Abstract
In a pre‐specified subgroup analysis of a 12‐week randomized multicenter study, we investigated effects of valsartan/amlodipine 80/5 mg single‐pill combination (n = 75) and nifedipine GITS 30 mg (n = 75) on ambulatory blood pressure (BP) and arterial stiffness assessed by brachial‐ankle pulse wave velocity (PWV) in patients with uncontrolled hypertension. At week 12, the between‐treatment mean differences in systolic/diastolic BP were smaller for 24‐hour and daytime (–2.1/–1.7 and −2.0/−1.5 mm Hg, respectively, P ≥ 0.22) but greater (P < 0.01) for nighttime (–4.0/‐2.8 mm Hg, P ≤ 0.09), especially in sustained uncontrolled hypertension (−5.0/−4.1 mm Hg, P ≤ 0.04) and non‐dippers (−6.5/−3.7 mm Hg, P ≤ 0.07), in favor of valsartan/amlodipine. At week 12, PWV was significantly reduced from baseline by valsartan/amlodipine (n = 59, P < 0.0001) but not nifedipine (n = 59, P = 0.06). The changes in PWV were significantly associated with that in ambulatory systolic BP and pulse pressure in the nifedipine (P ≤ 0.0008) but not valsartan/amlodipine group (P ≥ 0.57), with a significant interaction (P ≤ 0.045). The valsartan/amlodipine combination was more efficacious than nifedipine GITS in lowering nighttime BP in sustained uncontrolled hypertension and non‐dippers, and in lowering arterial stiffness independent of BP lowering.
Keywords: ambulatory blood pressure, amlodipine, arterial stiffness, single‐pill combination, valsartan
1. INTRODUCTION
The new American1 and European2 hypertension guidelines unequivocally advocate early use of combination antihypertensive therapy in the management of hypertension, including single‐pill combination. The latter approach of combination antihypertensive therapy does have advantages, such as high adherence,3 low cost,3, 4 more efficacy,5, 6 less side effects,5, 6 and so on.7, 8 Moreover, co‐administration of two or more drugs with different modes of action may have improved clinical outcomes.9, 10 In the Avoiding Cardiovascular Events through COMbination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial, the single‐pill combination of amlodipine and benazepril reduced the risk of the composite cardiovascular endpoints by 20%, in comparison with that of hydrochlorothiazide and benazepril, with similar blood pressure reductions in the two groups.11 The mechanism for the clinical outcome differences observed in the ACCOMPLISH trial remains under investigation. It is possible that co‐administration of a dihydropyridine calcium‐channel blocker and an inhibitor of renin‐angiotensin system may potentiate cardiovascular protection of these two classes of antihypertensive drugs.11
In a recent randomized multicenter study, we found that the valsartan/amlodipine single‐pill combination, compared with nifedipine GITS, significantly improved systolic/diastolic blood pressure lowering efficacy by 5.8/4.0 mm Hg at 12 weeks of treatment.12 In five participating hospitals of this multicenter study, a sub‐study was conducted on ambulatory blood pressure and arterial stiffness as measured by brachial‐ankle pulse wave velocity (PWV). In the present pre‐specified subgroup analysis, we investigated effects of the valsartan/amlodipine 80/5 mg per day single‐pill combination and nifedipine GITS 30 mg per day on ambulatory blood pressure and arterial stiffness, and explored the interrelationship between the treatment‐induced changes in ambulatory blood pressure and arterial stiffness.
2. METHODS
2.1. General study design
The present sub‐study was a pre‐specified subgroup of a multicenter, open‐label, randomized, actively‐controlled, parallel‐group study in patients with hypertension inadequately controlled with initiation‐dose monotherapy (ClinicalTrials.gov number, NCT01167153). The study protocol of the main trial was described in detail previously.12 Briefly, potentially eligible patients should have been previously treated with initiation‐dose of antihypertensive monotherapy for at least 4 weeks. If at a screening visit, their clinic systolic/diastolic blood pressure was uncontrolled (140‐159/90‐99 mm Hg or 130‐159/80‐99 in diabetes mellitus), they entered a 1‐ to 2‐week run‐in period with their preexisting initiation‐dose monotherapy. Patients with uncontrolled clinic blood pressure at the end of the run‐in period were asked to stop their prior antihypertensive monotherapy and randomized to receive once‐daily oral dose of valsartan/amlodipine 80/5 mg or nifedipine GITS 30 mg. Patients were assigned to treatment groups by unique identification numbers. A central randomization scheme was prepared by Gleneagles CRC (Beijing, China) and sent to each study site. Subjects were assigned to the treatment groups by enrolling sequence.12 Patients were followed up at 2, 4, 8, and 12 weeks of treatment. During the 12‐week randomized treatment period, patients were instructed to take the medication at 8:00 o'clock in the morning. Clinic blood pressure and pulse rate were measured at each of the follow‐up visits. Ambulatory blood pressure monitoring and measurements of brachial‐ankle PWV were performed at randomization and at the final visit.12
The study was performed under the guidance of the International Conference on Harmonization Guidelines for Good Clinical Practice local regulations and the ethical principles of the Declaration of Helsinki. The protocol of this study was approved by the ethics committees of all participating hospitals. All patients gave written informed consent.
2.2. Inclusion and Exclusion Criteria
Eligible subjects were men and women aged 18‐65 years, with uncontrolled blood pressure after antihypertensive monotherapy for at least 4 weeks. To be eligible for inclusion in the study, a patient should also have a systolic blood pressure in the range from 140 to 159 mm Hg (130‐159 mm Hg for patients with diabetes mellitus) and/or a diastolic blood pressure in the range from 90 to 99 mm Hg (80‐99 mm Hg for patients with diabetes mellitus) both at the screening visit and at the end of the run‐in period. The exclusion criteria included the use of more than one antihypertensive drug or a therapeutic regimen higher than the initiation dose, an elevated serum creatinine concentration (>176.8 μmoI/L), a history of nephrotic syndrome or diabetes mellitus requiring insulin treatment or poorly controlled (glycosylated hemoglobin A1c [HbA1c] >8.0%). Women who were pregnant, lactating or of childbearing potential without adequate contraception were also excluded.12
2.3. Ambulatory and clinic blood pressure measurements
Validated ambulatory blood pressure monitors (SpaceLabs 90207, SpaceLabs Inc, Redmond, WA) were programmed to obtain blood pressure readings every 20 minutes in the daytime (6:00 am to 10:00 pm) and every 30 minutes at night (10:00 pm to 6:00 am). A recording was considered valid, if it had a total duration of at least 20 hours, more than 80% of the expected readings, and at least 14 readings and 10 readings in the daytime and at night, respectively.13 In the present analysis, we applied the short‐clock time approach to define daytime (8:00 am to 6:00 pm) and nighttime (11:00 pm to 6:00 am), which required at least 14 and 7 readings, respectively. Blood pressure load was the percentage of blood pressure values reaching or exceeding 135 mm Hg systolic or 85 mm Hg diastolic during daytime or 120 mm Hg systolic or 70 mm Hg diastolic during nighttime.
