Abstract
Recent studies have revealed 2 peaks in the onset of cardiovascular events, 1 in the morning and another in the evening. We evaluated whether blood pressure (BP) also rises in the morning/evening and identified the determinants of evening BP rise using 24‐hour ambulatory BP monitoring for 7 consecutive days. We identified 2 BP peaks, 1 in the morning (0‐3 hours after waking) and 1 in the evening (9‐12 hours after waking). Subjects were subclassified according to the extent of evening BP rise: those in the top quartile (≥6.45 mm Hg, n = 34; ER group) vs all others. After adjustment for age, sex, and 24‐hour systolic BP, evening BP rise was associated with the use of antihypertensive medications [odds ratio (OR), 3.57; 95% confidence interval (CI), 1.46‐8.74; P = .01] and estimated glomerular filtration rate (OR, 0.96; 95% CI, 0.93‐0.99; P = .04), confirming its association with antihypertensive medication use and renal dysfunction.
Keywords: blood pressure, circadian rhythm, evening rise
1. INTRODUCTION
Previous studies have reported a high incidence of acute cardiovascular events in the morning.1, 2, 3, 4, 5, 6 The reasons for the high incidence of acute cardiovascular events during the morning have been widely discussed.7, 8, 9, 10 An abrupt and dramatic surge in blood pressure (BP) on waking has been suggested as an important trigger of cardiovascular events.7, 8 We previously reported that the occurrence of acute myocardial infarction showed 2 peaks, 1 in the morning and another in the evening.11 Some epidemiological studies also have revealed an increase in acute cardiovascular in the evening,2, 5, 6, 11, 12, 13, 14 but little attention has been paid to BP variation in the evening.
Twenty‐four‐hour ambulatory BP monitoring (ABPM) is useful in evaluating circadian BP variations,15 but some limitations have been demonstrated.16, 17, 18 Previous studies indicated that ABPM for just 24 hours might be insufficient for a proper diagnosis of hypertension.17 The pressor effect due to wearing an ABPM device for the first time increases BP for the first 6 to 8 hours of monitoring. This “ABPM effect” may mask the second BP rise in the evening. We hypothesized that 24‐ hour ABPM over several days could enable the detection of detailed circadian BP variations using a time averaging method.
In addition, the brachial‐ankle pulse wave velocity (baPWV) measurements were also performed. The baPWV is a marker related to the severity of atherosclerosis.19
In this study, we examined whether BP might also increase in the evening and whether the evening rise in BP might be associated with risk factors for cardiovascular disease using 24‐hour ABPM for 7 consecutive days.
2. METHODS
2.1. Subjects
A total of 140 subjects were initially recruited for this study. All subjects were residents of a rural Japanese town who had participated in free health screening, counseling, and educational services offered by the town office. We obtained their medical history, including use of medications, and the latest laboratory data. Subjects with definite neurological diseases, such as Parkinson disease and stroke, and those who were too severely ill to stand without help, were excluded from the study.
Subjects were also asked about their lifestyle, such as smoking status, habitual alcohol drinking, and daily siesta habits. Smokers were defined as active smokers at the time of the study. Habitual drinkers were defined as moderate or heavy drinkers. This study was approved by the Medical Ethics Committee of the Tokyo Women's Medical University as Clinical Study #2912, titled ‘‘Health assessment of community‐dwelling elderly in Japan”. All subjects provided written informed consent.
