Abstract
Insulin resistance (IR) plays a crucial role in the development of hypertension, so early recognition of IR is of substantial clinical importance for the management of hypertension. But traditional IR indexes are invasive, complex, and impractical. We aimed to evaluate the associations between three simple IR indexes and hypertension in different body mass index (BMI) categories. A total of 142 005 adults who did not take antihypertensive medication were included in this analysis. The ratio of triglycerides to high‐density lipoprotein cholesterol (TG/HDLc), the product of fasting triglycerides and glucose (TyG), and metabolic score for IR (METS‐IR) were calculated according to the corresponding formulas. The associations between them and hypertension were analyzed by logistic regression. Among the three indicators, only METS‐IR had positive correlations with blood pressure levels (all P < 0.001). After full adjustment, METS‐IR was significantly associated with hypertension in the normal BMI group but not in the elevated BMI group. The OR for hypertension in the normal BMI group in the highest quartile of METS‐IR was 2.884 (95% CI: 2.468‐3.369) in the total sample, 1.915 (95% CI: 1.614‐2.271) in females and 2.083 (95% CI: 1.717‐2.527) in males. Our findings indicate that METS‐IR, a simple and cost‐effective IR index, was strongly associated with hypertension in normal‐weight Chinese subjects. It could help monitor and manage hypertension in normal‐weight individuals.
Keywords: hypertension, insulin resistance, METS‐IR, TG/HDLc, TyG index
1. INTRODUCTION
Hypertension and type 2 diabetes (T2D), which frequently coexist in the same individual, have become the leading risk factors for global disease burden.1 Insulin resistance (IR) has been speculated to be a common aspect of the pathophysiology of T2D and hypertension. As early as 1966, hyperinsulinemia was observed in normoglycemic patients with hypertension.2 Much evidence shows that IR plays a crucial role in the development of hypertension.3, 4 Therefore, early and accurate recognition of IR is of substantial clinical importance for implementing preventive strategies and optimizing the management of hypertension. This is more important for normal‐weight individuals, whose metabolic abnormalities are more easily overlooked.
The most common direct method for measuring IR is the hyperinsulinemic/euglycemic clamp (HEC) technique, which is invasive, complex, and impractical.5 The homeostasis model assessment for IR (HOMA‐IR) index, the most widely used indirect method, is easily affected by the accuracy of insulin measurement and has poor reproducibility.6 Therefore, a more simple, accurate, and practical IR index is needed. In recent years, several non‐insulin‐based IR indexes, which can be calculated by some simple routine biochemical indicators, have been developed, such as the ratio of triglycerides to high‐density lipoprotein cholesterol (TG/HDLc), the product of fasting triglycerides and glucose (TyG), and the metabolic score for IR (METS‐IR).
Although some studies have investigated the correlations between these non‐insulin‐based IR indexes and hypertension,7, 8 their relationship has not been evaluated simultaneously in large samples, especially in different body mass index (BMI) categories. Therefore, the objective of the present study was to assess the associations between the three non‐insulin‐based IR indexes and hypertension in a large sample of Chinese adults in different BMI categories.
2. METHODS
2.1. Subjects
This study was based on the data of adults who received a routine physical examination between November 2015 and July 2018 in China. A total of 174 695 subjects had complete data. Because the three non‐insulin‐based IR indexes to be explored in this study contained fasting plasma glucose (FPG) and lipid parameters, to avoid the interference of medication, we excluded people who self‐reported the use of antihypertensive medication, lipid‐lowering agents, or hypoglycemic drugs. Finally, 142 005 adults were included in this study. Ethical approval was obtained from the local ethics committee.
2.2. Clinical measurement
Basic medical history and medication use were collected. Anthropometric indicators (height, weight, waist circumference [WC], hip circumference [HC]) were measured by well‐trained examiners. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were obtained three times on the right arm after at least 5 minutes of rest using an automatic blood pressure (BP) monitor (HEM‐1000; OMRON). The blood samples of subjects were collected after a minimum of 8 hours of overnight fasting. Serum levels of FPG, plasma uric acid (UA), total cholesterol (TC), TG, low‐density lipoprotein cholesterol (LDLc), and HDLc were determined by a biochemical autoanalyzer.