Clinic blood pressure was measured using a validated automated electronic blood pressure monitor (HEM‐7112, Omron Healthcare, Kyoto, Japan) with an appropriately sized cuff. After resting for at least 5 minutes in the sitting position, blood pressure was measured three times consecutively with 2 to 3 minutes time interval.
In these randomized patients with uncontrolled clinic hypertension at baseline, we defined white‐coat uncontrolled hypertension as a normal daytime systolic/diastolic blood pressure (<135/85 mm Hg) and sustained uncontrolled hypertension as an elevated daytime systolic/diastolic blood pressure (≥135/85 mm Hg). We computed night‐to‐day systolic and diastolic ratios by dividing the nighttime blood pressure by daytime blood pressure. We defined dippers and non‐dippers as a night‐to‐day ratio of 90% or less and >90%, respectively.13
2.4. Brachial‐ankle PWV
Brachial‐ankle PWV was measured with the oscillometric Vascular Profiler‐1000 device (Omron Healthcare, Kyoto, Japan).14 After the study subjects had rested in the supine position for 5‐10 minutes with specially designed upper arm and lower leg cuffs, pulse waveforms were collected on the four limbs. The pulse transit time was estimated by comparing these simultaneously collected waveforms. The path length of the pulse waves was estimated using a formula with body height as a factor. Brachial‐ankle PWV was calculated as the ratio of the path length to the transit time between the brachial and ankle arterial sites. The brachial‐ankle PWV values on both sides were averaged for analysis.
2.5. Statistical analysis
The SAS software (version 9.2, SAS Institute, Cary, NC) was used for data management and statistical analysis. Means and proportions were compared using Student's t test and chi‐square test, respectively. Analysis of covariance was performed to calculate the least square mean change (±standard error) from baseline and between‐group differences (95% confidence interval) with baseline values as covariate and treatment as a factor. For brachial‐ankle PWV, the analysis was also adjusted for sex and the baseline age, body mass index, mean arterial pressure, and pulse rate. Scatter plot and correlation analysis were used to analyze the interrelationship between changes in brachial‐ankle PWV and the changes in ambulatory systolic blood pressure and pulse pressure.
3. RESULTS
3.1. Characteristics of the randomized patients
Of the 162 randomized patients in five participating hospitals, 150 (92.6%) patients had valid ambulatory blood pressure recordings, and 118 (72.8%) patients had measurements of brachial‐ankle PWV (Figure S1). The demographics and baseline characteristics were comparable between the 150 patients included in the present analysis and those excluded because of no ambulatory blood pressure monitoring data (n = 363), except that age was 6.0 years greater in the former subgroup (Table S1). The demographics and baseline characteristics were comparable between the valsartan/amlodipine 80/5 mg single‐pill combination (n = 75) and the nifedipine GITS 30 mg monotherapy groups (n = 75, Table 1), except that brachial‐ankle PWV was 0.9 m/s higher in the single‐pill combination group.
Table 1.
Characteristics of the randomized patients at baseline
| Characteristic | Valsartan/amlodipine (n = 75) | Nifedipine GITS (n = 75) | P b |
|---|---|---|---|
| Men, n (%) | 36 (48.0%) | 39 (52.0%) | 0.62 |
| Age, y | 55.5 ± 7.4 | 53.8 ± 8.5 | 0.36 |
| Body mass index, kg/m2 | 25.8 ± 3.3 | 26.4 ± 3.5 | 0.28 |
| Duration of hypertension, years | 14.7 ± 7.0 | 15.2 ± 8.0 | 0.65 |
| Previous antihypertensive treatment, n (%) | |||
| Calcium channel blockers | 36 (48.0%) | 41 (54.7%) | 0.41 |
| Angiotensin‐converting enzyme inhibitors | 11 (14.7%) | 10 (13.3%) | 0.81 |
| Angiotensin‐receptor blockers | 19 (25.3%) | 17 (22.7%) | 0.70 |
| β‐blockers | 4 (5.3%) | 2 (2.7%) | 0.40 |
| Diuretics | 2 (2.7%) | 3 (4.0%) | 0.65 |
| Other | 3 (4.0%) | 2 (2.7%) | 0.65 |
| Clinic BP, mm Hg | |||
| Systolic | 146.3 ± 6.1 | 145.1 ± 6.6 | 0.22 |
| Diastolic | 83.4 ± 8.3 | 83.9 ± 9.1 | 0.19 |
| Clinic pulse rate, beats/min | 73.2 ± 11.3 | 74.2 ± 9.3 | 0.54 |
| Ambulatory BP recording | |||
| 24‐h systolic BP, mm Hg | 130.5 ± 13.1 | 128.7 ± 12.4 | 0.38 |
| 24‐h diastolic BP, mm Hg | 83.1 ± 14.0 | 82.7 ± 12.1 | 0.86 |
| 24‐h pulse rate, beats/min | 70.5 ± 8.8 | 72.2 ± 7.3 | 0.24 |
| Daytime systolic BP, mm Hg | 134.7 ± 15.3 | 132.2 ± 13.2 | 0.29 |
| Daytime diastolic BP, mm Hg | 86.3 ± 15.2 | 85.5 ± 12.7 | 0.73 |
| Daytime pulse rate, beats/min | 76.6 ± 9.5 | 77.4 ± 8.5 | 0.63 |
| Nighttime systolic BP, mm Hg | 122.8 ± 14.5 | 121.0 ± 13.7 | 0.44 |
| Nighttime diastolic BP, mm Hg | 77.7 ± 14.7 | 77.2 ± 12.5 | 0.85 |
| Nighttime pulse rate, beats/min | 61.3 ± 9.6 | 63.2 ± 7.4 | 0.22 |
| 24‐h systolic BP load, % | 51.6 ± 29.4 | 49.0 ± 29.5 | 0.61 |
| 24‐h diastolic BP load, % | 55.5 ± 33.2 | 59.4 ± 32.7 | 0.49 |
| Daytime systolic BP load, % | 50.0 ± 32.3 | 47.0 ± 30.8 | 0.58 |
| Daytime diastolic BP load, % | 51.3 ± 35.6 | 54.9 ± 35.3 | 0.55 |
| Nighttime systolic BP load, % | 54.6 ± 36.4 | 52.5 ± 33.7 | 0.72 |
| Nighttime diastolic BP load, % | 64.3 ± 36.8 | 69.2 ± 32.9 | 0.41 |
| Night‐to‐day systolic BP ratio, % | 91.7 ± 10.3 | 91.7 ± 7.0 | 0.98 |
| Night‐to‐day diastolic BP ratio, % | 90.4 ± 10.4 | 90.5 ± 7.6 | 0.94 |
| Diabetes mellitus, n (%) | 4 (5.3%) | 10 (13.3%) | 0.16 |
| Coronary artery disease, n (%) | 1 (1.3%) | 3 (4.0%) | 0.61 |
| eGFR, ml/min·1.73 m2 a | |||
| Mean ± SD | 87.8 ± 14.1 | 91.9 ± 14.6 | 0.09 |
| <60, n (%) | 4 (5.3%) | 5 (6.7%) | 0.99 |
| Proteinuria on urine routine test, n (%) | 7 (9.3%) | 5 (6.7%) | 0.55 |
| Serum total cholesterol, mmol/L | 5.1 ± 0.9 | 5.1 ± 1.1 | 0.98 |
| Brachial‐ankle PWV, m/s | 16.5 ± 2.6 | 15.6 ± 2.2 | 0.03 |
BP, blood pressure; eGFR, estimated glomerular filtration rate; PWV, pulse wave velocity.