2.2. Twenty‐four‐hour ABPM for seven consecutive days
Noninvasive ABPM was performed using an oscillometric monitor (TM‐2431, A & D Co.) to record systolic BP (SBP), diastolic BP (DBP), and heart rate. The recorder was programmed to take readings at 30‐minute intervals between 07:00 and 22:00 and at 60‐min intervals between 22:00 and 07:00 for 7 consecutive days. At the town office, all subjects were fitted with a recorder and asked to revisit the office 7 days later. Subjects were taught how to attach and remove the recorder and were instructed to remove the recorder while taking a bath. Each time a reading was taken; subjects were instructed to remain motionless and to then record their activity in a diary. Stored data were retrieved on a personal computer using commercially available software for the oscillometric monitor (TM‐2430‐15, A & D Co.). Data collected from 24‐hour ABPM over 7 consecutive days were divided into 7 intervals. The first interval was from the time of recorder fitting until the time of waking up the next morning. The second to the seventh intervals were from the time of getting out of bed until the time of waking up the next morning on the consecutive days. To exclude the novelty effect of ABPM, BP values for the first 2 hours after recorder fitting were not included in the analysis. We calculated the average BP per unit of the time for 7 days by the time averaging method (Figure 1). “Awake BP” was defined as the mean of BP results obtained while the subject was awake. “Asleep BP” was defined as the mean of BP results obtained while the subject was asleep. If daily BP readings showed an error of >25%, that day was excluded from analysis. Editing criteria of the recordings were SBP levels between 70 and 250 mm Hg, DBP levels between 30 and 130 mm Hg, and pulse pressure between 20 and 160 mm Hg.20
Figure 1.
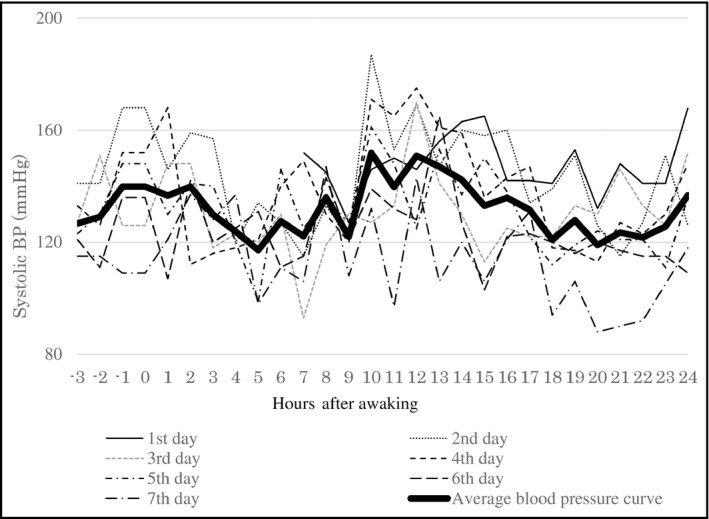
A case of circadian blood pressure variation profile by analysis of consecutive seven‐day (24‐h) ambulatory blood pressure monitoring
2.3. Pulse wave velocity
The brachial‐ankle Pulse wave velocity (baPWV) was measured using a volume‐plethysmographic apparatus (Form PWV/ABI; Nippon Colin Co., Ltd.). This device can record the baPWV, BP, electrocardiograms, and heart sounds simultaneously. The methodology for baPWV measurement has been previously described.19 In brief, subjects were examined while resting in the supine position. Cuffs were wrapped around both upper arms and ankles. The baPWV values were calculated by measuring the time for the pulse wave to travel between the brachial and posterior tibial arteries.
2.4. Laboratory measurements
The serum levels of total cholesterol, high‐density lipoprotein cholesterol, triglycerides, creatinine, and fasting plasma glucose were measured enzymatically using blood samples obtained from the subjects. The eGFR was calculated using the modification of diet in renal disease equation21:
2.5. Statistical analysis
Data were expressed as the mean ± SD or percentage. Between‐group differences for continuous variables were assessed using unpaired t tests. Categorical data were tested using the chi‐squared test. Two‐way analysis of variance was used to test for differences in the results for each period, and Tukey's test was performed for multiple comparisons of the means for each period. Stepwise multivariate logistic regression analyses were performed to calculate odds ratio with 95% CI.
Differences with a P‐value of <.05 were considered to be statistically significant. All statistical analyses were performed using commercially available statistical software (Ekuseru‐Toukei 2015; Social Survey Research Information Co. Ltd.).
