Abstract
To prevent and treat hypertension, it is important to restrict salt in one's diet since adolescence. However, an effective salt‐reduction education system has yet to be established. Besides accurate evaluation, we believe that the frequent usage of a measurement device may motivate individuals to avoid high salt intake. The present study evaluated the use of a urinary salt excretion measurement device for salt‐reduction education in a parallel randomized trial of two groups. The sample comprised 100 university students who provided consent to participate. A survey with 24‐hour home urine collection and blood pressure measurement was conducted. Participants in the self‐monitoring group measured their own urinary salt excretion level for 4 weeks, using the self‐measurement device. Analyses were conducted on 51 participants in the control group and 49 in the self‐monitoring group. At baseline, there was no significant difference between the two groups in terms of their characteristics and 24‐hour urinary salt excretion levels. After intervention, 24‐hour urinary sodium/potassium ratio showed no change in the control group [baseline score: 4.1 ± 1.5; endline score: 4.2 ± 2.0; P = 0.723], but it decreased significantly in the self‐monitoring group [baseline score: 4.0 ± 1.7; endline score: 3.5 ± 1.4; P = 0.044]. This change was significant even after adjusting for baseline and endline differences between groups using analysis of covariance (P = 0.045). The self‐monitoring urinary salt excretion measurement device improved the 24‐hour urinary sodium/potassium ratio. The device is a useful and practical tool for educating young individuals about dietary salt reduction.
Keywords: eating behavior, salt‐intake, self‐monitoring, urinary salt excretion, young individuals
1. INTRODUCTION
Excess salt intake is a major problem that leads to the development and progression of hypertension, and the development of cardiovascular diseases.1, 2, 3, 4 Consuming too much salt in the early stages of life results in higher systolic and diastolic blood pressure, which is reported to lead to hypertension or pre‐hypertension.5, 6 According to a national study on daily salt intake among 1043 Japanese women aged 18‐22 years, the mean values of salt intake based on 24‐hour urine collection and a dietary questionnaire were 9.8 g and 9.2 g, respectively,7, 8 which substantially exceeded the Japanese target salt‐intake value of 7.0 g.9 We also reported that the mean estimated daily salt‐intake value for female university students (mean age of 17.9 years) who entered university from 1995 to 2015 was 8.1 g, and that only 31.3% of the students10 consumed more salt than the Japanese target value of 7.0 g.9 A study estimating the decrease in the mortality rate by reducing salt intake showed that the earlier the age at which salt restriction was started, the more effective it was in decreasing the mortality rate.11 These reports suggest that promoting the commencement of salt reduction at a younger age in Japan, where excessive salt is consumed, is an important factor in motivating lifestyle changes to prevent and treat hypertension. However, an effective salt‐reduction education system has yet to be established. The 24‐hour urine collection approach is the gold standard for measuring salt intake. However, this is difficult for young individuals to conduct it themselves, and it is not a practical method for supporting salt‐reduction education.
We have previously reported the usefulness of managing salt intake by using a urinary salt excretion measurement device (KME‐03, Kohno ME institute)12 to estimate 24‐hour urinary salt excretion based on overnight (ON) salt excretion. This easy‐to‐use device allows individuals to repeatedly measure their own urinary salt excretion level at home. The results obtained from this device are reliable, and according to our previous careful salt in‐out measurements, they correlate well with measurements based on 24‐hour urine collection.13, 14, 15 Moreover, this device is useful for supporting salt‐reduction education and it can reduce overnight salt excretion in middle‐ to old‐aged individuals for a short period.16, 17 However, it is unclear whether this device can be used as a salt‐reduction education tool for young individuals.
The present study aimed to evaluate the hypothesis that repetitive usage of a self‐monitoring urinary salt excretion measurement device is useful in conducting salt‐reduction education for female college students, because such interventions would help establish effective salt‐intake management from an early age.
2. MATERIALS AND METHODS
2.1. Participants
The target population comprised 144 female college students who provided informed consent after verbal and written explanation was conducted for 151 females who gathered at a poster proposal on the present study at the following universities during the indicated period: Nakamura Gakuen University and Fukuoka Woman's University (from March to April, 2017) and Nishikyushu University (from August to September, 2017). The target age was limited to 18‐25 years. Additionally, potential participants who fulfilled the following criteria were explained at the study proposal and explanatory meetings that they were not eligible for the study, and they were excluded from the sample: having a physical, mental, or social difficulty or disadvantage in everyday life; taking anti‐hypertensive drugs; being pregnant; and being edematous or hypertensive, or having renal disease.