Values are mean ± standard deviation or number of patients (% of column total).
eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula.27
The P value is for the comparison between the valsartan/amlodipine combination and nifedipine GITS groups.
3.2. Treatment effects on ambulatory and clinic blood pressure
Ambulatory blood pressure was significantly (P ≤ 0.046) reduced from baseline to 12 weeks of treatment in both treatment groups (Table 2 and Figure 1), except for the daytime systolic/diastolic blood pressure in the nifedipine GITS group (P ≥ 0.21). The between‐treatment difference was greater for the nighttime systolic/diastolic blood pressure (−4.0/−2.8 mm Hg, P ≤ 0.09) and smaller for the daytime systolic/diastolic blood pressure (−2.0/−1.5 mm Hg, P ≥ 0.34), in favor of the valsartan/amlodipine group, but did not reach statistical significance (P = 0.06 to 0.36, Table 2). Accordingly, the night‐to‐day ratio in both systolic and diastolic blood pressure was significantly reduced from baseline to 12 weeks of treatment in the valsartan/amlodipine (P ≤ 0.004) but not nifedipine GITS group (P ≥ 0.35). The between‐treatment difference, however, did not reach statistical difference (P ≥ 0.12, Table 2). If blood pressure loads, instead of mean level, were assessed, the results remained similar (Table 2).
Table 2.
Mean change at 12 weeks of treatment from baseline in clinic and ambulatory blood pressure and pulse rate by randomization
| Variable | Valsartan/amlodipine (n = 75) | Nifedipine GITS (n = 75) | Between‐group difference (95% CI) | P |
|---|---|---|---|---|
| Clinic BP, mm Hg | ||||
| Systolic | −15.1 ± 1.2** | −11.9 ± 1.4** | −3.2 (−6.9, 0.4) | 0.08 |
| Diastolic | −5.4 ± 1.0** | −3.3 ± 1.0* | −2.1 (−4.9, 0.7) | 0.14 |
| Clinic pulse rate, beats/min | −1.9 ± 1.1 | −2.5 ± 1.1* | 0.6 (−2.4, 3.5) | 0.70 |
| Ambulatory BP recording | ||||
| 24‐h systolic BP, mm Hg | −4.8 ± 1.5* | −2.7 ± 1.2* | −2.1 (−5.9, 1.6) | 0.26 |
| 24‐h diastolic BP, mm Hg | −3.5 ± 1.2* | −1.8 ± 0.8* | −1.7 (−4.5, 1.1) | 0.22 |
| 24‐h pulse rate, beats/min | 2.0 ± 1.0* | 0.4 ± 1.0 | 1.5 (−1.2, 4.2) | 0.27 |
| Daytime systolic BP, mm Hg | −3.8 ± 1.6* | −1.7 ± 1.4 | −2.0 (−6.3, 2.2) | 0.34 |
| Daytime diastolic BP, mm Hg | −2.6 ± 1.3* | −1.1 ± 1.0 | −1.5 (−4.6, 1.7) | 0.36 |
| Daytime pulse rate, beats/min | 1.0 ± 1.1 | 0.2 ± 1.1 | 0.8 (−2.4, 4.0) | 0.62 |
| Nighttime systolic BP, mm Hg | −7.2 ± 1.7** | −3.2 ± 1.3* | −4.0 (−8.2, 0.2) | 0.06 |
| Nighttime diastolic BP, mm Hg | −5.2 ± 1.3** | −2.4 ± 1.0* | −2.8 (−6.0, 0.4) | 0.09 |
| Nighttime pulse rate, beats/min | 2.2 ± 1.1 | 1.0 ± 1.2 | 1.2 (−2.0, 4.4) | 0.46 |
| 24‐h systolic BP load, % | −12.4 ± 3.4* | −6.5 ± 3.4 | −5.9 (−15.4, 3.5) | 0.22 |
| 24‐h diastolic BP load, % | −10.3 ± 3.2* | −5.2 ± 3.1 | −5.0 (−13.8, 3.7) | 0.26 |
| Daytime systolic BP load, % | −9.8 ± 3.6* | −5.0 ± 3.6 | −4.8 (−14.9, 5.3) | 0.35 |
| Daytime diastolic BP load, % | −8.8 ± 3.5* | −5.1 ± 3.5 | −3.6 (−13.4, 6.1) | 0.46 |
| Nighttime systolic BP load, % | −18.9 ± 4.3** | −9.4 ± 4.2* | −9.5 (−21.4, 2.3) | 0.11 |
| Nighttime diastolic BP load, % | −14.2 ± 4.1* | −5.7 ± 4.0 | −8.6 (−19.9, 2.8) | 0.14 |
| Night‐to‐day systolic BP ratio, % | −3.5 ± 1.3* | −0.9 ± 1.0 | −2.6 (−5.8, 0.7) | 0.12 |
| Night‐to‐day diastolic BP ratio, % | −3.6 ± 1.4* | −1.2 ± 1.1 | −2.4 (−5.9, 1.0) | 0.17 |
Values are least square mean ± standard error, unless otherwise indicated.