3. RESULTS
Seven subjects were excluded due to incomplete ABPM data. One hundred and thirty‐three subjects were eligible for this study. The mean age of subjects was 57 ± 10 years, and the proportion of men was 45.4%. The average body mass index was 24.5 ± 2.6 kg/m2. Of the subjects, 37 (28.6%) were smokers, 79 (60.3%) were habitual drinkers, and 42 (31.8%) were receiving antihypertensive medications. Most subjects were prescribed long‐acting antihypertensive drugs once a day after breakfast or twice a day after breakfast and dinner. No subjects were prescribed only one short‐acting antihypertensive drug.
The waking time of this study population was 4:00 am‐8:00 am Figure 2 shows the circadian variation in the average SBP and DBP every 1 hour after waking in the total study population. There were 2 peaks in BP. The first BP peak was observed in the early morning (between 0 and 3 hours after waking), and the second BP peak was observed in the early evening (between 9 and 12 hours after waking). Average BPs during the first 3 hours after waking were significantly higher than those during the 3 hours just before waking (SBP: 133.0 ± 16.8 mm Hg vs 115.8 ± 18.3 mm Hg, P < .01, and DBP: 82.0 ± 9.9 mm Hg vs 71.4 ± 10.0 mm Hg, P < .01), and average BPs between 9 and 12 hours after waking were significantly higher than those between 6 and 9 hours after waking (SBP: 137.0 ± 16.9 mm Hg vs 133.8 ± 16.6 mm Hg, P < .01, and DBP: 84.1 ± 10.1 mm Hg vs 82.0 ± 10.1 mm Hg, P < .01).
Figure 2.
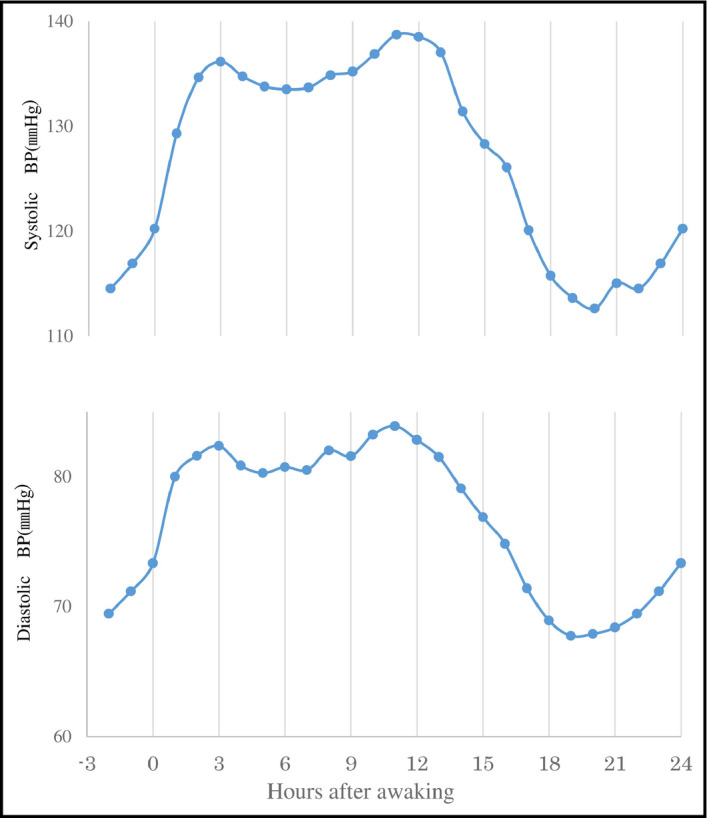
Circadian variations in SBP and DBP in the total study population. DBP; diastolic blood pressure; SBP; systolic blood pressure
Prewaking morning BP surge was defined as the average SBP during the first 3 hours after waking minus the average SBP during the 3 hours just before waking. Subjects were subclassified according to the extent of the prewaking morning BP surge. The cutoff value for identifying the top quartile of prewaking morning BP surge was 22.3 mm Hg. Subjects were divided into 2 groups as follows: the top quartile of prewaking morning BP surge (≥22.3 mm Hg, n = 34; the MS group) vs all others (<22.3 mm Hg, n = 99, the non‐MS group). The prevalence of habitual smoking and alcohol drinking were significantly higher in the MS group than in the non‐MS group (both P < .01) (Table 1). The prevalence of habitual smoking and alcohol drinking was independent determinants of a prewaking morning BP surge (P = .01 and P = .04, respectively) (Table 2).