2.3. Definitions
Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.9 BMI was calculated as weight divided by the square of height. Elevated BMI was defined as a BMI ≥ 24 kg/m2, and normal BMI was defined as a BMI = 18.5‐23.9 kg/m2.10 WC divided by HC was the waist‐to‐hip ratio (WHR), and WC divided by height was the waist‐to‐height ratio (WHtR).11 Non‐insulin‐based IR indexes were calculated by the following formulas: TyG = Ln [fasting TG (mg/dL)*FPG (mg/dL)/2]12; TG/HDLc = TG divided by HDLc13; METS‐IR = Ln [(2*FPG) + TG]*BMI)/(Ln[HDLc]).14
2.4. Statistical analysis
Data are expressed as numbers (percentage) or means ± SD. Statistical analysis was performed using SPSS 18.0 (SPSS Inc). The independent sample t test and chi‐square test were used to compare continuous and categorical variables between groups, respectively. Partial correlation was applied to examine the correlation between BP levels and these non‐insulin‐based IR indexes, which were adjusted for age. Logistic regression analyses were applied to explore the associations of non‐insulin‐based IR indexes with hypertension. TG/HDLc, TyG, and METS‐IR were divided into four quartiles, and the lowest quartile was used as a reference. Age and smoking status were adjusted. P < 0.05 was considered statistically significant.
3. RESULTS
Table 1 shows the clinical characteristics of 142 005 participants without self‐reported use of antihypertensive medication, lipid‐lowering agents, or hypoglycemic drugs. In the normal BMI group, the proportion of hypertension was 9.3%, and it was 24.2% in the elevated BMI group. The subjects with hypertension in both the normal and elevated BMI groups were older and had higher BMI and WC, WHR, WHtR, heart rate, and METS‐IR than those without. The subjects with hypertension in the normal BMI group had lower LDLc, UA, and TyG; in the elevated BMI group, subjects with hypertension had higher HDLc and lower TG, TG/HDLc, and TyG.
Table 1.
Clinical characteristics of the participants according to blood pressure status and body mass index category
| Characteristics | Normal BMI | Elevated BMI | ||||
|---|---|---|---|---|---|---|
| Non‐hypertension | Hypertension | P values | Non‐hypertension | Hypertension | P values | |
| No., n (%) | 77 201 (90.7) | 7938 (9.3) | <0.001 | 43 132 (75.8) | 13 734 (24.2) | <0.001 |
| Age, year | 41.0 ± 11.1 | 50.0 ± 14.1 | <0.001 | 44.0 ± 10.9 | 48.6 ± 11.9 | <0.001 |
| Female, n (%) | 42 977 (55.7) | 2804 (35.3) | <0.001 | 10 599 (24.6) | 2425 (17.7) | <0.001 |