BP, blood pressure; CI, confidence interval.
Significance of the difference from baseline,
P < 0.05,
P < 0.0001.
Figure 1.
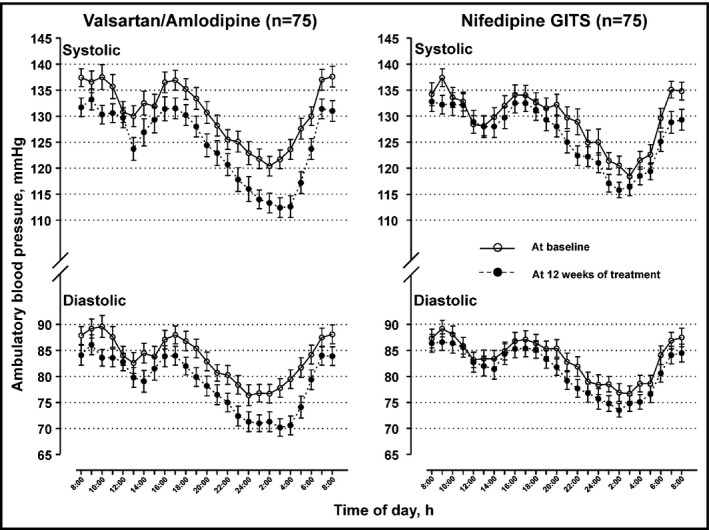
24‐h blood pressure profile at baseline (circle) and 12 weeks of treatment (dot) with the valsartan/amlodipine 80/5 mg single‐pill combination or nifedipine GITS 30 mg. Symbols denote hourly mean. Vertical lines denote standard error
The mean changes from baseline to 12 weeks of treatment in clinic systolic/diastolic blood pressure (−15.1 to −11.9/−5.4 to −3.3 mm Hg, P ≤ 0.002) were much greater than that in ambulatory blood pressure in both groups. However, the between‐treatment difference in clinic systolic/diastolic blood pressure was only −3.2/−2.1 mm Hg and did not reach statistical significance (P ≥ 0.08, Table 2).
3.3. Treatment effects in white‐coat and sustained uncontrolled hypertension and in dippers and non‐dippers
Clinic blood pressure was significantly (P ≤ 0.01) reduced from baseline in both valsartan/amlodipine and nifedipine GITS groups in patients with white‐coat (n = 53) as well as sustained uncontrolled hypertension (n = 97, Table S2). However, ambulatory blood pressure was only reduced from baseline in sustained uncontrolled hypertension (P ≤ 0.07) but not white‐coat uncontrolled hypertension (P ≥ 0.10). In sustained uncontrolled hypertension, the between‐treatment difference in ambulatory systolic/diastolic blood pressure was –4.9/–3.8 mm Hg (P ≤ 0.03), –4.7/−3.9 mm Hg (P ≤ 0.048) and −5.0/−4.1 mm Hg (P ≤ 0.04) for 24 hours, daytime and nighttime, respectively, in favor of the valsartan/amlodipine group (Table 3).
Table 3.
Mean change at 12 weeks of treatment from baseline in clinic and ambulatory blood pressure and pulse rate in patients with white‐coat or sustained uncontrolled hypertension by randomization
| Variable | Valsartan/amlodipine | Nifedipine GITS | Between‐group difference (95%CI) | P |
|---|---|---|---|---|
| White‐coat uncontrolled hypertension | n = 29 | n = 24 | ||
| Clinic BP, mm Hg | ||||
| Systolic | −16.2 ± 1.9** | −14.8 ± 2.2** | −1.4 (−7.2, 4.4) | 0.63 |
| Diastolic | −4.7 ± 1.7* | −3.7 ± 2.0* | −1.0 (−6.3, 4.2) | 0.69 |
| Clinic pulse rate, beats/min | −2.3 ± 1.8 | −3.9 ± 1.9 | 1.6 (−3.7, 6.8) | 0.55 |
| Ambulatory BP recording | ||||
| 24‐h systolic BP, mm Hg | 1.2 ± 2.3 | −0.1 ± 2.2 | 1.3 (−5.2, 7.8) | 0.69 |
| 24‐h diastolic BP, mm Hg | 1.4 ± 1.6 | 0.6 ± 1.3 | 0.7 (−3.5, 4.9) | 0.74 |
| 24‐h pulse rate, beats/min | −0.5 ± 2.1 | 0.9 ± 2.1 | −1.4 (−7.5, 4.7) | 0.65 |
| Daytime systolic BP, mm Hg | 2.6 ± 2.4 | 0.7 ± 2.9 | 1.9 (−5.6, 9.5) | 0.61 |
| Daytime diastolic BP, mm Hg | 2.8 ± 1.7 | 1.0 ± 1.7 | 1.8 (−3.1, 6.7) | 0.46 |
| Daytime pulse rate, beats/min | −2.1 ± 2.5 | 0.01 ± 2.4 | −2.1 (−9.1, 4.9) | 0.54 |
| Nighttime systolic BP, mm Hg | −2.5 ± 2.9 | 0.9 ± 2.0 | −3.4 (−10.5, 3.6) | 0.33 |
| Nighttime diastolic BP, mm Hg | −0.8 ± 2.2 | 0.8 ± 1.3 | −1.6 (−6.8, 3.5) | 0.52 |
| Nighttime pulse rate, beats/min | 0.6 ± 2.6 | 1.5 ± 2.5 | −0.8 (−8.3, 6.6) | 0.82 |
| Sustained uncontrolled hypertension | n = 46 | n = 51 | ||
| Clinic BP, mm Hg | ||||
| Systolic | −14.4 ± 1.7** | −10.5 ± 1.6** | −3.9 (−8.6, 0.7) | 0.10 |
| Diastolic | −5.9 ± 1.2** | −3.1 ± 1.2* | −2.7 (−6.1, 0.6) | 0.11 |
| Clinic pulse rate, beats/min | −1.7 ± 1.4 | −1.9 ± 1.3 | 0.2 (−3.5, 3.9) | 0.93 |
| Ambulatory BP recording | ||||
| 24‐h systolic BP, mm Hg | −8.5 ± 1.7** | −3.6 ± 1.3* | −4.9 (−9.2, −0.7) | 0.02 |
| 24‐h diastolic BP, mm Hg | −6.6 ± 1.5** | −2.8 ± 1.0* | −3.8 (−7.3, −0.3) | 0.03 |
| 24‐h pulse rate, beats/min | 2.9 ± 1.0* | 0.2 ± 1.1 | 2.6 (−0.4, 5.6) | 0.08 |
| Daytime systolic BP, mm Hg | −7.8 ± 1.8** | −2.9 ± 1.6 | −4.7 (−9.8, −0.3) | 0.045 |
| Daytime diastolic BP, mm Hg | −6.0 ± 1.7** | −2.1 ± 1.3 | −3.9 (−7.7, −0.03) | 0.048 |
| Daytime pulse rate, beats/min | 2.2 ± 1.2 | 0.3 ± 1.3 | 1.9 (−1.6, 5.4) | 0.29 |
| Nighttime systolic BP, mm Hg | −9.9 ± 1.9** | −4.9 ± 1.4* | −5.0 (−9.7, −0.3) | 0.04 |
| Nighttime diastolic BP, mm Hg | −7.9 ± 1.5** | −3.7 ± 1.1* | −4.1 (−7.8, −0.5) | 0.03 |
| Nighttime pulse rate, beats/min | 2.8 ± 1.2* | 0.8 ± 1.3 | 2.0 (−1.5, 5.5) | 0.27 |
Values are least square mean ± standard error, unless otherwise indicated.