2.2. Intervention schedule
Immediately after their enrollment in the study, we asked participants to fill out a check sheet to estimate their everyday average salt‐intake level18 and a questionnaire17 on eating behaviors based on the transtheoretical model.19 Additionally, to minimize the effect of participants' menstrual cycle on fluctuations in body sodium levels, we asked them to hand in a sample from a 24‐hour home urine collection immediately after menstruation, during the follicular phase. We asked them to consume meals that they usually do, and to measure their blood pressure before and after urine collection, using a sphygmomanometer that we lent them. The resulting baseline body mass index (BMI), blood pressure, and 24‐hour urinary salt excretion levels were used as criteria for stratified randomization, and participants were assigned to self‐monitoring and control groups, in a 1:1 proportion. We instructed participants in the self‐monitoring group to use the self‐monitoring urinary salt excretion measurement device for 3‐4 weeks from the end of menstruation to the next menstruation. The control group was asked to behave as they usually did. After 4 weeks, on the day immediately after menstruation, we asked all participants to conduct 24‐hour urine collection at home, check their blood pressure, and fill out the salt check sheet and eating behavior questionnaire, for use as endline data. No nutritional education was conducted in either group. Results of the 24‐hour urinary salt excretion measurement were shared with participants after their participation ended.
2.3. Outcomes
The primary outcomes of this study were (a) the difference in 24‐hour urinary salt excretion level and sodium/potassium ratio after intervention, and (b) the difference between the intervention and control groups. Since salt intake differs greatly between individuals,20 it is recommended that 24‐hour urine collection be performed on multiple days.21 However, the compliance rate typically decreases after multiple iterations,13 resulting in a low number of samples available for analysis. Thus, in the present study, we instructed participants to collect 24‐hour urine samples only at the baseline and endline measurements.
The secondary outcomes were changes in the salt check sheet score and dietary behavior stage after 4 weeks of intervention.
2.4. Twenty‐four‐hour urine collection
A partition cup (proportional sampling method) was used for the 24‐hour home urine collection, and 1/50th of the total urine volume was collected.22 Reports comparing the partition cup and total urine method show a high correlation index of urine volume (r = 0.97) and urinary salt excretion level (r = 0.98).22 To collect urine accurately using the partition cup, participants were provided verbal and written instructions, and they participated in a simulation before the actual collection. Additionally, they were specifically reminded to collect urine during defecation.
Participants were instructed to empty their bladders at the first urination in the morning, specifically at 6:00 AM, which is the recommended standard urine collection time. Thereafter, they were asked to collect all the urine excreted throughout the day, using the partition cup. Finally, they were asked to complete another scheduled urination at 6:00 AM the next day, regardless of the desire to urinate. The total volume collected up to that time was designated as the 24‐hour home urine collection.13, 15, 17, 23, 24 Urine samples of all participants were collected and analyzed at baseline and endline. SRL Co. Ltd. analyzed the total urine volume, as well as urine creatinine, sodium, and potassium levels. Specifically, the creatinine level was analyzed by enzyme assay, and the sodium and potassium levels were analyzed by the electrode method. Urine samples with 24‐hour creatinine levels within 30% of the estimated levels were included in further analyses. Those outside this range were eliminated.13, 15, 17, 23, 24
2.5. Self‐monitoring device
A self‐monitoring urinary salt excretion measurement device (KME‐03; Kohno ME Laboratory) was used in this study. This device can estimate the 24‐hour salt excretion level from 8‐hour ON urine samples collected in a special container. Therefore, participants were asked to urinate 8 hours before their pre‐arranged waking time (6:00 AM). Urine excreted during the sleeping period was collected in a dedicated cup. If an unscheduled urination was needed between 10:00 PM and 6:00 AM, the urine was added to morning's first urine and analyzed.
The principle of the measurement device is as follows. The volume sensor consists of 50 small resistant chips and a conductivity sensor comprising two gold‐coated nickel plates. These sensors measure urine volume, chloride concentration, and temperature. These values are then integrated, and the sodium chloride (NaCl) concentration is estimated using an algorithm. In the present study, because conductivity‐based concentration measurement is affected by other electrolytes such as potassium, the NaCl concentration was adjusted using a correlation formula, such that the value ranged between the values obtained using ion electrode and conductivity methods. The self‐monitoring device estimated 24‐hour salt excretion from ON urine samples, using the following formula: Y = 5.76 × (X) × 0.53 (g/day), where Y is the estimated 24‐hour urinary salt excretion level and X is the sodium content of the ON urine sample.12 This formula was derived from a regression analysis on 24‐hour salt excretion level and the value estimated using the self‐monitoring device to analyze ON urine samples. Moreover, others have reported that the 24‐hour salt excretion level correlates well with the ON urinary salt excretion level (r = 0.72, P < 0.001; n = 224).12 We measured urinary salt excretion among 33 men and women (aged 39.6 ± 16.7 years) using the 24‐hour urine collection method and the ON salt excretion level measurement using the salt‐monitoring device on the same day, for 3 days. The present device showed underestimated levels at high values and overestimated levels at low values on the 24‐hour urinary salt excretion level measurement. On the other hand, the mean ± SD (g/day) of measurement errors of ON salt excretion level minus 24‐hour urinary salt excretion level for each day (Day 1:0 ± 1.9, Day 2: −0.4 ± 2.5, Day 3:0 ± 3.1) showed no significant difference between the two methods, which confirmed that the ON salt excretion level measurement using the present device was quite accurate.13 Additionally, in and out studies of salt using salt‐managed meals showed that the ON salt excretion level measured using the present device correlated with salt intake, showing that it is a reliable tool for assessing changes in individuals' salt intake.14, 15
