Abstract
The efficacy and safety of blood pressure (BP) lowering initiated at baseline systolic BP (SBP) of 130‐140 mm Hg in patients with diabetes remain controversial. The authors aimed to investigate the benefits and harms of BP lowering initiated at these levels for patients with diabetes. Medline and EMBASE were searched from inception to March 10, 2018. The primary outcome was major cardiovascular events. Random‐effects model was used to pool all the estimates. Six trials with 21 574 diabetics were included. In diabetics, initiating BP lowering at baseline SBP of 130 and 140 mm Hg did not reduce the rate of major cardiovascular events (RR, 1.01 [95% CI, 0.93‐1.10]), finding that was consistent in subgroup and sensitivity analyses. Moreover, BP lowering did not reduce the risks of myocardial infarction (RR, 0.99 [95% CI, 0.85‐1.16]), stroke (0.83 [95% CI, 0.54‐1.27]), heart failure (0.91 [95% CI, 0.79‐1.04]), albuminuria (0.93 [95% CI, 0.84‐1.04]), end‐stage renal disease (0.93 [95% CI, 0.70‐1.24]), cardiovascular death (1.25 [95% CI, 0.90‐1.74]) and all‐cause death (1.05 [95% CI, 0.94‐1.17]) in patients with diabetes and baseline SBP of 130‐140 mm Hg but possibly increase the risks of serious adverse events (RR, 2.00 [95% CI, 1.33‐3.01]) and hypotension (5.30 [95% CI, 0.99‐28.40]). In diabetics, initiating BP lowering at baseline SBP of 130‐140 mm Hg may not produce any benefit but probably increase the risks of serious adverse events and hypotension. It may not be recommended to initiate BP lowering at a threshold of SBP lower than 140 mm Hg for diabetics.
Keywords: blood pressure lowering, cardiovascular outcome, diabetes, hypertension
1. INTRODUCTION
Diabetes mellitus and hypertension often coexist. Patients with hypertension at the time of diabetes mellitus diagnosis exhibited nearly twofold higher risk of all‐cause death and cardiovascular disease (CVD) compared with normotensive patients with diabetes mellitus.1 In diabetic individuals, the association of blood pressure (BP) with disease outcomes is shown to be continuous, with increasing risks of cardiovascular events occurring in parallel with increasing systolic blood pressure (SBP) levels from as low as 114 mm Hg.2, 3 However, in clinical practice, the threshold for initiation of BP‐lowering treatment for patients with diabetes is not at SBP of 114 mm Hg, whose value is changed with time and still uncertain in patients with diabetes. In patients with diabetes and baseline SBP level larger than 140 mm Hg, initiating BP lowering has been demonstrated to produce certain cardiovascular benefit,4, 5 while in patients with diabetes and baseline SBP level of 130 and 140 mm Hg, the effect of initiating BP lowering remains controversial.
Recently, the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guideline6 and the 2018 Canada's hypertension guideline7 revise the threshold for initiation of BP‐lowering treatment from baseline SBP level of 140 mm Hg to that of 130 mm Hg in patients with diabetes, while the 2018 European Society of Hypertension/European Society of Cardiology (ESC/ESH) hypertension guideline8 still retains the threshold of SBP level of 140 mm Hg. The inconsistency of recommendations was mainly due to a paucity of evidence to support having a threshold for initiation of BP lowering in patients with diabetes. Relevant large‐scale randomized controlled trials (RCTs) on this topic reported inconsistent results,9, 10 and consecutive meta‐analyses mainly focused on the target rather than the initiation of BP lowering treatment.11, 12 Only two meta‐analyses provided evidence for selecting initiation of BP‐lowering treatment in patients with diabetes, but they came to different conclusions.4, 5 Moreover, they have been criticized for the inclusion of patients with heart failure whose benefit from lipid‐lowering drugs is not due to antihypertensive effects. Therefore, we conducted a meta‐analysis by collecting the latest RCTs performed on diabetics without heart failure to investigate the efficacy and safety of BP lowering initiated at baseline SBP of 130‐140 mm Hg in patients with diabetes.
2. METHODS
We performed a meta‐analysis of RCTs in accordance with the (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) PRISMA statement.16
2.1. Data sources and searches
Relevant studies were identified from Medline and EMBASE, using the following search terms: (antihypertensive agent OR BP lowering) and (diabetes OR diabetes mellitus). Studied were searched from inception to March 10, 2018, with no language restriction. Studies were limited to RCTs performed on human adults. The additional search was performed by manually searching reference lists of trials, reviews, meta‐analyses and abstracts from major cardiovascular conferences (European Society of Cardiology Congress and American College of Cardiology Congress) held in the past 2 years.