| BMI (kg/m2) | 21.2 ± 1.8 | 21.9 ± 1.6 | <0.001 | 26.3 ± 1.9 | 27.0 ± 2.3 | <0.001 |
| WC (cm) | 72.5 ± 6.8 | 75.9 ± 6.5 | <0.001 | 86.0 ± 6.9 | 88.5 ± 7.3 | <0.001 |
| WHR | 0.80 ± 0.05 | 0.83 ± 0.05 | <0.001 | 0.87 ± 0.05 | 0.89 ± 0.05 | <0.001 |
| WHtR | 0.43 ± 0.03 | 0.45 ± 0.03 | <0.001 | 0.51 ± 0.03 | 0.52 ± 0.04 | <0.001 |
| Heart rate (beats/min) | 71.6 ± 3.9 | 73.9 ± 4.1 | <0.001 | 75.3 ± 3.7 | 77.8 ± 4.5 | <0.001 |
| SBP (mm Hg) | 114.5 ± 11.9 | 145.3 ± 11.8 | <0.001 | 121.1 ± 10.4 | 158.4 ± 1433.6 | <0.001 |
| DBP (mm Hg) | 70.2 ± 8.6 | 88.8 ± 9.4 | <0.001 | 75.0 ± 8.0 | 91.4 ± 9.1 | <0.001 |
| FPG (mmol/L) | 5.73 ± 1.20 | 5.71 ± 1.19 | 0.198 | 5.70 ± 1.17 | 5.71 ± 1.23 | 0.790 |
| TC (mmol/L) | 4.77 ± 0.90 | 4.75 ± 0.89 | 0.199 | 4.74 ± 0.89 | 4.74 ± 0.88 | 0.680 |
| TG (mmol/L) | 1.55 ± 1.32 | 1.54 ± 1.33 | 0.509 | 1.51 ± 1.29 | 1.47 ± 1.21 | 0.013 |
| HDLc (mmol/L) | 1.48 ± 0.33 | 1.49 ± 0.34 | 0.091 | 1.49 ± 0.34 | 1.51 ± 0.34 | 0.001 |
| LDLc (mmol/L) | 2.70 ± 0.77 | 2.66 ± 0.74 | 0.001 | 2.68 ± 0.76 | 2.67 ± 0.75 | 0.251 |
| UA (μmol/L) | 343.3 ± 88.5 | 341.2 ± 89.6 | 0.046 | 339.1 ± 88.4 | 338.8 ± 88.8 | 0.681 |
| TG/HDLc | 1.21 ± 1.45 | 1.21 ± 1.37 | 1.000 | 1.17 ± 1.42 | 1.14 ± 1.35 | 0.032 |
| TyG | 8.66 ± 0.63 | 8.64 ± 0.63 | 0.049 | 8.62 ± 0.63 | 8.61 ± 0.62 | 0.015 |
| METS‐IR | 30.75 ± 3.81 | 31.75 ± 3.80 | <0.001 | 37.95 ± 4.41 | 38.88 ± 4.83 | <0.001 |
Normal BMI, BMI = 18.5‐23.9 kg/m2; Elevated BMI, BMI ≥ 28.0 kg/m2.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDLc, high‐density lipoprotein cholesterol; LDLc, low‐density lipoprotein cholesterol; METS‐IR, metabolic score for insulin resistance; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TyG, triglyceride and glucose index; UA, plasma uric acid; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
The three cut points of the quartiles of TG/HDLc, TyG, and METS‐IR in different groups are shown in Table S1. The changes in BP value by quartiles of TG/HDLc, TyG, and METS‐IR in the normal and elevated BMI groups are shown in Table 2. The SBP and DBP levels were significantly elevated from the lowest to the highest quartile of METS‐IR, but the same was not true of TG/HDLc or TyG. The correlations between BP values and the three non‐insulin‐based IR indexes are shown in Table 3. After controlling for age, only METS‐IR showed positive correlations with SBP and DBP in both the elevated and normal BMI groups (all P < 0.001).
Table 2.