2.6. Salt check sheet
The salt check sheet is a single A4 sheet with 13 questions. It takes 3‐5 minutes to complete. The 13 questions are categorized as follows: seven items evaluate the intake of salty meals such as miso soup, pickles, and noodles; four evaluate the use of salty sauces (eg, soy sauce), eating out, and home‐meal replacement; and two evaluate the seasoning content and size of homemade meals. Each question is scored on a 3‐point scale, with a total of 35 points. The resulting scores on salt consumption level are categorized as low (0‐8 points), medium (9‐13 points), high (14‐19 points), or very high (>20 points).18 The validity of the values obtained from the salt check sheet was confirmed in studies involving patients with hypertension and those involving local middle‐aged participants.18, 24
2.7. Questionnaire on salt‐reducing eating behavior
Generally, desirable health behavior is attained in the following five stages, as evaluated by the transtheoretical model: (1) pre‐contemplation stage, in which a patient has no intention of changing in the foreseeable future; (2) contemplation stage, in which a patient intends to change, but not soon; (3) preparation stage, in which a patient intends to change during the next month; (4) action stage, in which a patient changes; and (5) maintenance stage, in which a patient maintains the change for at least 6 months.19 We referred to a previous report in which the transtheoretical model showed that spot urine measurement is comparable with 24‐hour urinary salt excretion level measurement.25 The same study categorized the eating behavior of each participant into the above five stages using a questionnaire.17
2.8. Blood pressure measurement
Participants measured their blood pressure after resting for 5 minutes, using a digital upper‐arm automatic sphygmomanometer (HEM‐7080 IC; Omron). They did so at the beginning and end of the 24‐hour urine collection period immediately after early morning urination. The mean of four consecutive measurements was recorded.13, 17
2.9. Anthropometric measurements
Fasting measurements of height and body weight were conducted after urine sampling on the last day of 24‐hour urine collection. Participants were dressed lightly, and they did not wear shoes or socks. Their body weight was self‐measured at home, using the same scale for measuring their weight at the baseline and endline. Their height was self‐declared, and body weight measurements were recorded to the nearest 0.1 kg. BMI was calculated using these height and body weight values.17
2.10. Statistical analysis
Statistical data were presented as means ± standard deviations. The chi‐square test was used to analyze qualitative variables, the paired t‐test was used to compare two paired groups, and the unpaired t‐test was used to compare two unpaired groups. Concerning urinary salt excretion level, BMI, blood pressure, and salt check sheet score, the difference between baseline and endline values was corrected as covariates using analysis of covariance (ANCOVA). Interactions between covariates and independent variables (parallel regression), and linear regressions between covariates and dependent variables (significance of regression coefficients) were checked prior to the analysis. Moreover, ordinal variables from the Questionnaire on Salt Reduction Eating Behaviour were evaluated by defining the following stages: pre‐contemplation stage = 1, contemplation stage = 2, preparation stage = 3, action stage = 4, and maintenance stage = 5, with equal intervals. Analyses were conducted to identify changes in these stages. For all analyses, the significance level was set at P < 0.05. The SPSS v22 statistical software was used to perform the analyses.
2.11. Ethical standards disclosure
The study protocol was approved by the ethics committee of Nakamura Gakuen University (Rinri‐16‐012), and the study was registered in the UMIN Clinical Trials Registry (UMIN000026775). It adhered to the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from participants, of their own free will. They confirmed that (a) they suffered no disadvantages from refusing to participate in the study, (b) they were participating freely, (c) there were no disadvantages from withdrawing consent after agreeing to participate, (d) they consented to public dissemination of the results at scientific meetings and in published manuscripts, and (e) their personal information would be protected.
3. RESULTS
After registering 144 participants as the target population, 24‐hour home urine collection was conducted for the baseline investigation. As 20 participants were excluded for failing to complete the 24‐hour urine collection, the remaining 124 participants were equally assigned to the control and self‐monitoring groups (62 participants each) by stratified randomization. Four weeks later, 24‐hour urine collection was conducted for the endline investigation, and data from 100 participants (control group: 49 participants; self‐monitoring group: 51 participants) were used in further analyses, due to the following losses: 21 participants failed to collect urine (control group: 11 participants; self‐monitoring group: 10 participants), two participants from the control group withdrew consent, and one participant from the self‐monitoring group exhibited especially bad compliance in measurement using the self‐monitoring urinary salt excretion level measurement device (Figure 1). Both groups showed no significant difference in age, height, body weight, BMI, or blood pressure (Table 1). No participants were current or past smokers. There was no difference between the two groups' anthropometric measurements, blood pressure, or 24‐hour salt excretion level at baseline. The difference in 24‐hour urinary sodium (salt) excretion level before and after intervention was 61.8 mg (0.2 g) for the control group and −315 mg (−0.8 g) for the self‐monitoring group, but this difference was not statistically significant (P = 0.074). The sodium/potassium ratio showed no difference in the control group (baseline score: 4.1 ± 1.5; endline score: 4.2 ± 2.0; P = 0.723) but it improved significantly in the self‐monitoring group (baseline score: 4.0 ± 1.7; endline score: 3.5 ± 1.4; P = 0.044). This change was significant even after adjusting for the differences between the two groups in their baseline and endline scores using an ANCOVA (P = 0.045). It should be noted that there was no difference in the level of 24‐hour potassium excretion between the two groups (Table 2). However, the self‐monitored ON salt excretion level of the self‐monitoring group decreased significantly [first day of measurement: 9.7 ± 2.4; last day of measurement: 8.8 ± 2.3, P = 0.013].