2.2. Study selection
Relevant articles were independently searched and screened by two authors of us. Trials deemed potentially eligible by either author were processed to full‐text screening. Any discrepancy on full‐text screening was resolved by consensus and discussion with a third author. Trials were included if they met the following criteria: (a) Participants had a history of diabetes mellitus; (b) the mean baseline SBP level of participants was between 130 and 140 mm Hg; (c) trials were comparing any antihypertensive agent against placebo, any two agents against one, or any BP target against another; (d) each randomized group should report at least 1000 person‐years; (e) each group at least reported one of the interesting outcomes listed below: major CVD, all‐cause death, cardiovascular death, myocardial infarction, stroke, heart failure, incident albuminuria, and end‐stage renal disease (ESRD). Trials were excluded for following reasons: (a) Trials comparing agents against each other were not eligible because they risk assessing BP‐independent effects of agents; (b) we excluded trials performed on diabetics with heart failure because in this group of patients taking BP‐lowering drugs is in view of other therapeutic properties other than antihypertensive effect.17
2.3. Data extraction and quality assessment
Two authors independently extracted data of each trial with a predefined structured form. Disagreements on data extraction were resolved according to the judgment of a third author. We obtained following information: baseline study and patient characteristics (published year, number of participants, mean age of participants, follow‐up duration, type of diabetes mellitus, BP‐lowering strategies in intervention and control group, baseline SBP and diastolic blood pressure (DBP), and mean‐in‐treatment difference SBP/DBP); cardiovascular events rate in control group; disease history of chronic kidney disease and CVD; mean follow‐up SBP level in each group; and number of outcome events in each group.
The primary outcome was the major cardiovascular events (MACE) defined as a composite of myocardial infarction, cerebral vascular accident, heart failure, or cardiovascular death. The secondary outcomes included myocardial infarction, stroke, heart failure, cardiovascular death, all‐cause death, albuminuria (defined as new onset of micro‐albuminuria or macroalbuminuria, or a progression from micro‐albuminuria to macroalbuminuria), and ESRD (including dialysis and transplant). Adverse events included serious adverse events associated with BP lowering, hypotension, adverse events leading to discontinuation of treatment, and dizziness.
Risk of bias tool recommended by Cochrane Collaboration was used to assess the risk of bias by two authors of us, including random sequence generation, allocation concealment, blinding of participants and outcome assessment, incomplete data, selective reporting, and other sources of bias. Any disagreements on quality assessment were judged by a third author.
2.4. Data synthesis and analysis
For each study, relative risk (RR) with 95% confidence interval (CI) for each outcome was calculated before pooling. We calculate heterogeneity across studies using Cochran Q test and I 2 statistic. I 2 > 50% indicated significant heterogeneity. Random‐effects DerSimonian model was used to summary estimates of RR in order to account for heterogeneity across studies. Evidences for heterogeneity in estimates of treatment effect attributed to the baseline characteristics of the trials were explored by comparing summary results obtained from subsets of studies grouped by sample size, mean age, follow‐up duration, cardiovascular event rate in control group, history of CVD, baseline SBP, and mean SBP difference between groups during follow‐up. The subgroup difference was explored by Cochran Q test and I 2 statistic. Meta‐regression analysis was supplemented to explore the potential heterogeneity from these baseline characteristics of the trials. A two‐sided P value less than 0.05 was regarded as statistical significance. Two authors of us independently assess the publication bias by visually inspecting into the funnel plot, and potential bias was reported if asymmetry of funnel plot was identified by either author. Publication bias was further assessed by Begg and Egger tests.
Additional sensitivity analysis was conducted by excluding trials one by one, then seeing whether the omitted trial could significantly change the outcome. All analyses were performed by Stata 12.0 (StataCorpLP), and RevMan 5.3 (Nordic Cochrane Centre).
3. RESULTS
The full search process was shown in Figure 1. A total of 1536 records were retrieved, including 703 records from Medline, 791 records from EMBASE, and 42 records from manual search. The list of trials, reviews, and meta‐analyses processed to manual search was shown in Table S1. Once duplicates were removed, 889 articles were processed to title and abstract screening, and 92 articles were selected for full‐text review. Finally, six RCTs10, 18, 19 with 21 574 participants were included for statistical analysis.
Figure 1.