The change of blood pressure level by quartiles of the three non‐insulin‐based IR indexes
| Variable | Normal BMI | Elevated BMI | ||
|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |
| TG/HDLc | ||||
| 1st Quartile | 117.5 ± 15.2 | 72.0 ± 10.3 | 127.4 ± 15.5 | 78.9 ± 11.0 |
| 2nd Quartile | 117.3 ± 14.8 | 71.8 ± 10.2 | 127.1 ± 15.3 | 79.0 ± 10.9 |
| 3rd Quartile | 117.3 ± 14.8 | 71.8 ± 10.1 | 127.1 ± 14.8 | 78.9 ± 10.7 |
| 4th Quartile | 117.3 ± 14.6 | 71.8 ± 10.1 | 127.0 ± 14.9 | 79.0 ± 10.7 |
| TyG | ||||
| 1st Quartile | 117.5 ± 15.2 | 71.9 ± 10.4 | 127.3 ± 15.4 | 78.9 ± 11.1 |
| 2nd Quartile | 117.4 ± 14.8 | 71.9 ± 10.2 | 127.1 ± 15.2 | 78.9 ± 10.9 |
| 3rd Quartile | 117.4 ± 14.9 | 71.9 ± 10.1 | 127.1 ± 17.6 | 78.9 ± 10.7 |
| 4th Quartile | 117.2 ± 14.6 | 71.9 ± 10.1 | 127.2 ± 14.2 | 79.1 ± 10.8 |
| METS‐IR | ||||
| 1st Quartile | 114.7 ± 14.5 | 70.3 ± 9.8 | 126.1 ± 16.8 | 76.7 ± 11.9 |
| 2nd Quartile | 118.0 ± 14.8a | 72.2 ± 10.2a | 124.7 ± 15.2a | 76.8 ± 10.7a |
| 3rd Quartile | 119.8 ± 14.8a | 73.4 ± 10.3a | 125.9 ± 15.1a | 78.1 ± 10.7a |
| 4th Quartile | 120.9 ± 14.7a | 74.0 ± 10.5a | 128.5 ± 15.0a | 80.0 ± 10.8a |
Normal BMI, BMI = 18.5‐23.9 kg/m2; Elevated BMI, BMI ≥ 28.0 kg/m2.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDLc, high‐density lipoprotein cholesterol; METS‐IR, metabolic score for insulin resistance; SBP, systolic blood pressure; TG, triglyceride; TyG, triglyceride and glucose index.
There is a significant increase in BP compared to the first quartile.
Table 3.
Partial correlations coefficients between the three non‐insulin‐based IR indexes and BP level
| Variable | Normal BMI | Elevated BMI | ||||
|---|---|---|---|---|---|---|
| Total | Female | Male | Total | Female | Male | |
| TG/HDLc | ||||||
| SBP | ||||||
| r | 0.002 | −0.005 | 0.004 | −0.010 | −0.014 | −0.018 |
| P values | 0.678 | 0.360 | 0.556 | 0.072 | 0.226 | 0.004 |
| DBP | ||||||
| r | 0.004 | 0.000 | 0.001 | −0.002 | −0.006 | −0.014 |
| P values | 0.362 | 0.938 | 0.827 | 0.688 | 0.588 | 0.018 |
| TyG | ||||||
| SBP | ||||||
| r | 0.001 | −0.009 | −0.001 | −0.006 | −0.016 | −0.020 |
| P values | 0.800 | 0.126 | 0.853 | 0.238 | 0.142 | 0.001 |
| DBP | ||||||
| r | 0.002 | −0.006 | −0.003 | 0.006 | −0.010 | −0.015 |
| P values | 0.588 | 0.321 | 0.688 | 0.290 | 0.369 | 0.015 |
| METS‐IR | ||||||
| SBP | ||||||
| r | 0.122 | 0.070 | 0.070 | 0.137 | 0.117 | 0.121 |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| DBP | ||||||
| r | 0.108 | 0.049 | 0.074 | 0.144 | 0.110 | 0.124 |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Normal BMI, BMI = 18.5‐23.9 kg/m2; Elevated BMI, BMI ≥ 28.0 kg/m2.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDLc, high‐density lipoprotein cholesterol; METS‐IR, metabolic score for insulin resistance; SBP, systolic blood pressure; TG, triglyceride; TyG, triglyceride and glucose index.
The proportions of hypertension by quartiles of TG/HDLc, TyG, and METS‐IR are shown in Figure 1. In the normal BMI group, the proportion of hypertension showed a significant increasing trend in ascending quartiles of METS‐IR, but TG/HDLc and TyG did not (Figure 1A). In the elevated BMI group, the proportion of hypertension did not show a significant increasing trend in ascending quartiles of TG/HDLc, TyG, or METS‐IR (Figure 1B).
Figure 1.