Figure 1.
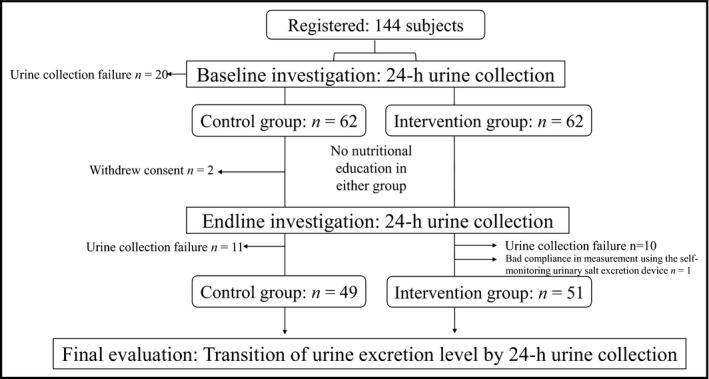
Process of assignment of participants to study groups. In total, 144 participants were registered for the present study. Of them, 62 each were assigned to the control and self‐monitoring groups using stratified randomization. Finally, 100 participants (control group: 49 participants; self‐monitoring group: 51 participants) were included in the data analysis
Table 1.
Characteristics of study participants
| All subjects | Control group | Self‐monitoring group | P value | |
|---|---|---|---|---|
| Number | 100 | 49 | 51 | |
| Age (y) | 20.8 ± 0.9 | 20.7 ± 0.9 | 20.9 ± 0.8 | 0.274 |
| Height (cm) | 158.6 ± 5.0 | 158.9 ± 4.6 | 158.2 ± 5.5 | 0.528 |
| Body weight (kg) | 51.4 ± 5.8 | 51.6 ± 6.0 | 51.2 ± 5.6 | 0.738 |
| BMI (kg/m2) | 20.4 ± 2.0 | 20.7 ± 0.9 | 20.9 ± 0.8 | 0.951 |
Data expressed as frequency (%) or mean (SD). Abbreviation: BMI: body mass index.
Table 2.
Change in 24‐h urine collection and blood pressure after 4 wk of intervention
| Baseline | Endline | Change from baseline | Adjusted difference (intervention vs control)a | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | P value | Mean | 95% CI | P value | |
| Sodium excretion (mg/d) | ||||||||
| Control group (n = 49) | 3258 ± 920 | 3319 ± 1352 | 61.8 | −333, 456 | 0.755 | 425 | −43, 893 | 0.074 |
| Self‐monitoring group (n = 51) | 3189 ± 1012 | 2874 ± 1046 | −315 | −692, 62 | 0.100 | |||
| P‐value | 0.725 | 0.068 | ||||||
| Salt excretion (g/d) | ||||||||
| Control group (n = 49) | 8.3 ± 2.4 | 8.5 ± 3.5 | 0.2 | −0.9, 1.2 | 0.755 | 1.1 | −0.1, 2.3 | 0.074 |
| Self‐monitoring group (n = 51) | 8.2 ± 2.6 | 7.4 ± 2.7 | −0.8 | −1.8, 0.2 | 0.100 | |||
| P value | 0.725 | 0.068 | ||||||
| Potassium excretion (mg/d) | ||||||||
| Control group (n = 49) | 1464 ± 451 | 1443 ± 466 | −21 | −182, 140 | 0.798 | −77 | −264, 315 | 0.413 |
| Self‐monitoring group (n = 51) | 1468 ± 510 | 1522 ± 531 | 54 | −102, 211 | 0.491 | |||
| P value | 0.966 | 0.432 | ||||||
| Sodium/potassium ratio | ||||||||
| Control group (n = 49) | 4.1±1.5 | 4.2 ± 2.0 | 0.1 | −0.5, 0.7 | 0.723 | 0.7 | 0.0, −1.3 | 0.045 |
| Self‐monitoring group (n = 51) | 4.0 ± 1.7 | 3.5 ± 1.4 | −0.5 | −1.1, 0.0 | 0.044 | |||
| P value | 0.870 | 0.049 | ||||||
| Body weight (kg) | ||||||||
| Control group (n = 49) | 51.6 ± 6.0 | 51.3 ± 5.8 | −0.2 | −0.5, 0.0 | 0.055 | −0.1 | −0.5, 0.3 | 0.602 |
| Self‐monitoring group (n = 51) | 51.1 ± 5.6 | 51.0 ± 5.7 | −0.1 | −0.4, 0.1 | 0.310 | |||
| P value | 0.737 | 0.804 | ||||||
| SBP (mm Hg) | ||||||||
| Control group (n = 49) | 101.9 ± 7.3 | 99.3 ± 6.9 | −2.7 | −4.0, −1.3 | 0.000 | −2.1 | −4.1, −0.1 | 0.035 |
| Self‐monitoring group (n = 51) | 100.9 ± 7.7 | 100.6 ± 8.1 | −0.3 | −1.9, 1,4 | 0.739 | |||
| P value | 0.477 | 0.378 | ||||||
| DBP (mm Hg) | ||||||||
| Control group (n = 49) | 63.9±6.6 | 61.5 ± 6.7 | −2.5 | −4.0, −0.9 | 0.002 | −1.3 | −3.2, 0.7 | 0.194 |
| Self‐monitoring group (n = 51) | 63.2 ± 6.5 | 62.2 ± 7.0 | −1.0 | −2.4, 0.4 | 0.146 | |||
| P value | 0.566 | 0.618 | ||||||
Data expressed as mean (SD). P values are for tests comparing mean differences between baseline and endline changes in the variables between the two groups. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Correction by baseline values using analysis of covariance.