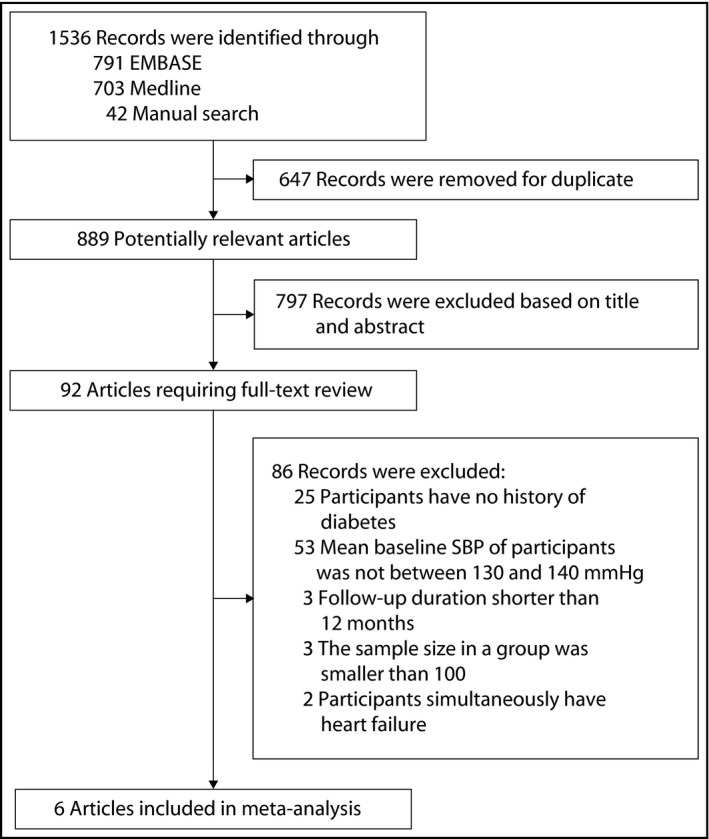
The process of study selection. The diagram summarizes search results from inception to March 10, 2018. Manual search was performed by screening the bibliographies of previously relevant trials, reviews, meta‐analyses, or abstracts from major cardiovascular conferences held in the past 2 years
The baseline characteristics of included trials were presented in Table 1. All the included trials were performed on patients with type 2 diabetes, with mean baseline SBP of participants ranged from 132.9 to 139.2 mm Hg and mean SBP difference between groups ranged from 1.3 to 14.2 mm Hg. The mean baseline DBP level of participants was between 72.7 and 84.4 mm Hg, and the mean DBP difference between groups ranged from 0.6 to 6.1 mm Hg. Of the six trials, only one trial22 recruited patients simultaneously with diabetes and nephropathy. The antihypertensive agents were different among the included trials, of which angiotensin converting enzyme inhibitor was the most common treatment. The mean sample size was 3596 (ranged from 480 to 8561), the mean age was 61 (ranged from 57 to 65), and the mean follow‐up was 3.8 years (ranged from 2.2 to 5.3 years). All the included trials had a low risk of bias (Table S2). The description of MACE for each trial was shown in Table S3.
Table 1.
Baseline characteristics of included trials
| Study ID | Published year | No. of participants | Mean age | Follow‐up (years) | Type of patients | Intervention group/control group | Baseline SBP/DBP (mm Hg ) | Mean‐in‐treatment difference SBP/DBP (mm Hg ) |
|---|---|---|---|---|---|---|---|---|
| ABCD‐N18 | 2002 | 480 | 59 | 5.3 | Type 2 diabetes mellitus | Intensive/moderate therapya | 136.4/84.4 | 9.0/6.0 |
| ACCORD BP10 | 2010 | 4733 | 62 | 4.7 | Type 2 diabetes mellitus | Intensive therapy/standard therapyb | 139.2/76.0 | 14.2/6.1 |
| ROADMAP19 | 2011 | 4447 | 58 | 3.2 | Type 2 diabetes mellitus | Olmesartan/placebo | 136.5/80.5 | 3.1/1.9 |
| DIRECT‐P220 | 2011 | 1905 | 57 | 4.7 | Type 2 diabetes mellitus | Candesartan/placebo | 132.9/78.0 | 3.3/1.3 |
| ALTITUDE21 | 2012 | 8561 | 65 | 2.7 | Type 2 diabetes mellitus | Aliskiren/placebo | 137.3/74.2 | 1.3/0.6 |
| VA NEPHRON‐D22 | 2013 | 1448 | 65 | 2.2 | Diabetic nephropathy | Losartan plus Lisinopril/Losartan | 137.0/72.7 | 1.5/1.0 |
ABCD‐N, appropriate blood pressure control in diabetes‐normotensive; ACCORD BP, the action to control cardiovascular risk in diabetes blood pressure trial; ALTITUDE, the aliskiren trial in type 2 diabetes using cardiorenal end‐points; CHF, chronic heart failure; CR/XL, controlled release/extended release; DBP, diastolic blood pressure; DIRECT‐P2, the Diabetic REtinopathy Candesartan Trials‐Protect 2 study; ROADMAP, the Randomized Olmesartan and Diabetes Microalbuminuria Prevention study; SBP, systolic blood pressure; VA NEPHRON‐D, The Veterans Affairs Nephropathy in Diabetes study.