Table 1.
Comparison of baseline clinical characteristics between non‐MS group and MS group
| Non‐MS group (N = 99) | MS group (N = 34) | P‐value | |
|---|---|---|---|
| Male, % | 40 | 61 | .07 |
| Age, yr | 57 ± 11 | 56 ± 10 | .56 |
| Body mass index, kg/m2 | 24.5 ± 2.6 | 23.7 ± 2.3 | .11 |
| Smoking habit, % | 22 | 50 | <.01 |
| Alcohol habit, % | 53 | 82 | <.01 |
| Hypertension, % | 37 | 53 | .12 |
| Diabetes mellitus, % | 5 | 3 | .68 |
| Heart disease, % | 6 | 12 | .39 |
| Antihypertensive drugs, % | 28 | 42 | .20 |
| No. of antihypertensive drugs | 1.5 ± 0.7 | 1.8 ± 1.0 | .31 |
| CCB, % | 85 | 86 | 1.00 |
| ACEI/ARB, % | 42 | 50 | .89 |
| β‐Blocker, % | 4 | 14 | .66 |
| Diuretics, % | 4 | 14 | .66 |
| Siesta habit, % | 42 | 40 | .88 |
| LDL cholesterol, mg/dL | 121.4 ± 25.7 | 117.4 ± 36.1 | .52 |
| HDL cholesterol, mg/dL | 56.7 ± 13.7 | 60.7 ± 14.1 | .17 |
| Triglyceride, mg/dL | 132.3 ± 69.6 | 132.6 ± 90.0 | .98 |
| Fasting blood sugar, mg/dL | 101.5 ± 26.0 | 97.9 ± 10.7 | .47 |
| eGFR, mL/min/1.73m2 | 61.6 ± 15.1 | 64.5 ± 14.5 | .37 |
| baPWV, mm/sec | 1546.4 ± 347.0 | 1435.4 ± 238.2 | .12 |
| 24 h‐ | |||
| Systolic BP, mm Hg | 126.7 ± 16.3 | 126.9 ± 13.8 | .93 |
| Diastolic BP, mm Hg | 77.4 ± 8.3 | 79.2 ± 9.7 | .31 |
| Heart rate, bpm | 68.9 ± 6.5 | 70.4 ± 9.0.4 | .32 |
| Awake‐ | |||
| Systolic BP, mm Hg | 131.8 ± 15.3 | 134.6 ± 13.1 | .34 |
| Diastolic BP, mm Hg | 80.1 ± 8.5 | 84.2 ± 9.7 | .02 |
| Heart rate, bpm | 72.2 ± 6.8 | 72.8 ± 10.1 | .72 |
| Asleep‐ | |||
| Systolic BP, mm Hg | 116.1 ± 17.3 | 108.6 ± 12.6 | .02 |
| Diastolic BP, mm Hg | 69.6 ± 9.6 | 67.8 ± 8.1 | .32 |
| Heart rate, bpm | 63.3 ± 15.3 | 63.1 ± 10.4 | .94 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin Ⅱ receptor blocker; baPWV, brachial‐ankle pulse wave velocity; BP, blood pressure; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MS, morning surge.
Table 2.