There was no difference in systolic or diastolic blood pressure at baseline and endline in both groups, but the changes in systolic and diastolic blood pressure from baseline to endline were significant in the control group. When these were adjusted by an ANCOVA, the change in systolic blood pressure was still significant, but that in diastolic blood pressure was not significant in the control group (Table 2). It should be noted that there was no difference in weight during the intervention period, but a decrease was observed in the control group.
Pertaining to changes in the salt check sheet before and after intervention, both groups showed no differences in scores on each section and the overall score. Further, there was no difference in the total score on frequency of consuming high‐salt meals (seven items) or using high‐salt seasoning and eating out (four items) before and after the 4‐week period in the control group, but these scores decreased significantly in the self‐monitoring group. Additionally, the total score on all 13 items decreased significantly. However, when the difference in baseline and endline scores of both groups was adjusted by an ANCOVA, the result became non‐significant (Table 3).
Table 3.
Change in salt check‐sheet points and dietary behavior stage after 4 wk of intervention
| Baseline | Endline | Change from baseline | Adjusted difference (intervention vs control)b | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | P value | Mean | 95% CI | P value | |
| Salt check‐sheet | ||||||||
| Frequency of intake of high‐salt diet (7 items: 0–17 points) | ||||||||
| Control group (n = 49) | 4.7 ± 2.3 | 4.4±2.0 | −0.3 | −0.8, 0.2 | 0.241 | 0.1 | −0.5, 0.7 | 0.704 |
| Self‐monitoring group (n = 51) | 5.0 ± 2.0 | 4.5 ± 2.0 | −0.5 | −0.9, −0.1 | 0.028 | |||
| P value | 0.605 | 0.918 | ||||||
| Additional seasoning, frequency of eating out, and home‐meal replacement (4 items: 0–12 points) | ||||||||
| Control group (n = 49) | 4.1 ± 1.7 | 3.9 ± 1.8 | −0.2 | −0.6, −0.2 | 0.429 | 0.2 | −0.4, 0.7 | 0.558 |
| Self‐monitoring group (n = 51) | 4.4 ± 1.5 | 3.9 ± 1.5 | −0.5 | −0.9, 0.0 | 0.043 | |||
| P value | 0.328 | 0.943 | ||||||
| Taste of your homemade dishes, amount of food (2 items: 0–6 points) | ||||||||
| Control group (n=49) | 2.4 ± 1.1 | 2.3 ± 1.3 | −0.1 | −0.5, 0.2 | 0.383 | 0.0 | −0.5, 0.6 | 0.879 |
| Self‐monitoring group (n=51) | 2.2 ± 1.4 | 2.1 ± 1.5 | −0.1 | −0.6, 0.4 | 0.743 | |||
| P value | 0.437 | 0.649 | ||||||
| Total (0‐35 points) | ||||||||
| Control group (n = 49) | 11.2 ± 3.1 | 10.6 ± 3.0 | −0.6 | −1.3, 0.2 | 0.121 | 0.3 | −0.7, 1.3 | 0.567 |
| Self‐monitoring group (n = 51) | 11.5 ± 3.0 | 10.5 ± 3.5 | −1.0 | −1.8, 0.2 | 0.015 | |||
| P value | 0.573 | 0.923 | ||||||
| Baseline | Endline | Change from baselinea | Adjusted difference (intervention vs. control)c | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency (%) | Frequency (%) | Mean | 95% CI | P value | Mean (stage) | 95% CI | P value | |
| Dietary behaviour stage | ||||||||
| Control group (n = 49) | ||||||||
| Precontemplation stage | 11 (22.4) | 12 (24.5) | 0.873 | |||||
| Contemplation stage | 16 (32.7) | 19 (38.8) | ||||||
| Preparation stage | 7 (14.3) | 4 (8.2) | ||||||
| Action stage | 10 (20.4) | 10 (20.4) | ||||||
| Maintenance stage | 5 (10.2) | 4 (8.2) | ||||||
| Self‐monitoring group (n = 51) | −0.7 | −1.1, −0.2 | 0.004 | |||||
| Precontemplation stage | 14 (27.5) | 9 (17.6) | 0.032 | |||||
| Contemplation stage | 15 (29.4) | 11 (21.6) | ||||||
| Preparation stage | 11 (21.6) | 6 (11.8) | ||||||
| Action stage | 5 (9.8) | 18 (35.3) | ||||||
| Maintenance stage | 6 (11.8) | 7 (13.7) | ||||||
| P value | 0.551 | 0.192 | ||||||
P values are for tests comparing mean differences between baseline and endline changes in the variables between the two groups.