Intensive therapy is enalapril or nisoldipine; moderate therapy is placebo, with enalapril or nisoldipine prescribed when needed.
For intensive group participants, medication dose titration or addition of another drug was indicated whenever SBP was ≥120 mm Hg; for standard BP group participants, medication dose titration or addition of another drug was indicated if SBP was >160 mm Hg at a single visit or >140 mm Hg at two successive visits.
Data about the effect of BP‐lowering treatment on MACE were available from six trials10, 18, 19 including 21 574 patients and 2312 cardiovascular events (Figure 2). Overall, BP‐lowering was not associated with reduced risk of MACE (RR, 1.01 [95% CI, 0.93‐1.10]), with no evidence of significant heterogeneity of effect size across the included trials (I 2 = 6%, P = 0.38).
Figure 2.
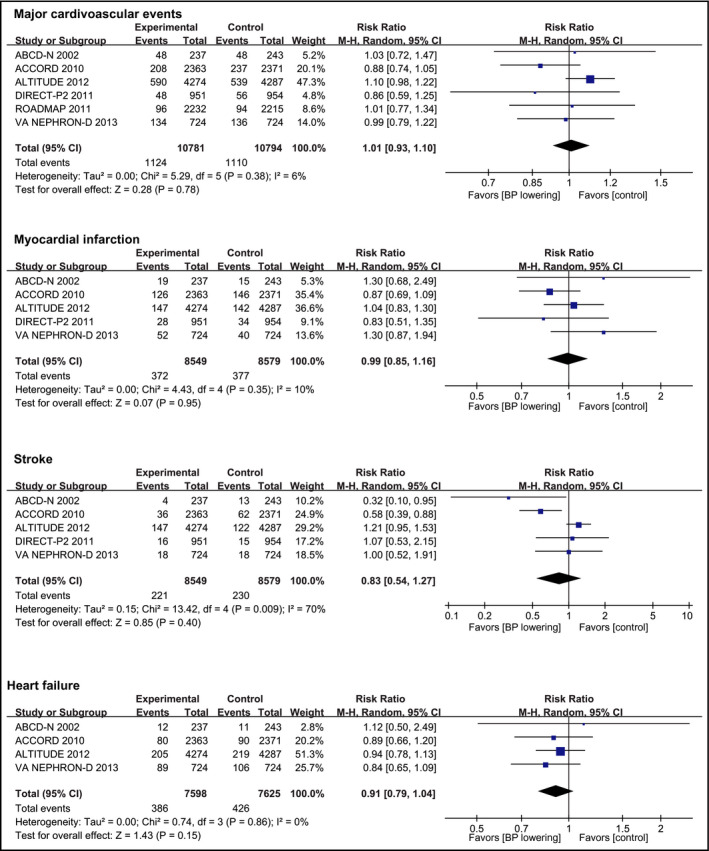
Effect of blood pressure lowering on risks of cardiovascular events. Forest plots of blood pressure‐lowering treatment vs control on major cardiovascular events, myocardial infarction, stroke, and heart failure. BP, blood pressure; CI, confidence interval; M‐H, Mantel‐Haenszel. A P value <0.05 represents a significant pooled point estimate of relative risk (RR). Boxes and horizontal lines represent RR and 95% CI for each trial. The size of each box is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of effect and are centered on pooled RR
Myocardial infarction was reported in five trials10, 18, 20, 21 including 17 128 participants, in whom 749 events were recorded. Compared with control group, BP‐lowering treatment did not reduce the risk of myocardial infarction (RR, 0.99 [95% CI, 0.85‐1.16]; Figure 2). Five trials10, 18, 20, 21 (17 128 participants) reported 451 stroke events. No significant reduction in stroke occurred with BP‐lowering treatment (RR, 0.83 [95% CI, 0.54‐1.27]; Figure 2). Heart failure was reported by four trials10, 18, 21, 22 with 15 223 participants and 812 events, and BP lowering was not associated with reduced risk of heart failure (RR, 0.91 [95% CI, 0.79‐1.04]; Figure 2). Two trials10, 18 reported data about progression of albuminuria (4859 participants with 2000 events) and shown that BP‐lowering did not reduce the risk of albuminuria progression (RR, 0.93 [95% CI, 0.84‐1.04]; Figure 3). Three trials10, 21, 22 including 14 742 participants recorded 421 ESRD events. Compared with control group, the BP‐lowering group did not significantly reduce the risk of ESRD (RR, 0.93 [95% CI, 0.70‐1.24]; Figure 3). Four trials10, 18, 19, 21 reported data about cardiovascular death (18 222 participants and 619 events) and showed that BP lowering did not reduce the risk of cardiovascular death compared with control group (RR, 1.25 [95% CI, 0.90‐1.74]; Figure 3). Six trials10, 18, 19 (21 574 participants) reported 1273 occurrences of all‐cause death with no significant reduction in this outcome in patients allocated to BP‐lowering group compared with control group (RR, 1.05 [95% CI, 0.94‐1.17]; Figure 3), with no significant heterogeneity in the magnitude of the effect. Although no publication bias was identified by Egger and Begg tests (Table S4), visual inspection suggested potential bias existed in analyzing the outcomes of stroke and cardiovascular death (Figure S1).