BP, blood pressure; CI, confidence interval.
Significance of the difference from baseline,
P < 0.05,
P < 0.0001.
We also performed subgroup analysis in dippers (n = 61) and non‐dippers (n = 89, Table S3). Clinic blood pressure was significantly (P ≤ 0.04) reduced from baseline in both treatment groups in dippers as well as non‐dippers. Ambulatory systolic/diastolic blood pressure was significantly (P ≤ 0.01) reduced from baseline in the valsartan/amlodipine group for daytime in dippers and for 24‐hour and nighttime in non‐dippers, and in the nifedipine GITS group for nighttime only in non‐dippers. Only for nighttime in non‐dippers, the between‐treatment difference in ambulatory systolic/diastolic blood pressure tended to be statistically significant (−6.5/‐3.7 mm Hg, P ≤ 0.07), in favor of the valsartan/amlodipine group (Table 4). In non‐dippers, the analysis on night‐to‐day ratio in systolic and diastolic blood pressure was confirmatory (Table 4).
Table 4.
Mean change at 12 weeks of treatment from baseline in clinic and ambulatory blood pressure and pulse rate in dippers and non‐dippers by randomization
| Variable | Valsartan/amlodipine | Nifedipine GITS | Between‐group difference (95% CI) | P |
|---|---|---|---|---|
| Dippers | n = 33 | n = 28 | ||
| Clinic BP, mm Hg | ||||
| Systolic | −15.0 ± 1.8** | −12.5 ± 2.2** | −2.5 (−8.1, 3.1) | 0.38 |
| Diastolic | −5.1 ± 1.2** | −4.0 ± 1.8* | −1.2 (−5.3, 3.0) | 0.57 |
| Clinic pulse rate, beats/min | −3.9 ± 1.6* | −3.7 ± 1.8* | −0.2 (−5.0, 4.6) | 0.94 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic BP, mm Hg | −3.8 ± 1.9 | −2.2 ± 2.1 | −1.6 (−7.3, 4.1) | 0.58 |
| 24‐h diastolic BP, mm Hg | −3.4 ± 1.6 | −1.5 ± 1.8 | −1.9 (−6.7, 3.0) | 0.44 |
| 24‐h pulse rate, beats/min | 3.0 ± 1.3* | −1.1 ± 1.4 | 4.1 (0.2, 8.1) | 0.04 |
| Daytime systolic BP, mm Hg | −5.9 ± 2.3* | −2.7 ± 1.9 | −3.2 (−9.2, 2.8) | 0.29 |
| Daytime diastolic BP, mm Hg | −4.4 ± 2.1* | −1.8 ± 1.6 | −2.6 (−7.9, 2.7) | 0.33 |
| Daytime pulse rate, beats/min | 1.1 ± 1.5 | −2.1 ± 1.7 | 3.2 (−1.3, 7.7) | 0.16 |
| Nighttime systolic BP, mm Hg | −1.0 ± 2.0 | 0.6 ± 2.1 | −1.7 (−7.5, 4.2) | 0.57 |
| Nighttime diastolic BP, mm Hg | −1.9 ± 1.7 | 0.3 ± 1.9 | −2.2 (−7.3, 2.9) | 0.39 |
| Nighttime pulse rate, beats/min | 4.0 ± 1.4* | −0.1 ± 1.5 | 4.1 (−0.1, 8.3) | 0.053 |
| Non‐dippers | n = 42 | n = 47 | ||
| Clinic BP, mm Hg | ||||
| Systolic | −15.2 ± 1.7** | −11.5 ± 1.8** | −3.7 (−8.6, 1.2) | 0.14 |
| Diastolic | −5.7 ± 1.4* | −2.9 ± 1.3* | −2.7 (−6.6, 1.1) | 0.16 |
| Clinic pulse rate, beats/min | −0.4 ± 1.4 | −1.8 ± 1.3 | 1.4 (−2.4, 5.2) | 0.47 |
| Ambulatory BP recording | ||||
| 24‐h systolic BP, mm Hg | −5.7 ± 1.8* | −3.0 ± 1.6 | −2.7 (−7.7, 2.3) | 0.29 |
| 24‐h diastolic BP, mm Hg | −3.7 ± 1.3* | −2.0 ± 1.2 | −1.7 (−5.1, 1.8) | 0.34 |
| 24‐h pulse rate, beats/min | 0.8 ± 1.4 | 1.7 ± 1.3 | −0.8 (−4.7, 3.0) | 0.66 |
| Daytime systolic BP, mm Hg | −2.1 ± 2.1 | −1.1 ± 2.0 | −0.9 (−6.8, 4.9) | 0.75 |
| Daytime diastolic BP, mm Hg | −1.1 ± 1.6 | −0.7 ± 1.2 | −0.4 (−4.4, 3.5) | 0.84 |
| Daytime pulse rate, beats/min | 0.9 ± 1.6 | 2.1 ± 1.6 | −1.1 (−5.6, 3.3) | 0.61 |
| Nighttime systolic BP, mm Hg | −12.0 ± 2.2** | −5.4 ± 1.7* | −6.5 (−11.9, −1.1) | 0.02 |
| Nighttime diastolic BP, mm Hg | −7.8 ± 1.5** | −4.0 ± 1.4* | −3.7 (−7.7, 0.2) | 0.07 |
| Nighttime pulse rate, beats/min | 0.3 ± 1.8 | 1.9 ± 1.7 | −1.6 (−6.5, 3.3) | 0.52 |
| Night‐to‐day systolic BP ratio, % | −7.8 ± 1.4** | −2.9 ± 1.4* | −4.9 (−8.8, −1.0) | 0.01 |
| Night‐to‐day diastolic BP ratio, % | −8.8 ± 1.8** | −3.9 ± 1.7* | −4.9 (−9.8, −0.0001) | 0.049 |
Values are least square mean ± standard error, unless otherwise indicated.