P‐value compares the ratio difference between the baseline and endline changes in the variables between the two groups by the chi‐square test.
Correction by baseline values using analysis of covariance.
Ordinal variables were converted to continuous variables; correction by baseline values using analysis of covariance.
The distribution of eating behavior stage during the intervention showed no difference at baseline in both groups. However, at the endline, no difference was found in the control group, but a significant increase was found in the self‐monitoring group (Table 3). When adjusted by an ANCOVA, the self‐monitoring group had a 0.7 stage improvement as compared to the control group (P = 0.004).
4. DISCUSSION
Japan is among the countries with the highest salt intake in the world,1, 26 and since this dietary behavior is formed since adolescence,7, 8, 10 it is necessary to establish salt management and a practical salt‐reduction education tool for young individuals. The present study was the first to report that frequent self‐monitoring using a self‐monitoring salt excretion level measurement device can improve 24‐hour urinary sodium/potassium ratio. Thus, it is a practical salt‐reduction educational tool for young individuals. In other words, in the self‐monitoring group using the self‐monitoring urinary salt excretion level measurement device, there was no difference in the 24‐hour potassium excretion level but there was a tendency to exhibit reduced 24‐hour urinary salt excretion, resulting in a significant reduction of the sodium/potassium ratio. Furthermore, the self‐monitoring group showed significant reduction in ON salt excretion. The absolute value may have a certain error range,13, 14, 15 but since the correlation of ON salt with daily salt intake has been confirmed by in and out tests,14, 15 we believe that the significant change in individual salt intake observed in this study is very important. This result shows the same tendency as that observed in the parallel randomized trial we conducted with a target population of men and women of a mean age of 58.1 years, using almost the same protocol as the present study.17
For the young individuals in the present study, as well as for middle‐ to old‐aged individuals, recognizing one's own urinary salt excretion level was probably an important subjective factor in beginning salt‐reduction behavior. We came to this conclusion due to the result that eating behavior stage improved only in the self‐monitoring group. This implied that self‐monitoring one's own ON salt excretion level with a self‐monitoring salt excretion level measurement device leads to behavioral change (Table 3). There was also a significant decrease in systolic blood pressure only in the control group, while no change was observed in urinary salt excretion level (Table 2). While the reason for this is unclear, we believe that some other factors besides nutritional intake, such as body weight, age, nutritional elements, a particular food item, physical activity, or other lifestyle factors were involved.27, 28, 29
The relationship between blood pressure and salt intake is complicated. Some reports showed no relationship, and that blood pressure is influenced by BMI and age rather than salt intake.27, 28, 29 Even in the DASH‐Sodium trial, where participants' salt intake or body weight were kept constant during the intervention period, it was observed that complex factors, such as measurement variables or environmental or behavioral stress, influenced participants' blood pressure.30 Our previous study also reported that, in a salt in‐out test of young individuals, some participants showed higher blood pressure with decreased salt intake and lower blood pressure with increased salt intake, and that the morning blood pressure did not always correlate with salt intake.15 In the present study, the target population was young women, and their blood pressure and BMI were low at baseline. This made it difficult to interpret the change in blood pressure in the two groups. Therefore, when managing salt intake in young individuals, it is important to estimate their salt intake objectively, irrespective of blood pressure. Evidently, it is necessary to monitor salt‐intake level regularly. To this end, the self‐monitoring salt excretion level measurement device is easy to use, and it facilitates repeated usage. Thus, it is an essential tool to facilitate salt‐reduction education and salt‐intake management in young individuals.
The importance of the need of an easily operated, cost‐beneficial salt‐monitoring device can also be found in other recent studies. For instance, a study on non‐overweight normotensive individuals (n = 889, mean age: 57.3 years) showed that the sodium excretion level estimated by spot urine tests correlated significantly with future systolic blood pressure (mean follow‐up period: 5.8 years).31 Similarly, a prospective cohort study of adults who were free of clinical cardiovascular disease (n = 6814, mean age: 62.0 years) showed that the sodium/potassium ratio estimated by spot urine tests predicted future incidents of stroke.32 Thus, a daily self‐monitoring urinary salt excretion level measurement device is the key to protecting us from diseases related to excess salt intake.