Determinants of a preawaking morning BP surge by multiple logistic regression analysis
| Odds ratio (95% Confidence Interval) | P‐value | |
|---|---|---|
| Smoking habit, % | 3.22 (1.33 ‐ 7.78) | .01 |
| Alcohol habit, % | 2.65 (1.01 ‐ 7.00) | .04 |
Evening BP rise was defined as the average SBP between 9 and 12 hours after waking minus the average SBP between 6‐ and 9‐ hour after waking. Subjects were also subclassified according to the extent of the evening BP rise. The cutoff value for identifying the top quartile of evening BP rise was 6.45 mm Hg. Subjects were divided into two groups as follows. The top quartile of evening BP rise (≥6.45 mm Hg, n = 34, the ER group) vs all others (<6.45 mm Hg, n = 99, the non‐ER group). Antihypertensive medications were used more frequently in the ER group than in the non‐ER group (P = .01). The prevalence of habitual alcohol drinking was significantly lower in the ER group than in the non‐ER group (P = .01). The estimated glomerular filtration rate (eGFR) was significantly lower in the ER group than in the non‐ER group (P = .01). The PWV measurements in the ER group tended to be higher in the ER group than in the non‐ER group (P = .07) (Table 3). Antihypertensive medications use and eGFR were independent determinants of the evening BP rise (P = .01 and P = .04, respectively) (Table 4).
Table 3.
Comparison of baseline clinical characteristics between non‐ER group and ER group
| Non‐ER group (N = 99) | ER group (N = 34) | P‐value | |
|---|---|---|---|
| Male, % | 49 | 35 | .23 |
| Age, yr | 56 ± 11 | 60 ± 8 | .12 |
| Body mass index, kg/m2 | 24.2 ± 2.5 | 24.6 ± 2.6 | .49 |
| Smoking habit, % | 32 | 21 | .31 |
| Alcohol habit, % | 67 | 41 | .01 |
| Hypertension, % | 36 | 55 | .10 |
| Diabetes mellitus, % | 5 | 4 | 1.00 |
| Heart disease, % | 7 | 10 | .80 |
| Antihypertensive drugs, % | 26 | 50 | .01 |
| No. of antihypertensive drugs | 1.5 ± 0.7 | 1.8 ± 1.0 | .31 |
| CCB, % | 84 | 88 | 1.00 |
| ACEI/ARB, % | 52 | 35 | .45 |
| β‐Blocker, % | 12 | 0 | .38 |
| Diuretics, % | 8 | 6 | 1.00 |
| Siesta habit, % | 40 | 46 | .77 |
| LDL cholesterol, mg/dL | 117.8 ± 29.8 | 126.8 ± 21.0 | .12 |
| HDL cholesterol, mg/dL | 58.8 ± 14.8 | 54.7 ± 10.5 | .14 |
| Triglyceride, mg/dL | 137.0 ± 101.2 | 137.5 ± 56.3 | .97 |
| Fasting blood sugar, mg/dL | 100.3 ± 25.3 | 101.9 ± 15.9 | .74 |
| eGFR, mL/min/1.73 m2 | 64.3 ± 14.6 | 56.4 ± 14.5 | .01 |
| baPWV, mm/sec | 1489.1 ± 332.2 | 1603.2 ± 306.2 | .07 |
| 24 h | |||
| Systolic BP, mm Hg | 125.9 ± 16.4 | 129.2 ± 13.1 | .28 |
| Diastolic BP, mm Hg | 77.4 ± 8.8 | 79.2 ± 8.4 | .28 |
| Heart rate, bpm | 69.2 ± 7.4 | 68.8 ± 6.6 | .77 |
| Awake‐ | |||
| Systolic BP, mm Hg | 131.7 ± 15.5 | 134.8 ± 12.3 | .31 |
| Diastolic BP, mm Hg | 80.6 ± 9.2 | 82.6 ± 8.4 | .25 |
| Heart rate, bpm | 71.9 ± 7.8 | 73.1 ± 7.2 | .44 |
| Asleep‐ | |||
| Systolic BP, mm Hg | 112.9 ± 16.2 | 117.9 ± 16.0 | 013 |
| Diastolic BP, mm Hg | 68.4 ± 9.1 | 71.5 ± 9.7 | .07 |
| Heart rate, bpm | 62.3 ± 7.8 | 61.4 ± 6.6 | .52 |
Abbreviation: ER, evening rise. Other abbreviations as in Table 1.
