;122:2142‐2151. [DOI] [PubMed] [Google Scholar]
- 49. Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372‐1407. [DOI] [PubMed] [Google Scholar]
- 50. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309‐1321. [DOI] [PubMed] [Google Scholar]
- 52. Antman EM, Cohen M, Bernink P, et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835‐842. [DOI] [PubMed] [Google Scholar]
- 53. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long‐term mortality post acute coronary syndrome. Am Heart J. 2007;153:29‐35. [DOI] [PubMed] [Google Scholar]
- 54. Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30‐day mortality in the era of reperfusion for acute myocardial infarction: results from an international trial of 41,021 patients. Circulation. 1995;91:1659‐1668. [DOI] [PubMed] [Google Scholar]
- 55. Lip G, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 56. Lip G, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173‐180. [DOI] [PubMed] [Google Scholar]