Figure 3.
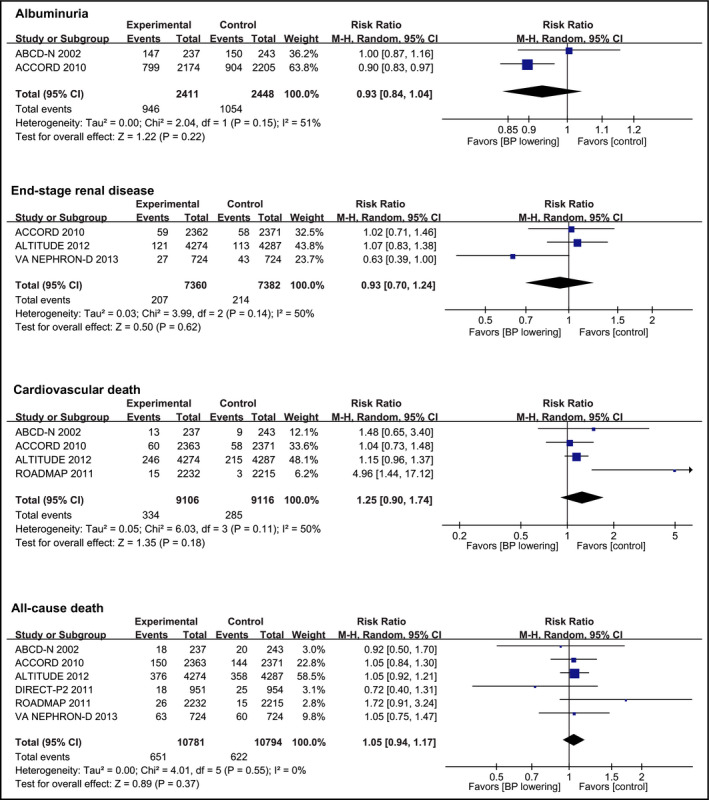
Effect of blood pressure lowering on the risks of renal events and death. Forest plots of blood pressure‐lowering treatment vs control on albuminuria, end‐stage renal disease, cardiovascular death, and all‐cause death. BP, blood pressure; CI, confidence interval; M‐H, Mantel‐Haenszel. A P value <0.05 represents a significant pooled point estimate of relative risk (RR). Boxes and horizontal lines represent RR and 95% CI for each trial. The size of each box is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of effect and are centered on pooled RR
There was no evidence that the observed effect of BP‐lowering treatment on the outcome of MACE was different across subgroups stratified according to baseline characteristics of included trials and participants (Table 2). In meta‐regression analyses, none of these baseline characteristics can explain the heterogeneity between trials in the analysis of MACE (Figure S2). In sensitivity analyses, no study exclusion significantly changed the result of MACE (Figure S3). Moreover, the exclusion of ALTITUDE trial did not significantly change the results of primary and secondary outcomes (Table S5).
Table 2.