Table 4.
Determinants of an evening BP rise identified by multiple logistic regression analysis
| Odds ratio (95% confidence interval) | P‐value | |
|---|---|---|
| Antihypertensive drugs, % | 3.57 (1.46 ‐ 8.74) | .01 |
| eGFR, mL/min/1.73m2 | 0.96 (0.93 ‐ 0.99) | .04 |
Abbreviations as in Table 1.
4. DISCUSSION
A major finding of our study was that 2 peaks in BP were observed in the early morning (between 0 and 3 hours after waking) and in the early evening (between 9 and 12 hours after waking up). Previous studies showed that the time of onset of acute cardiovascular events was related to the time of waking, instead of clock time.22, 23 Therefore, we conducted our analyses based on the subject's wake‐up time as the start of each day.
4.1. Morning BP surge
The relationship between the prewaking morning BP surge and risk of cardiovascular events has been controversial. Verdecchia et al24 reported that a blunted prewaking morning BP surge was an independent risk factor for cardiovascular events. On the other hand, Li et al8 reported that an excessive prewaking morning BP surge was an independent risk factor for cardiovascular events. It is assumed that several factors such as ethnic differences, the differences in the composition of study populations, and the confounding influence of nocturnal BP fall are associated with this discrepancy.25, 26, 27 In our study, smoking and drinking habits were independent determinants of the preawaking morning surge. These results are consistent with those reported in previous studies.28, 29
4.2. Evening BP rise
The evening BP rise was observed between 9 and 12 hours after waking up. Peters et al12 observed a second peak in acute myocardial infarction between 11 and 12 hours after waking up, in the Cardiac Arrhythmia Suppression Trial. Muller et al2 demonstrated the existence of a second increase in the incidence of sudden cardiac death between 18:00 and 19:00. Other epidemiological studies have also shown that a second peak in the incidence of cardiovascular disease was observed in the evening.5, 6, 11, 12, 13, 14 The evening BP rise might be a trigger for cardiovascular events, but few studies have focused on BP variations in the evening. We demonstrated a second BP rise in the evening with the use of a 24‐ hour ABPM for 7 consecutive days.
The causal factors of the evening BP rise remain unclear; however, some hypotheses have been suggested. It has been suggested that the second BP rise may occur in parallel with an acceleration of physical activity. The level of physical activity in the early evening might be accelerated by going home and preparation of dinner. Stergious et al30 demonstrated that a BP rise after daytime napping might be an important triggering factor for a cardiovascular event. However, no relationship between the evening rise in BP and the habit of daytime napping was found in this study.
Shea et al31 showed that the endogenous circadian rise in BP, unlike that of platelet agreeability, neurohormonal factors, and fibrinolytic activity, occurred in the evening. The evening BP rise might reflect the endogenous circadian BP rhythms.
We demonstrated that taking antihypertensive medications was an important determinant of the evening BP rise. Most of the subjects treated with hypertensive medications in this study were taking antihypertensive medications in the morning. The efficacy of their antihypertensive medications might therefore have been attenuated in the evening. The eGFR level of subjects was also an important determinant of the evening BP rise. It is well known that abnormal BP circadian rhythms are very common in chronic kidney disease patients.32, 33, 34 Chronic kidney disease is closely associated with a non‐dipping BP pattern,35, 36 but high 24‐hour BP variability and a morning BP surge are also associated with chronic kidney disease.37, 38
Previous studies have demonstrated that this may be caused by decreased renal excretion of water and sodium,39 predominance of nocturnal sympathetic nerve activity,40 progression of arterial stiffness,41 and disturbances in the circadian rhythm of the intrarenal renin‐angiotensin system.42 These factors could contribute to a BP rise in the evening.