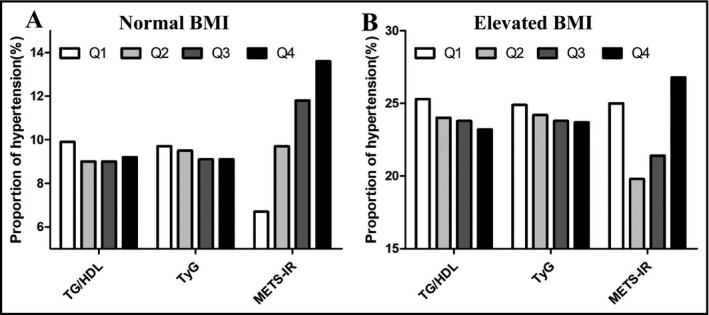
The proportion of hypertension by quartiles of the three non‐insulin‐based IR indexes. HDLc, high‐density lipoprotein cholesterol; METS‐IR, metabolic score for insulin resistance; Q, quartile; TG, triglycerides; TyG, TG and glucose index; Normal BMI, BMI = 18.5‐23.9 kg/m2; Elevated BMI, BMI ≥ 28.0 kg/m2
In the logistic regression analysis, the ORs for hypertension in the highest quartile of the three non‐insulin‐based IR indexes are shown in Figure 2, and the ORs in the second quartile and third quartile are shown in Table S2. After full adjustment, only METS‐IR was significantly associated with hypertension in the normal BMI group, irrespective of gender (Figure 2A), but METS‐IR also lost its significant association with hypertension in the elevated BMI group because of the wider OR span (Figure 2B). In the normal BMI group, the OR for hypertension in the highest quartile of METS‐IR was 2.884 (95% CI: 2.468‐3.369) in the total sample, 1.915 (95% CI: 1.614‐2.271) in females and 2.083 (95% CI: 1.717‐2.527) in males.
Figure 2.
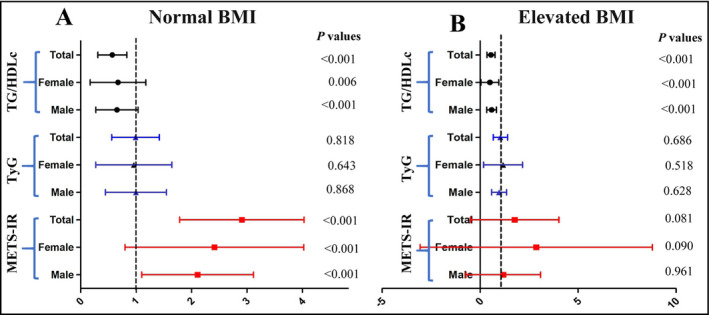
The ORs for hypertension in the highest quartiles of the three non‐insulin‐based IR indexes. HDLc, high‐density lipoprotein cholesterol; METS‐IR, metabolic score for insulin resistance; TG, triglycerides; TyG, TG and glucose index. Normal BMI, BMI = 18.5‐23.9 kg/m2; Elevated BMI, BMI ≥ 28.0 kg/m2
4. DISCUSSION
To the best of our knowledge, this is the first large‐scale cross‐sectional study to investigate the relationships between the three non‐insulin‐based IR indexes and hypertension and to compare the strengths of the associations between them and hypertension in different BMI categories. We found that METS‐IR outperformed the other two indexes and was positively associated with hypertension only in individuals with normal BMI.
Theoretically, IR is a core pathological feature of metabolic syndrome and a risk factor for the development of hypertension. IR‐compensatory hyperinsulinemia induces carotid body overactivation, leading to an increase in sympathetic nervous system activity, which can increase peripheral vascular resistance.15 IR can also promote BP elevation by activating the renin‐angiotensin‐aldosterone system and increasing the synthesis and release of endothelin.16, 17 Obesity is a significant risk factor for IR, so many individuals with normal BMI are often assumed not to have IR and to be metabolically healthy.18 However, a large number of people with normal BMI have IR and are metabolically unhealthy.19 Therefore, the recognition of IR, especially in normal‐weight individuals, is of great significance for the prevention and treatment of hypertension.