Moreover, the radical increase in smartphone users worldwide has led to the development of information and communication technology based health care for diseases and nutritional education with emerging outcomes. Mobile applications for weight loss, like those involving self‐monitoring of weight, diet, and activity,33, 34 help choosing lower‐salt foods,35 and they provide real‐time feedback of energy and nutrient intake.36 These applications are likely to evolve and expand in the near future, with systems linking to devices with biological information such as that obtained from the salt‐monitoring device tested in the present study. These methods will contribute to lowering the risks and costs related to cerebrovascular and renal diseases.37, 38, 39
The limitations of the present study are that the decrease in the salt excretion level and salt check sheet score was not significant, and a related blood pressure change was not observed due to the short study period and the recruitment of a small target population with normal mean blood pressure. Therefore, a longer study period and the examination of outcomes with a group of young individuals with high blood pressure are necessary.
However, despite these limitations, the present finding that the use of a self‐monitoring salt excretion level measurement device can improve salt management among young individuals is important in that the sodium/potassium ratio can be improved by the mere use of the device.
5. CONCLUSIONS
Frequent self‐monitoring of ON salt excretion using the self‐monitoring urinary salt excretion level measurement device tested in the present study contributed to improving participants' 24‐hour urinary sodium/potassium ratio and eating behavior stage. Thus, it is a useful and practical tool to educate young individuals about reducing their salt intake.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
We thank Ms Tomomi Kajiyama, Ms Haruka Suzuki, Ms Chiharu Tajiri, Ms Haruka Masuda, Ms Ikumi Koga, Kyoko Shisa, and Ms Yoriko Iwasaka for their excellent technical assistance. We also thank Editage (www.editage.jp) for the English language editing.
Yasutake K, Umeki Y, Horita N, et al. A self‐monitoring urinary salt excretion level measurement device for educating young women about salt reduction: A parallel randomized trial involving two groups. J Clin Hypertens. 2019;21:730–738. 10.1111/jch.13545
REFERENCES
- 1. Intersalt Cooperative Research Group . Intersalt. An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. Am J Hypertens. 2002;15:222‐229. [DOI] [PubMed] [Google Scholar]
- 3. Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. New Eng J Med. 2014;371:601‐611. [DOI] [PubMed] [Google Scholar]
- 4. Umesawa M, Iso H, Date C, et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan collaborative cohort study for evaluation of cancer risks. Am J Clin Nutr. 2008;88:195‐202. [DOI] [PubMed] [Google Scholar]
- 5. Yang Q, Zhang Z, Kuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatr. 2012;130:611‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta‐analysis of controlled trials. Hypertension. 2006;48(5):861‐869. [DOI] [PubMed] [Google Scholar]
- 7. Murakami K, Sasaki S, Uenishi K. Japan dietetic students' study for nutrition and biomarkers group. The degree of misreporting of the energy‐adjusted intake of protein, potassium, and sodium does not differ among under‐, acceptable, and over‐reporters of energy intake. Nutr Res. 2012;32:741‐750. [DOI] [PubMed] [Google Scholar]
- 8. Murakami K, Livingstone MB, Sasaki S, Uenishi K. Japan Dietetic Students' Study for Nutrition and Biomarkers Group . Ability of self‐reported estimates of dietary sodium, potassium and protein to detect an association with general and abdominal obesity: Comparison with the estimates derived from 24 h urinary excretion. Br J of Nutr. 2015;113:1308–1318. [DOI] [PubMed] [Google Scholar]
- 9. Ministry of Health, Labor and Welfare of Japan . Dietary Reference Intakes for Japanese, 2015 (in Japanese). Tokyo, Japan: Dai‐ichi Shuppan Co; 2014. 247 p.