Subgroup analyses stratified by baseline characteristics of included trials and participants
| Subgroup | Trials (n) | Relative risk (95% CI) | P a | I 2 statistic (%) | |
|---|---|---|---|---|---|
| Sample size (n) | <2000 | 3 | 0.97 (0.82, 1.14) | 0.50 | 0.0 |
| ≥2000 | 3 | 1.04 (0.93, 1.15) | |||
| Mean age (years) | <65 | 4 | 0.95 (0.84, 1.08) | 0.14 | 53.9 |
| ≥65 | 2 | 1.07 (0.97, 1.18) | |||
| Duration (years) | <4 | 3 | 1.07 (0.97, 1.17) | 0.13 | 55.3 |
| ≥4 | 3 | 0.94 (0.82, 1.08) | |||
| CV rate in control group | <15% | 5 | 1.03 (0.95, 1.12) | 0.69 | 0.0 |
| ≥15% | 1 | 0.99 (0.79, 1.22) | |||
| Baseline SBP (mm Hg ) | <135 | 1 | 0.86 (0.56, 1.25) | 0.34 | 0.0 |
| ≥135 | 5 | 1.03 (0.96, 1.12) | |||
| SBP difference (mm Hg) | <5 | 4 | 1.05 (0.96, 1.15) | 0.26 | 22.6 |
| ≥5 | 2 | 0.95 (0.82, 1.11) | |||
CI, confidence interval; CV, cardiovascular; SBP, systolic blood pressure.
P value for subgroup difference assessed by Cochran Q test.
The data for adverse events were limitedly reported among the included trials. Three trials10, 19, 22 reported data for severe adverse events associated with BP‐lowering treatment (10 628 participants and 566 events), showing BP‐lowering treatment significantly increased the risk of severe adverse events (RR, 2.00 [95% CI, 1.33‐3.01]; Figure 4). Three trials10, 19, 21 reported severe hypotension outcomes (17 741 participants, with 594 events in the BP lowering vs 364 in the control group), with BP lowering associated with borderline increase in hypotension ((RR, 5.30 [95% CI, 0.99‐28.40]; Figure 4; P = 0.05). BP lowering was to a trend to increase the risk of dizziness (three trials10, 19, 21 with 13 976 participants and 1110 events; RR, 1.18 [95% CI, 0.95‐1.46]; Figure 4). Finally, no trial reported the rate of drug discontinuation.
Figure 4.
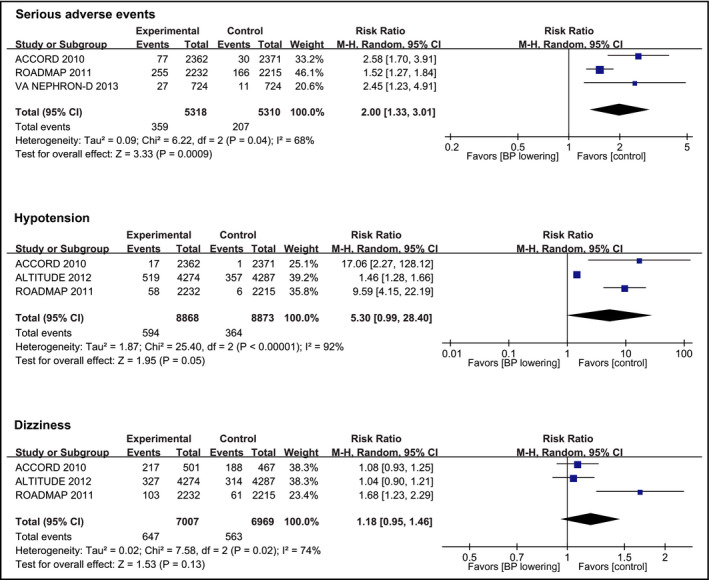
Effect of blood pressure lowering on the risks of adverse events. Forest plots of blood pressure‐lowering treatment vs control on serious adverse events associated with BP lowering, hypotension, and dizziness. BP, blood pressure; CI, confidence interval; M‐H, Mantel‐Haenszel. A P value <0.05 represents a significant pooled point estimate of relative risk (RR). Boxes and horizontal lines represent RR and 95% CI for each trial. The size of each box is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of effect and are centered on pooled RR
4. DISCUSSION
Unlike previous meta‐analyses, our meta‐analysis focused on the initiation rather than the target of BP‐lowering treatment for patients with diabetes and found that BP lowering initiated at baseline SBP of 130‐140 mm Hg was not associated with any reduction of cardiovascular or renal events (including MACE, myocardial infarction, stroke, heart failure, cardiovascular death, all‐cause death, albuminuria, and ESRD). On the contrary, initiating BP lowering at baseline SBP of 130‐140 mm Hg probably increased the risks of several adverse events and hypotension for patients with diabetes.