A wide variety of methods can be used to assess IR, including reference techniques and simple indexes, and each method has its own advantages and limitations.20 The HEC technique is the gold standard and frequently sampled intravenous glucose tolerance (FSIVGTT) the silver standard in estimating IR21 However, because they are costly, time‐consuming, invasive, and unphysiological methods, HEC and FSIVGTT are not appropriate in epidemiological studies. Some simple indexes of IR, such as HOMA‐IR and the quantitative insulin sensitivity check index, do not need the intravenous administration of exogenous insulin or glucose and are the most commonly used tools in clinical and epidemiological studies.22, 23 However, the calculation of these simple indexes requires an insulin assay, which is likely to cause significant bias. Factors affecting the insulin assay include the choice of kit, calibration setup in the kit, and conversions between units (mIU/L to pmol/L).21 One study showed that the HOMA2‐IR estimated by 11 insulin kits varied by up to twofold.24 To remedy the shortcomings of the aforementioned indicators, some non‐insulin‐based IR indexes have emerged. These non‐insulin‐based IR indexes only incorporate some simple biochemical or anthropometric indicators and do not require insulin values.
Although TG/HDLc is a useful surrogate of IR,25, 26 it varies according to sex and ethnicity.27, 28 The existing literature shows that TG/HDLc is associated with hypertension.29, 30 However, in this study, we did not find that TG/HDLc was significantly associated with hypertension, even in subjects with elevated BMI. The reason for the inconsistent results may be, on the one hand, the interethnic and sample size differences and, on the other hand, publication bias.
Since 2008, TyG has been compared to HOMA‐IR and HEC, and studies have suggested that TyG could be a surrogate for identifying IR12, 31 Subsequently, a series of clinical studies confirmed a strong association between TyG and T2D, metabolic syndrome, hypertension, and cardiometabolic diseases.32, 33 Zheng et al conducted a longitudinal study with 4686 subjects followed up for 9 years and demonstrated that TyG could predict incident hypertension among the Chinese population.34 A cross‐sectional study involving 1777 participants over 40 years old showed that TyG was associated with isolated systolic hypertension but not isolated diastolic hypertension.7 Unlike previous studies, the present study did not find a significant association between TyG and hypertension in obese or normal‐weight individuals. The basic characteristics of the selected populations may have caused the inconsistent results. For example, the previous study was a longitudinal study started in 2006. However, the past decade has been a period of rapid economic growth in China, and people's lifestyles have undergone tremendous changes. The spectra of weight, biochemical indicators, and BP in the Chinese have undergone corresponding changes over the past 10 years.
Metabolic score for IR is a novel surrogate of IR that incorporates conventional parameters (BMI, FPG, TG, HDLc) and demonstrates well consistent with EHC and FSIVGTT.13 To date, there has been no research on the correlation between METS‐IR and hypertension. A study comparing the ability of TyG and METS‐IR to identify metabolic syndrome demonstrated that the value of METS‐IR was unremarkable.33 In the present study, only METS‐IR was significantly associated with hypertension among the three non‐insulin‐based IR indexes. In addition, the close correlation between METS‐IR and hypertension was only apparent in normal‐weight individuals but not in overweight/obese subjects, which is very surprising to us because the METS‐IR's calculations include BMI.
This unexpected result also shows the need to think further about the role of IR in the increased BP, especially in different obese phenotypes. In nonobese individuals, the relationship between IR and hypertension is more direct and obvious. Some studies have provided genetic evidence that some groups of genetic variants indeed lead to higher visceral‐to‐subcutaneous adipose tissue ratios and fasting insulin, which can increase the risk of hypertension in the absence of elevated BMI.35 However, the connection between IR and obesity‐induced hypertension is not as straightforward.36 One experimental study demonstrated that obesity‐induced hypertension (mediated through α1‐ and/or β‐adrenoceptors) and obesity‐induced IR (mediated through α2‐adrenoceptors) are not directly linked.37
The strength of the present study is its relatively large sample size, which also makes the sample size of the normal‐weight group sufficient. The main limitation of this cross‐sectional study is that we cannot show a causal relationship between any of the three non‐insulin‐based IR indexes and hypertension, so we are also unable to determine whether the METS‐IR is a suitable predictive index of hypertension. Second, because the insulin assay was not included in routine physical examinations, we cannot assess how consistent METS‐IR is with HOMA‐IR in East Asians. Third, because the data in this study are from Chinese adults, the applicability of the findings to other ethnic groups is uncertain.