4.3. Limitations
This study had some potential limitations. First, this study was a cross‐sectional study and the number of subjects was relatively small. It was difficult to elucidate whether the evening BP rise was the cause or a consequence of renal dysfunction in this study. We hope to clarify this in a larger prospective study. Second, we could not perform a quantitative evaluation of physical activity. A quantitative evaluation of physical activity may provide important information about the evening BP rise. Third, the magnitude of the evening BP rise was small compared with that of the morning BP surge. However, the average BPs between 9 and 12 hours after waking were significantly higher than for other periods (Figure 2), and therefore, the second increase in cardiovascular events might have been observed in the evening.
5. CONCLUSIONS
In conclusion, our study demonstrated there were 2 peaks in BP, 1 in the morning and another in the early evening. The evening BP rise was associated with antihypertensive medication use and renal function. Subjects with impaired renal function and subjects taking antihypertensive medication might have to pay particular attention to factors influencing BP, such as bathing and intense exercise in the evening.
CONFLICT OF INTEREST
The authors report no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
SM performed an analysis of data and wrote the initial draft of the manuscript. KO designed the study and contributed to analysis and interpretation of data. KT contributed to assist in the preparation of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Yutaka Kubo and Dr Takashi Yamanaka for useful discussions.
Murakami S, Otsuka K, Kono T. Repeated ambulatory monitoring reveals an evening rise in blood pressure in a Japanese population. J Clin Hypertens. 2019;21:1675–1681. 10.1111/jch.13709
REFERENCES
- 1. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315‐1322. [DOI] [PubMed] [Google Scholar]
- 2. Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 3. Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60(10):801‐806. [DOI] [PubMed] [Google Scholar]
- 4. Marler JR, Price TR, Clark GL, et al. Morning increase in the onset of ischemic stroke. Stroke. 1989;20(4):473‐476. [DOI] [PubMed] [Google Scholar]
- 5. Marsh EE 3rd, Biller J, Adams HP Jr, et al. Circadian variation in onset of acute ischemic stroke. Arch Neurol. 1990;47(11):1178‐1180. [DOI] [PubMed] [Google Scholar]
- 6. Wroe SJ, Sandercock P, Bamford J, Dennis M, Slattery J, Warlow C. Diurnal variation in incidence of stroke: oxfordshire community stroke project. BMJ. 1992;304(6820):155‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107(10):1401‐1406. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Thijs L, Hansen TW, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55(4):1040‐1048. [DOI] [PubMed] [Google Scholar]
- 9. Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316(24):1514‐1518. [DOI] [PubMed] [Google Scholar]
- 10. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha‐sympathetic vasoconstrictor activity. N Engl J Med. 1991;325(14):986‐990. [DOI] [PubMed] [Google Scholar]
- 11. Kono T, Morita H, Nishina T, et al. Circadian variations of onset of myocardial infarction and efficacy of thrombolytic therapy. J Am Coll Cardiol. 1996;27(4):774‐778. [DOI] [PubMed] [Google Scholar]
- 12. Peters RW, Zoble RG, Liebson PR, Pawitan Y, Brooks MM, Proschan M. Identification of a secondary peak in myocardial infarction onset 11 to 12 hours after awaking: the Cardiac Arrhythmia Suppression Trial (CAST) experience. J Am Coll Cardiol. 1993;22(4):998‐1003. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi S, Toyoshima H, Tanabe N, Miyanishi K. Daily peak in the incidence of sudden cardiac death and fatal stroke in Niigata Prefecture. Jpn Circ J. 1996;60(4):193‐200. [DOI] [PubMed] [Google Scholar]
- 14. Tsukada T, Ikeda T, Ishiguro H, et al. Circadian variation in out‐of‐hospital cardiac arrests due to cardiac cause in a Japanese patient population. Circ J. 2010;74(9):1880‐1887. [DOI] [PubMed] [Google Scholar]
- 15. Pickering TG, Shimbo D, Haas D. Ambulatory blood pressure monitoring. N Engl J Med. 2006;354(22):2368‐2374. [DOI] [PubMed] [Google Scholar]