The previous meta‐analysis by Emdin et al5 summarized trials performed on type 2 diabetes and found that BP lowering was probably associated with reduced risks of albuminuria and stroke when initiating BP lowering at baseline SBP of less than 140 mm Hg. Recently, the post hoc analysis of SPRINT trial9 focused on the subgroup of patients with prediabetes and mean baseline SBP of 139 mm Hg also identified significantly cardiovascular benefit from BP‐lowering treatment. Based on these, the 2017 ACC/AHA hypertension guideline recommended a threshold of 130 mm Hg to initiate BP‐lowering treatment for patients with diabetes.6 However, the cardiovascular benefit of BP lowering observed in Emdin et al study5 having been questioned due to the usage of standardized analysis method. As the Brunström et al study23 found, standardized analysis method results in increased heterogeneity in meta‐analyses and disrupts the weight assigned to each trial. These strongly suggest a biased effect estimate in the standardized model. Moreover, the post hoc analysis of SPRINT trial9 focused on patients with prediabetes rather than diabetes. Possible interactions between diabetes and antihypertensive treatment effect go through the many complications related to high blood glucose (advanced glycation end products, vascular remodeling, arterial stiffness, decreased kidney function, etc). Such complications depend to a large extent on glycemic control and disease duration. Prediabetics, with not‐so‐deranged blood glucose for not‐so‐long time, actually do not experience possible deleterious effects of BP‐lowering treatment in diabetes. Additionally, in the clinical scenario, the cardiovascular preventive strategies in patients with advanced diabetes are very different from patients with prediabetes. Thus, the evidence obtained from prediabetics in SPRINT trial directly applied to diabetics may be inappropriate. In view of these, the evidence supporting a threshold of SBP of 130 mm Hg for initiation of BP lowering in patients with diabetes may be insufficient. In comparison, the current study used non‐standardized data, with specific focus on patients with diabetes without enrollment of prediabetics. We found that in patients with diabetes, BP lowering initiated at baseline SBP of 130‐140 mm Hg was not associated with cardiovascular or renal benefits. These findings were consistent with that of previous target‐oriented meta‐analyses that showed that BP lowering in patients with diabetes shown little or no further benefit by lowering SBP below 130 mm Hg.
Inconsistent with our results, the previous meta‐analysis by Brunström et al4 found that BP lowering increased the risk of cardiovascular death in patients with diabetes if baseline SBP was less than 140 mm Hg while our study did not find an increase in cardiovascular mortality. Although we both used the same non‐standardized model to pool all estimates, there are some problems in inclusion/exclusion criteria of Brunström et al study may explain the diverse results between us. To investigate the BP‐lowering effect at very low level, Brunström et al study not only included the trials with mean baseline SBP of 130‐140 mm Hg but also included those with mean baseline SBP lower than 130 mm Hg. Thus, more participants of their study may have a normotension or even have a SBP lower than 114 mm Hg. At these levels, BP lowering may do more harm than good. Moreover, their study included trials with person‐year less than 1000, which however might lead to potential bias from small study. Most importantly, Brunström et al4 meta‐analysis included diabetics with heart failure. This is a major source of bias, because the mechanism for treatment effect of renin‐angiotensin‐aldosterone system (RAAS) and beta‐blockers in heart failure is not the same as in hypertension. Patients with heart failure commonly have low BP due to systolic dysfunction of the left ventricle. RAAS inhibitors and beta‐blockers reduce the strain on the left ventricle through neurohormonal mechanisms, vasodilation, negative chronotropy, etc This may in fact induce a paradoxical increase in BP over time when/if the left ventricle recovers. These facts suggest that trials assessing the effect of RAAS inhibitors or beta‐blockers on heart failure cannot inform us about the effect of BP lowering. Such trials may give us false information about the effect of BP lowering in diabetics.
A major concern regarding the robustness of our findings is the inclusion of ALTITUDE trial.21 This was a trial designed to investigate the effect of aliskiren, a renin inhibitor, in diabetics who were already on RAAS inhibitors treatment.21 It is no longer recommended double‐RAAS blocker treatment as standard treatment in any patients. We therefore performed a sensitivity analysis by excluding ALTITUDE trial from the analyses of primary and secondary outcomes and tested its impact. However, this sensitivity analysis did not change the point estimate and its confidence intervals, indicating that the treatment effect is consistent across trials.
We additionally analyzed the clinical outcomes of serious adverse events that had not been analyzed by previous meta‐analyses4, 5 in order to fully reflect the efficacy and safety of BP lowering. However, we found that significant increase in the risk of the serious adverse events associated with BP lowering did occur in patients received BP‐lowering treatment. Moreover, BP lowering was also associated with a borderline increase in hypotension. Collectively, in diabetics with baseline SBP of 130‐140 mm Hg BP lowering may not bring any benefit but on the contrary may increase the risks of some adverse events. By weighing the pros and cons, it may not be recommended to initiate BP lowering in patients with diabetes when baseline SBP was between 130 and 140 mm Hg. These findings tend to support the recommendations of 2018 ESC/ESH hypertension guideline8 that recommend a threshold of SBP of 140 mm Hg for initiation of BP lowering in patients with diabetes.