In conclusion, the results of the present study suggest that METS‐IR is significantly associated with hypertension in normal‐weight Chinese adults. Therefore, we propose that METS‐IR is a cost‐effective and simple index for the prevention and management of hypertension, especially for people with normal weight.
CONFLICT OF INTEREST
No conflicts of interest to disclose.
AUTHOR CONTRIBUTION
All authors were involved in developing the study concept and design, data acquisition, data management, and interpretation of results. XZL and JF wrote the manuscript. JF and SJP helped establish the database and undertook all the statistical analysis of the data. XSB involved in designing, editing, and review. All authors have approved the final version of this submission.
Supporting information
ACKNOWLEDGMENTS
In the preparation and implementation of this study, we get a lot of selfless help. All of our authors thank all those who have helped us, especially the help of Xiao Su Bai (People's Hospital of Longhua, Shenzhen) in the revision stage of the manuscript.
Liu XZ, Fan J, Pan SJ. METS‐IR, a novel simple insulin resistance indexes, is associated with hypertension in normal‐weight Chinese adults. J Clin Hypertens. 2019;21:1075–1081. 10.1111/jch.13591
Xing Zhen Liu and Jie Fan contributed equally to this work.
REFERENCES
- 1. GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welborn TA, Breckenridge A, Rubinstein AH, Dollery CT, Fraser TR. Serum‐insulin in essential hypertension and in peripheral vascular disease. Lancet. 1966;1(7451):1336‐1337. [DOI] [PubMed] [Google Scholar]
- 3. Sung KC, Lim S, Rosenson RS. Hyperinsulinemia and homeostasis model assessment of insulin resistance as predictors of hypertension: a 5‐year follow‐up study of Korean sample. Am J Hypertens. 2011;24(9):1041‐1045. [DOI] [PubMed] [Google Scholar]
- 4. Xun P, Liu K, Cao W, Sidney S, Williams OD, He K. Fasting insulin level is positively associated with incidence of hypertension among American young adults: a 20‐year follow‐up study. Diabetes Care. 2012;35(7):1532‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic‐euglycemic clamps. Diabetes Care. 2012;35(7):1605‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrillo‐Larco RM, Miranda JJ, Gilman RH, et al. The HOMA‐IR performance to identify new diabetes cases by degree of urbanization and altitude in Peru: the CRONICAS Cohort Study. J Diabetes Res. 2018;2018:7434918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jian S, Su‐Mei N, Xue C, Jie Z, Xue‐Sen W. Association and interaction between triglyceride‐glucose index and obesity on risk of hypertension in middle‐aged and elderly adults. Clin Exp Hypertens. 2017;39(8):732‐739. [DOI] [PubMed] [Google Scholar]
- 8. Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high‐density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. 2012;26(9):525‐532. [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV, Bakris GL, Black HR, et al The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 10. Li HH, Fan J, Huang S, Liu XZ. The prevalence of obesity and metabolic abnormalities in eastern China: a cross‐ sectional study. Int J Diabetes Dev Ctries. 2019. 10.1007/s13410-019-00725-2. [Epub ahead of print]. [DOI] [Google Scholar]
- 11. Li HH, Huang S, Liu XZ, Zou DJ. Applying the China‐PAR risk algorithm to assess 10‐year atherosclerotic cardiovascular disease risk in populations receiving routine physical examinations in Eastern China. Biomed Environ Sci. 2019;32(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 12. Simental‐Mendía LE, Rodríguez‐Morán M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299‐304. [DOI] [PubMed] [Google Scholar]
- 13. Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides×glucose versus triglyceride/high‐density lipoprotein cholesterol. Metabolism. 2011;60(12):1673‐1676. [DOI] [PubMed] [Google Scholar]
- 14. Bello‐Chavolla OY, Almeda‐Valdes P, Gomez‐Velasco D, et al. METS‐IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533‐544. [DOI] [PubMed] [Google Scholar]