BP, blood pressure; CI, confidence interval.
Significance of the difference from baseline,
P < 0.05,
P < 0.0001.
3.4. Treatment effects on Brachial‐ankle PWV
Brachial‐ankle PWV was significantly reduced in the valsartan/amlodipine (n = 59, −1.1 ± 0.3 m/s, P < 0.0001) but not nifedipine GITS group (n = 59, –0.5 ± 0.3 m/s, P = 0.06), with a between‐treatment difference of −0.6 m/s (95% CI −1.3 to 0.1 m/s, P = 0.10) in favor of the valsartan/amlodipine group (Figure 2).
Figure 2.
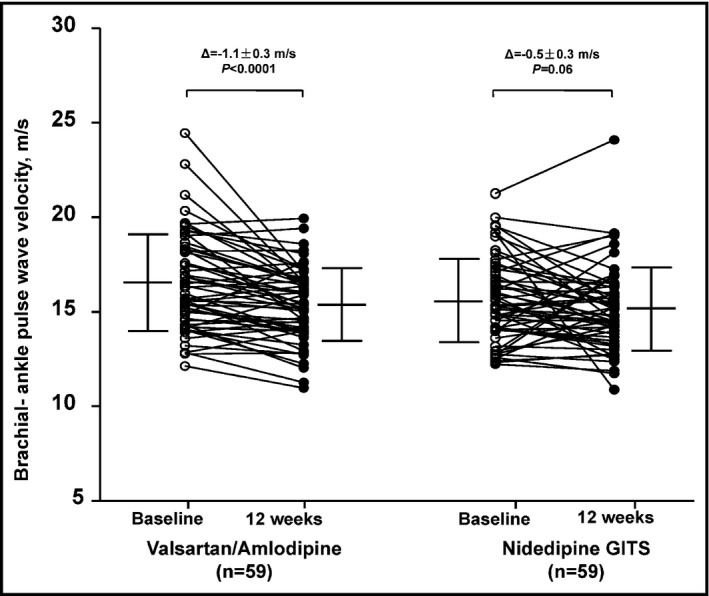
Changes in brachial‐ankle pulse wave velocity from baseline (circle) to 12 weeks of treatment (dot). Mean values are given alongside with standard deviation (vertical line). The least square mean change ± standard error and P values were adjusted for sex and the baseline age, body mass index, mean arterial pressure, pulse rate, and brachial‐ankle pulse wave velocity, and are given for each randomized group above the graph
Further analysis showed that the changes in brachial‐ankle PWV was significantly associated with the changes in ambulatory systolic blood pressure and pulse pressure in the nifedipine GITS group (P ≤ 0.0008) but not in the valsartan/amlodipine group (P ≥ 0.57), with a significant interaction between treatment assignment and treatment‐induced changes in ambulatory blood pressure (P ≤ 0.045, Figure 3).
Figure 3.
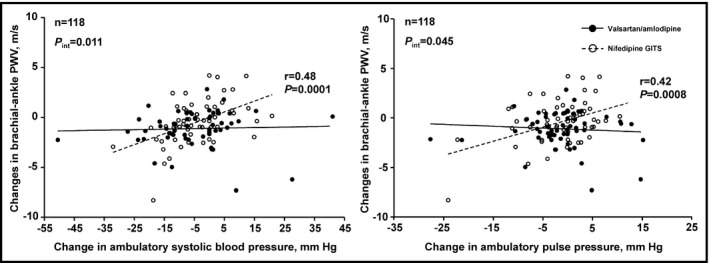
Scatter plot for the interrelationship of the changes in brachial‐ankle pulse wave velocity with the changes in ambulatory systolic blood pressure (left panel) and pulse pressure (right panel). Regression lines were drawn for the valsartan/amlodipine 80/5 mg combination group (dot) and the nifedipine GITS 30 mg group (circle) separately. Pearson correlation r values and P values are given for the regression line of the nifedipine GITS group (P) and for the interaction between randomized treatment and the changes in ambulatory systolic blood pressure or pulse pressure in relation to the changes in brachial‐ankle pulse wave velocity (P int)
3.5. Safety
The incidence rate of all adverse events was not statistically significant between the valsartan/amlodipine and nifedipine GITS groups, though quantitatively much higher in the nifedipine GITS monotherapy group (2.7% vs 8.0%, P = 0.27). Of particular note, symptomatic hypotension was not reported in either group, and the incidence rate of dizziness was similar in the valsartan/amlodipine and nifedipine GITS groups (1.3% vs 2.7%, P = 0.99).
4. DISCUSSION
The key finding of our randomized multicenter study was twofold. First, the valsartan/amlodipine single‐pill combination was more efficacious than nifedipine GITS in lowering ambulatory blood pressure, particularly nighttime blood pressure in patients with sustained uncontrolled hypertension or non‐dippers by approximately 5.0 to 6.5/3.7 to 4.1 mm Hg. Second, the single‐pill combination lowered brachial‐ankle PWV more than nifedipine GITS by 0.6 m/s, over and beyond its blood pressure lowering effect.