- 10. Yasutake K, Moriguchi R, Kajiyama T, et al. Interannual study of spot urine‐evaluated sodium excretion in young Japanese women. J Clin Hypertens (Greenwich). 2017;19:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bibbins‐Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. New Eng J Med. 2010;362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamasue K, Tochikubo O, Kono E, Maeda H. Self‐monitoring of home blood pressure with estimation of daily salt intake using a new electrical device. J Hum Hypertens. 2006;20:593–598. [DOI] [PubMed] [Google Scholar]
- 13. Yasutake K, Horita N, Umeki Y, et al. Self‐management of salt intake: clinical significance of urinary salt excretion estimated using a self‐monitoring device. Hypertens Res. 2015;39:127–132. [DOI] [PubMed] [Google Scholar]
- 14. Yasutake K, Sawano K, Shono N, Tsuchihashi T. Validation of a self‐monitoring device for estimating 24‐hour urinary salt excretion. Asia Pac J Clin Nutr. 2013;22:25–31. [DOI] [PubMed] [Google Scholar]
- 15. Yasutake K, Horita N, Murata Y, Koyama S, Enjoji M, Tsuchihashi T. Estimated urinary salt excretion by a self‐monitoring device is applicable to education of salt restriction. Hypertens Res. 2015;38:143–148. [DOI] [PubMed] [Google Scholar]
- 16. Yasutake K, Sawano K, Yamaguchi S, Sakai H, Amadera H, Tsuchihashi T. Self‐monitoring urinary salt excretion in adults: a novel education program for restricting dietary salt intake. Exp Ther Med. 2011;2:615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasutake K, Miyoshi E, Misumi Y, et al. Self‐monitoring of urinary salt excretion as a method of salt‐reduction education: a parallel, randomized trial involving two groups. Pub Health Nutr. 2018;21:2164–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuchihashi T, Masuda K, Oniki H, et al. Assessment of salt intake by using a simple check sheet in hypertensive patients. J Blood Pressure (in Japanese). 2013;20:1239–1243. [Google Scholar]
- 19. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. [DOI] [PubMed] [Google Scholar]
- 20. Fukumoto A, Asakura K, Murakami K, et al. Within‐ and between‐individual variation in energy and nutrient intake in Japanese adults: effect of age and sex differences on group size and number of records required for adequate dietary assessment. J Epidemiol Assoc. 2013;23:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwahori T, Miura K, Ueshima H. Time to consider use of the sodium‐to‐potassium ratio for practical sodium reduction and potassium increase. Nutrients. 2017;9:E700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tochikubo O, Uneda S, Kaneko Y. Simple portable device for sampling a whole day's urine and its application to hypertensive outpatients. Hypertension. 1983;5:270–274. [DOI] [PubMed] [Google Scholar]
- 23. Ohta Y, Tsuchihashi T, Fujii K, et al. Improvement of blood pressure control in a hypertension clinic: a 10‐year follow‐up study. J Hum Hypertens. 2004;18:273–278. [DOI] [PubMed] [Google Scholar]
- 24. Yasutake K, Miyoshi E, Kajiyama T, et al. Comparison of a salt check sheet with 24‐h urinary salt excretion measurement in local residents. Hypertens Res. 2016;39:879–885. [DOI] [PubMed] [Google Scholar]
- 25. Tamaki J, Kikuchi Y, Yoshita K, et al. Stages of change for salt intake and urinary salt excretion: baseline results from the high‐risk and population strategy for occupational health promotion (HIPOP‐OHP) study. Hypertens Res. 2004;27:157–166. [DOI] [PubMed] [Google Scholar]
- 26. Anderson C, Appel LJ, Okuda N, et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 2010;110:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uhernik AI, Erceg M, Milanovic SM. Association of BMI and nutritional habits with hypertension in the adult population of Croatia. Public Health Nutr. 2008;12:97–104. [DOI] [PubMed] [Google Scholar]
- 28. Lelong H, Galan P, Kesse‐Guyot E, Fezeu L, Hercberg S, Blacher J. Relationship between nutrition and blood pressure: a cross‐sectional analysis from the NutriNet‐Santé Study, a French web‐based cohort study. Am J Hypertens. 2015;28:362–371. [DOI] [PubMed] [Google Scholar]
- 29. Vernay M, Aıdara M, Salanave B, et al. Diet and blood pressure in 18–74‐year‐old adults: The French nutrition and health survey (ENNS 2006–2007). J Hypertens. 2012;30:1920–1927. [DOI] [PubMed] [Google Scholar]
- 30. Obarzanek E, Proschan MA, Vollmer WM, et al. Individual blood pressure responses to changes in salt intake: results from the DASH‐sodium trial. Hypertens. 2003;42:459–467. [DOI] [PubMed] [Google Scholar]
- 31. Umesawa M, Yamagishi K, Noda H, et al. The relationship between sodium concentrations in spot urine and blood pressure increases: a prospective study of Japanese general population: the circulatory risk in communities study (CIRCS). BMC Cardiovasc Disord. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Averill MM, Young RL, Wood AC, et al. Spot urine sodium‐to‐potassium ratio is a predictor of stroke. Stroke. 2019;50(2):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity (Silver Spring). 2017;25:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eyles H, McLean R, Neal B, Doughty RN, Jiang Y, Ni MC. Using mobile technology to support lower‐salt food choices for people with cardiovascular disease: protocol for the SaltSwitch randomized controlled trial. BMC Public Health. 2014;14:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee JE, Song S, Ahn JS, Kim Y, Lee JE. Use of a mobile application for self‐monitoring dietary intake: feasibility test and an intervention study. Nutrients. 2017;9(7):E748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He FJ, Brinsden HC, MacGregor GA. Salt reduction in the United Kingdom: a successful experiment in public health. J Hum Hypertens. 2014;28:345–352. [DOI] [PubMed] [Google Scholar]
- 38. Martikainen JA, Soini EJ, Laaksonen DE, Niskanen L. Health economic consequences of reducing salt intake and replacing saturated fat with polyunsaturated fat in the adult Finnish population: estimates based on the FINRISK and FINDIET studies. Eur J Clin Nutr. 2011;65:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schorling E, Niebuhr D, Kroke A. Cost‐effectiveness of salt reduction to prevent hypertension and CVD: a systematic review. Public Health Nutr. 2017;20:1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