- 16. Hermida RC, Calvo C, Ayala DE, Fernandez JR, Ruilope LM, Lopez JE. Evaluation of the extent and duration of the “ABPM effect” in hypertensive patients. J Am Coll Cardiol. 2002;40(4):710‐717. [DOI] [PubMed] [Google Scholar]
- 17. Cuspidi C, Meani S, Salerno M, et al. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens. 2004;22(2):273‐280. [DOI] [PubMed] [Google Scholar]
- 18. Hernández‐del Rey R, Martin‐Baranera M, et al. Reproducibility of the circadian blood pressure pattern in 24‐h versus 48‐h recordings: the Spanish Ambulatory Blood Pressure Monitoring Registry. J Hypertens. 2007;25(12):2406‐2412. [DOI] [PubMed] [Google Scholar]
- 19. Yamashina A, Tomiyama A, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359‐364. [DOI] [PubMed] [Google Scholar]
- 20. JCS Joint Working Group . Guideline for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS 2010). Circ J. 2012;76(2):508‐519. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 22. Willich SN, Goldberg RJ, Maclure M, et al. Increased onset of sudden cardiac death in the first three hours after awaking. Am J Cardiol. 1992;70(1):65‐68. [DOI] [PubMed] [Google Scholar]
- 23. White WB. Cardiovascular risk and therapeutic intervention for the early morning surge in blood pressure and heart rate. Blood Pressure Monit. 2001;6(2):63‐72. [DOI] [PubMed] [Google Scholar]
- 24. Verdecchia P, Angeli F, Mazzotta M, et al. Day‐night dip and early‐morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 25. Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66(4):750‐756. [DOI] [PubMed] [Google Scholar]
- 26. Pierdomenico SD, Pierdomenico AM, Coccina F, et al. Prognosis value of nondipping and morning surge in elderly treated hypertensive patients with controlled ambulatory blood pressure. Am J Hypertens. 2017;30(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 27. Bilo G, Grillo A, Guida V, Parati G. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr Blood Press Control. 2018;11:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohira T, Tanigawa T, Tabata T, et al. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension. 2009;53(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 29. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56(5):765‐773. [DOI] [PubMed] [Google Scholar]
- 30. Stergiou GS, Vemmos KN, Pliarchopoulou KM, Synetos AG, Roussias LG, Mountokalakis TD. Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke. 2002;33:1480‐1486. [DOI] [PubMed] [Google Scholar]
- 31. Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 33. Manios E, Tsagalis G, Tsivgoulis G, et al. Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens. 2009;27(11):2244‐2248. [DOI] [PubMed] [Google Scholar]
- 34. Mojón A, Ayala DE, Pineiro L, et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1‐2):145‐158. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recording in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175‐1180. [DOI] [PubMed] [Google Scholar]
- 36. Minutolo R, Agarwal R, Borrelli S, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171(12):1090‐1098. [DOI] [PubMed] [Google Scholar]
- 37. Tanner RM, Shimbo D, Dreisbach AW, Carson AP, Fox ER, Muntner P. Association between 24‐hour blood pressure variability and chronic kidney: a cross‐sectional analysis of African Americans participating in the Jackson heart study. BMC Nephrol. 2015;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turak O, Afsar B, Siriopol D, et al. Morning blood pressure surge as a predictor of development of chronic kidney disease. J Clin Hypertens (Greenwich). 2016;18(5):444‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fukuda M, Munemura M, Usami T, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65(2):621‐625. [DOI] [PubMed] [Google Scholar]
- 40. Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14(12):3239‐3244. [DOI] [PubMed] [Google Scholar]
- 41. Velasquez MT, Beddhu S, Nobakht E, Rahman M, Raj DS. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int Rep. 2016;1(2):94‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohnishi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin‐angiotensin system in the kidney. Hypertens Res. 2017;40(5):413‐422. [DOI] [PubMed] [Google Scholar]