Our finding on the preferential nocturnal blood pressure lowering of the valsartan/amlodipine single‐pill combination was observed in a Turkish study in non‐dippers,15 but not in two other previous studies in patients with clinic hypertension16 or ambulatory daytime hypertension.17 These previous studies similarly compared the valsartan/amlodipine single‐pill combination with a long‐acting calcium‐channel blocker (amlodipine) monotherapy.15, 16 In the 8‐week Turkish study in non‐dipping (<10% nocturnal blood pressure fall) hypertensive patients (clinic blood pressure ≥140/90 mm Hg and ambulatory blood pressure ≥130/80, 135/85 and/or 120/70 mm Hg over 24 hours, daytime or nighttime), the valsartan/amlodipine 160/5 mg single‐pill combination (n = 48), compared with amlodipine 10 mg per day (n = 47), reduced 24‐hour and nighttime systolic blood pressure by 2.0 and 3.5 mm Hg, respectively.15
In two other studies, however, daytime and nighttime blood pressures were similarly reduced more by the valsartan/amlodipine single‐pill combination, in comparison with amlodipine monotherapy.16, 17 In the ambulatory blood pressure monitoring sub‐study (n = 82) of an 8‐week, randomized, double‐blinded trial in Asian hypertensive patients (n = 696), the valsartan/amlodipine 80/5 mg combination therapy, compared with amlodipine 5 mg per day, significantly reduced 24‐hour, daytime and nighttime ambulatory systolic/diastolic blood pressure by 7.1/6.6 mm Hg, 7.2/6.8 mm Hg and 6.3/6.0 mm Hg, respectively.16 In a larger (n = 211) 8‐week, open‐label, randomized study in Korean patients with daytime ambulatory hypertension (daytime systolic/diastolic blood pressure ≥135/85 mm Hg), the valsartan/amlodipine 160/5 mg combination therapy, compared with amlodipine 10 mg per day, significantly reduced 24‐hour, daytime and nighttime ambulatory systolic/diastolic blood pressure by 5.5/4.4, 5.1/4.0, and 4.1/2.0 mm Hg, respectively.17
Taken the results of our study and these previous studies together, the preferential nighttime blood pressure reduction of the valsartan/amlodipine single‐pill combination may have several possible explanations. First, because of increased treatment intensity, the combination therapy is more efficacious than monotherapy in reducing blood pressure, whenever blood pressure is elevated, regardless of sustained hypertension or nocturnal non‐dipping. Second, nifedipine GITS 30 mg per day used in our study is insufficiently long‐acting in comparison with amlodipine 5 or 10 mg per day. Third, co‐administration of two drugs offered by the single‐pill combination may be clinically relevant in lowering nighttime blood pressure, especially when administered in the morning. In a randomized study, Fujiwara demonstrated that administration of the valsartan/amlodipine single‐pill combination in the morning was more efficacious than bedtime dosing in reducing nighttime blood pressure by 3.2 mm Hg.18 It is possible that co‐administration of a calcium‐channel blocker with an inhibitor of the renin‐angiotensin system reduces daytime blood pressure but does not compromise urinary sodium excretion in the daytime. According to the pressure‐natriuresis theory, blood pressure at night is therefore not required to elevate for urinary sodium excretion.19, 20
Our observation on the disproportionate blood pressure reductions from baseline between office and ambulatory measurements in white‐coat uncontrolled hypertension is in keeping with the results of previous studies with a much larger sample size.21, 22 In 251 white‐coat hypertensive patients enrolled in the European Lacidipine Study on Atherosclerosis (ELSA) and treated with either atenolol or lacidipine‐based antihypertensive regimen, office blood pressure was reduced similarly as those with sustained hypertension (n = 1670) by about 19/9 mm Hg, whereas ambulatory blood pressure did not change or even showed a slight increase.21 Similar results were observed in our previous study in 202 white‐coat hypertensive patients treated with either 2.5 mg or 5.0 mg of S‐(‐)‐amlodipine.22 The disproportionate changes between office and ambulatory measurements can be to some extent explained by the higher office and lower ambulatory blood pressure levels at baseline. However, dysregulation of blood pressure in white‐coat hypertension must also play a part, and requires further investigation.
Our observation on the treatment‐induced changes in brachial‐ankle PWV with the valsartan/amlodipine single‐pill combination was quantitatively similar to that was observed in a randomized study on carotid‐femoral PWV (1 m/s reduction from baseline).23 However, why valsartan/amlodipine combination reduces arterial stiffness independent of blood pressure lowering is incompletely understood. Several previous studies demonstrated that the angiotensin‐receptor blocker valsartan was more efficacious in reducing brachial‐ankle PWV in hypertensive patients than various calcium channel blockers, such as amlodipine,24 nifedipine coat‐core,25, 26 or cilnidipine.26
Our study has to be interpreted within the context of its limitations. First, the present analysis was performed in a subgroup of patients of a randomized controlled trial. Sample size estimation of this sub‐study was not performed. The participation in the study was restricted to those sites with measurement devices for both ambulatory blood pressure monitoring and brachial‐ankle PWV. Although all patients in the participating hospitals of the sub‐study were considered for inclusion in the sub‐study, and the randomization procedure was stratified for study sites, the possibility of selection bias cannot be entirely ruled out. Another source of selection bias was the high use of calcium‐channel blockers at baseline. Addition of an angiotensin‐receptor blocker valsartan on a calcium channel blocker in the combination therapy group might exert greater antihypertensive treatment effect in the non‐responders to calcium‐channel blockers than the continuation of a calcium‐channel blocker. Second, our sub‐study had a relatively small sample size and short follow‐up time. The non‐significant between‐treatment differences could be the consequence of inadequate power or short duration of treatment. Third, the relatively high proportion of white‐coat uncontrolled hypertension may have also diminished the between‐treatment differences in ambulatory blood pressure in the overall study population.
In conclusion, our study showed that valsartan/amlodipine 80/5 mg single‐pill combination therapy was more efficacious in lowering ambulatory blood pressure than nifedipine GITS 30 mg monotherapy in sustained uncontrolled hypertension and non‐dippers. Over and beyond its blood pressure lowering effect, the combination therapy of valsartan/amlodipine was also more efficacious in reducing arterial stiffness. Future studies should address whether the single‐pill combination of a calcium‐channel blocker with an inhibitor of the renin angiotensin system is superior to that of other drug classes. Such comparison trials are ongoing in several different ethnic populations in China, India, and South Africa (personal communications).
CONFLICT OF INTEREST
Ji‐Guang Wang was financially supported by grants from the National Natural Science Foundation of China (grants 81170245 and 91639203), Beijing, China and the Shanghai Commissions of Science and Technology (grant 15XD1503200) and Health and Family Planning (grant 15GWZK0802 and a special grant for “Leading Academics”), Shanghai, China, and reports receiving lecture and consulting fees from Astra‐Zeneca, Bayer, Daiichi‐Sankyo, Novartis, Omron, Pfizer, Sanofi, Servier, and Takeda. The other authors declare no conflicts of interest.