4.1. Limitations of this review
One limitation of the current meta‐analysis is that it was based on trial level rather individual level, thus was unable to perform detail subgroup analyses stratified according to the baseline characteristics of duration of treatment, drug type, age of the patients, co‐morbidities, etc, to assess the reliability of BP‐lowering effect. Moreover, the strategies studied in the current analysis were mainly BP‐lowering agents; it is unknown whether others antihypertensive strategies such as weight loss and lifestyle modification also achieve similar cardiovascular benefit as BP‐lowering agents in patients with diabetes and baseline SBP level between 130 and 140 mm Hg. In addition, few trials have achieved a large reduction in BP between randomized groups; the results of some outcomes may be affected by this. Finally, since the individual patient data of each trial are unavailable for us, we cannot guarantee that the baseline SBP of each individual enrolled was between 130 and 140 mm Hg; thus, the findings in our study only represent the group of participants with mean SBP level between 130 and 140 mm Hg, but not the individuals with SBP level between 130 and 140 mm Hg.
5. CONCLUSIONS
This meta‐analysis found that BP‐lowering treatment was not associated with any cardiovascular or renal benefit but possibly associated with increased risks of several adverse events and hypotension in patients with diabetes and baseline SBP level between 130 and 140 mm Hg. It may not be recommended to initiate BP‐lowering treatment for patients with diabetes when baseline SBP level is 140 mm Hg or lower.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
SF.W and JP.B conceived and designed this meta‐analysis. SF.W, NM.D, and YX.L carried out the literature search and quality assessment. SF.W, NM.D, and HRL extracted the data. SF.W, NM.D, and JP.B performed the statistical analyses and data interpretations. SF.W and JP.B drafted the manuscript. WJ.L and YL.L provided supervision. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was supported by grants to Jianping Bin from the National Natural Science Foundation of China (No. 81771857, No. 81571698, and No. 81271640).
Wang S, Djama NM, Lai Y, et al. Cardiovascular outcomes in patients with diabetes when initiating blood pressure lowering at baseline SBP between 130 and 140 mm Hg: A meta‐analysis. J Clin Hypertens. 2019;21:220–229. 10.1111/jch.13471
Wang and Djama contribute equally to this work
REFERENCES
- 1. Chen G, McAlister FA, Walker RL, et al. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asia Pacific Cohort Studies Collaboration , Kengne AP, Patel A, et al. Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia‐Pacific region. J Hypertens. 2007;25:1205‐1213. [DOI] [PubMed] [Google Scholar]
- 4. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta‐analyses. BMJ. 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2015;313:603‐615. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13‐e115. [DOI] [PubMed] [Google Scholar]
- 7. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506‐525. [DOI] [PubMed] [Google Scholar]
- 8. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 9. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017;40:1401‐1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ACCORD Study Group , Cushman WC, Evans GW, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence in hypertension: 10 ‐ Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta‐analyses of randomized trials. J Hypertens. 2017;35:922‐944. [DOI] [PubMed] [Google Scholar]
- 12. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta‐analysis. JAMA Cardiol. 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta‐analysis. Lancet. 2016;387:435‐443. [DOI] [PubMed] [Google Scholar]
- 14. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013:CD008277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random‐effects meta‐analyses of randomized trials. Circulation. 2011;123:2799‐2810. [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. J Hypertens. 2014;32:2285‐2295. [DOI] [PubMed] [Google Scholar]
- 18. Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086‐1097. [DOI] [PubMed] [Google Scholar]
- 19. Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907‐917. [DOI] [PubMed] [Google Scholar]
- 20. Tillin T, Orchard T, Malm A, et al. The role of antihypertensive therapy in reducing vascular complications of type 2 diabetes. Findings from the DIabetic REtinopathy Candesartan Trials‐Protect 2 study. J Hypertens. 2011;29:1457‐1462. [DOI] [PubMed] [Google Scholar]
- 21. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204‐2213. [DOI] [PubMed] [Google Scholar]
- 22. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892‐1903. [DOI] [PubMed] [Google Scholar]
- 23. Brunstrom M, Carlberg B. Standardization according to blood pressure lowering in meta‐analyses of antihypertensive trials: comparison of three methodological approaches. J Hypertens. 2018;36:4 ‐ 15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