- 15. Arauz‐Pacheco C, Lender D, Snell PG, et al. Relationship between insulin sensitivity, hyperinsulinemia, and insulin‐mediated sympathetic activation in normotensive and hypertensive subjects. Am J Hypertens. 1996;9(12 Pt 1):1172‐1178. [DOI] [PubMed] [Google Scholar]
- 16. Fonseca VA. Insulin resistance, diabetes, hypertension, and renin‐angiotensin system inhibition: reducing risk for cardiovascular disease. J Clin Hypertens. 2006;8(10):713‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khalil RA. Modulators of the vascular endothelin receptor in blood pressure regulation and hypertension. Curr Mol Pharmacol. 2011;4(3):176‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim SM, Choi DP, Rhee Y, Kim HC. Association between obesity indices and insulin resistance among healthy Korean Adolescents: the JS High School Study. PLoS ONE. 2015;10(5):e0125238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gómez‐Ambrosi J, Silva C, Galofré JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2012;36(2):286‐294. [DOI] [PubMed] [Google Scholar]
- 20. Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudvik A, Månsson M. Evaluation of surrogate measures of insulin sensitivity‐correlation with gold standard is not enough. BMC Med Res Methodol. 2018;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487‐1495. [DOI] [PubMed] [Google Scholar]
- 23. Ijzerman RG, Stehouwer CD, Serné EH, et al. Incorporation of the fasting free fatty acid concentration into quantitative insulin sensitivity check index improves its association with insulin sensitivity in adults, but not in children. Eur J Endocrinol. 2009;160(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 24. Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53(5):922‐932. [DOI] [PubMed] [Google Scholar]
- 25. Manley SE, Luzio SD, Stratton IM, Wallace TM, Clark PM. Preanalytical, analytical, and computational factors affect homeostasis model assessment estimates. Diabetes Care. 2008;31(9):1877‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giannini C, Santoro N, Caprio S, et al. The triglyceride‐to‐HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He J, He S, Liu K, Wang Y, Shi D, Chen X. The TG/HDL‐C ratio might be a surrogate for insulin resistance in Chinese Nonobese Women. Int J Endocrinol. 2014;2014:105168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim‐Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high‐density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59(2):299‐304. [DOI] [PubMed] [Google Scholar]
- 29. Kang B, Yang Y, Lee EY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes. 2017;41(5):789‐792. [DOI] [PubMed] [Google Scholar]
- 30. Sánchez‐Íñigo L, Navarro‐González D, Pastrana‐Delgado J, Fernández‐Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34(7):1257‐1265. [DOI] [PubMed] [Google Scholar]
- 31. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347‐3351. [DOI] [PubMed] [Google Scholar]
- 32. Wang B, Zhang M, Liu Y, et al. Utility of three novel insulin resistance‐related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J Diabetes. 2018;10(8):641‐652. [DOI] [PubMed] [Google Scholar]
- 33. Yu X, Wang L, Zhang W, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: a nationwide study. J Diabetes Investig. 2018. 10.1111/jdi.12975. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9‐year longitudinal population‐based study. Lipids Health Dis. 2017;16(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yaghootkar H, Scott RA, White CC, et al. Genetic evidence for a normal‐weight "metabolically obese" phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63(12):4369‐4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lytsy P, Ingelsson E, Lind L, Arnlöv J, Sundström J. Interplay of overweight and insulin resistance on hypertension development. J Hypertens. 2014;32(4):834‐839. [DOI] [PubMed] [Google Scholar]
- 37. Rocchini AP, Yang JQ, Gokee A. Hypertension and insulin resistance are not directly related in obese dogs. Hypertension. 2004;43(5):1011‐1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