Supporting information
ACKNOWLEDGMENT
The study was financially supported by Novartis (Beijing, China). The authors gratefully acknowledge the voluntary contribution of the study subjects and the active participation of the investigators from the participating hospitals. The principal investigators are listed as follows in the descending order of the number of randomized patients for this ambulatory blood pressure monitoring sub‐study: Liang‐Long Chen (Fujian Medical University Affiliated Union Hospital, n = 51), Ji‐Guang Wang (Shanghai Ruijin Hospital, n = 38), Yong Huo (Peking University First Hospital, n = 31), Ming Yang (Beijing Fuxing Hospital, n = 23), and Yuqing Zhang (Beijing Fuwai Hospital, n = 19).
Xu S‐K, Huang Q‐F, Zeng W‐F, Sheng C‐S, Li Y, Wang J‐G. A randomized multicenter study on ambulatory blood pressure and arterial stiffness in patients treated with valsartan/amlodipine or nifedipine GITS. J Clin Hypertens. 2019;21:252–261. 10.1111/jch.13457
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269‐1324. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953‐2041. [DOI] [PubMed] [Google Scholar]
- 3. Machnicki G, Ong SH, Chen W, Wei ZJ, Kahler KH. Comparison of amlodipine/valsartan/hydrochlorothiazide single pill combination and free combination: adherence, persistence, healthcare utilization and costs. Curr Med Res Opin. 2015;31:2287‐2296. [DOI] [PubMed] [Google Scholar]
- 4. Kawalec P, Holko P, Stawowczyk E, Borowiec Ł, Filipiak KJ. Economic evaluation of single‐pill combination of indapamide and amlodipine in the treatment of arterial hypertension in the Polish setting. Kardiol Pol. 2015;73:768‐780. [DOI] [PubMed] [Google Scholar]
- 5. Fleig SV, Weger B, Haller H, Limbourg FP. Effectiveness of a fixed‐dose, single‐pill combination of perindopril and amlodipine in patients with hypertension: a non‐interventional study. Adv Ther. 2018;35:353‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Billecke SS, Marcovitz PA. Long‐term safety and efficacy of telmisartan/amlodipine single pill combination in the treatment of hypertension. Vasc Health Risk Manag. 2013;9:95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briasoulis A, Bakris G. Initial single‐pill combination therapy for cardiovascular risk factor management: it is not just convenience. J Hypertens. 2013;31:1537‐1538. [DOI] [PubMed] [Google Scholar]
- 8. Wang TD, Chen YH, Huang CH, Chen WJ, Chen MF. Bidirectional adherence changes and associated factors in patients switched from free combinations to equivalent single‐pill combinations of antihypertensive drugs. Hypertension. 2014;63:958‐967. [DOI] [PubMed] [Google Scholar]
- 9. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417‐2428. [DOI] [PubMed] [Google Scholar]
- 10. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895‐906. [DOI] [PubMed] [Google Scholar]
- 11. Poulter NR, Wedel H, Dahlöf B, et al. Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA). Lancet. 2005;366:907‐913. [DOI] [PubMed] [Google Scholar]
- 12. Wang JG, Zeng WF, He YS, et al. Valsartan/amlodipine compared to nifedipine GITS in patients with hypertension inadequately controlled by monotherapy. Adv Ther. 2013;30:771‐783. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821‐848. [DOI] [PubMed] [Google Scholar]
- 14. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359‐364. [DOI] [PubMed] [Google Scholar]
- 15. Ke Y, Zhu D, Hong H, et al. Efficacy and safety of a single‐pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin. 2010;26:1705‐1713. [DOI] [PubMed] [Google Scholar]
- 16. Erdogan D, Icli A, Aksoy F, et al. The effect of fixed‐dose combination of valsartan and amlodipine on nighttime blood pressure in patients with non‐dipper hypertension. Turk Kardiyol Dern Ars. 2016;44:404‐413. [DOI] [PubMed] [Google Scholar]
- 17. Sung J, Jeong JO, Kwon SU, et al. Valsartan 160 mg/amlodipine 5 mg combination therapy versus amlodipine 10 mg in hypertensive patients with inadequate response to amlodipine 5 mg monotherapy. Korean Circ J. 2016;46:222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujiwara T, Hoshide S, Yano Y, Kanegae H, Kario K. Comparison of morning vs bedtime administration of the combination of valsartan/amlodipine on nocturnal brachial and central blood pressure in patients with hypertension. J Clin Hypertens (Greenwich). 2017;19:1319‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science. 1991;252:1813‐1816. [DOI] [PubMed] [Google Scholar]
- 20. Zou J, Li Y, Yan CH, Wei FF, Zhang L, Wang JG. Blood pressure in relation to interactions between sodium dietary intake and renal handling. Hypertension. 2013;62:719‐725. [DOI] [PubMed] [Google Scholar]
- 21. Mancia G, Facchetti R, Parati G, Zanchetti A. Effect of long‐term antihypertensive treatment on white‐coat hypertension. Hypertension. 2014;64:1388‐1398. [DOI] [PubMed] [Google Scholar]
- 22. Chen Q, Huang QF, Kang YY, et al. Efficacy and tolerability of initial high vs low doses of S‐(‐)‐amlodipine in hypertension. J Clin Hypertens (Greenwich). 2017;19:973‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine‐valsartan combination decreases central systolic blood pressure more effectively than the amlodipine‐atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314‐1322. [DOI] [PubMed] [Google Scholar]
- 24. Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M. Effects of amlodipine and valsartan on vascular damage and ambulatory blood pressure in untreated hypertensive patients. J Hum Hypertens. 2006;20:787‐794. [DOI] [PubMed] [Google Scholar]
- 25. Munakata M, Nagasaki A, Nunokawa T, et al. Effects of valsartan and nifedipine coat‐core on systemic arterial stiffness in hypertensive patients. Am J Hypertens. 2004;17:1050‐1055. [DOI] [PubMed] [Google Scholar]
- 26. Takami T, Shigemasa M. Efficacy of various antihypertensive agents as evaluated by indices of vascular stiffness in elderly hypertensive patients. Hypertens Res. 2003;26:609‐614. [DOI] [PubMed] [Google Scholar]
- 27. Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


