Abstract
Background:
Diagnosing heart failure with preserved ejection fraction (HFpEF) can be challenging. The H2FPEF and HFA-PEFF scores have recently been developed to estimate the likelihood that HFpEF is present among patients with unexplained dyspnea.
Objectives:
To describe characteristics and risk of adverse outcomes associated with each score among participants in the community with unexplained dyspnea.
Methods:
We studied 4,892 Atherosclerosis Risk in Communities (ARIC) participants aged 67–90 at Visit 5 (2011–2013) without other cardiopulmonary causes of dyspnea. We categorized participants as asymptomatic (76.6%), known HFpEF (10.3%), and tertiles of each score among those with ≥moderate, self-reported dyspnea (13.1%). H2FPEF≥6 and HFA-PEFF≥5
Results:
Mean age was 75±5 years, 58% were women, and 22% were black. After a mean follow-up of 5.3±1.2 years, rates (95% CI) of HF hospitalization or death per 1000 person-years for asymptomatic and known HFpEF were 20.7 (18.9–22.7) and 71.6 (61.6–83.3). Among 641 participants with unexplained dyspnea, rates were 27.7 [18.2–42.1], 44.9 [34.9–57.7], and 47.3 [36.5–61.3] (tertiles of H2FPEF-score); and 31.8 [20.3–49.9], 32.4 [23.4–44.9], and 54.3 [43.8–67.3] (tertiles of HFA-PEFF score). Participants with unexplained dyspnea and scores above the diagnostic threshold suggested for each algorithm, H2FPEF score ≥6 and HFA-PEFF score ≥5, had equivalent risk of HF hospitalization or death compared with known HFpEF. Among those with unexplained dyspnea, 28% had “discordant” findings (only high risk by 1 algorithm), while 4% were high risk by both.
Conclusions:
Participants with unexplained dyspnea and higher H2FPEF or HFA-PEFF scores face substantial risks of HF hospitalization or death. A significant fraction of patients are classified discordantly by using both algorithms.
Keywords: H(2)FPEF score, HFA-PEFF score, diagnosis, heart failure with preserved ejection fraction, hospitalization, mortality, prognosis, risk scores
CONDENSED ABSTRACT
We studied 4,892 community-based participants using the H2FPEF and HFA-PEFF algorithms, which non-invasively estimate the likelihood of HFpEF among patients with a clinical suspicion of possible HFpEF. For both diagnostic scores, higher values were associated with elevated risk of HF hospitalization or death. Suggested thresholds for each algorithm identified patients with unexplained dyspnea with equivalent risk to known HFpEF. The diagnostic scores discordantly classified risk in 28% of participants with unexplained dyspnea.
INTRODUCTION
Heart failure (HF) affects 6.2 million people in the United States alone and carries high risk of morbidity and mortality (1). Approximately 50–70% of HF patients have preserved ejection fraction (EF), and the proportion of HF with preserved EF (HFpEF) to HF with reduced EF (HFrEF) is growing (2). Factors contributing to the increase in HFpEF prevalence include an aging population, increasing prevalence of obesity, greater clinical recognition of HFpEF, and advancements in diagnostic modalities (3,4).
The ‘gold standard’ for HFpEF diagnosis has generally been considered to be presence of signs and symptoms of HF, a preserved left ventricular (LV) EF, and elevated LV diastolic pressure at rest or exercise by cardiac catheterization (5,6). However, invasive hemodynamic evaluation is neither universally available nor uniformly desirable in all patients presenting with possible HFpEF. 2D- and Doppler-echocardiography have been used to non-invasively estimate LV filling pressure and incorporated into diagnostic algorithms for HFpEF in guidelines, but suffer from limited ability to accurately identify HFpEF when compared to invasive exercise testing (7–9). In this setting, the H2FPEF score was developed to non-invasively estimate the likelihood of HFpEF in patients presenting with unexplained dyspnea using 6 clinical and echocardiographic characteristics (10). The H2FPEF score was derived from patients referred for invasive stress testing at a quaternary care center with expertise in HFpEF assessments. More recently, the European Society of Cardiology (ESC) has published a separate diagnostic algorithm (Heart Failure Association [HFA]-PEFF score), constructed by expert consensus and comprised of several echocardiographic parameters and natriuretic peptides. While these scores have individually been evaluated externally in small cohorts (11,12), they have yet to be studied and compared in a large community-based population.
The Atherosclerosis Risk in the Communities (ARIC) study is an ongoing community-based epidemiologic study with longitudinal data on cardiovascular co-morbidities, incident cardiovascular events, and comprehensive echocardiographic data. We compare clinical characteristics and incidence of HF hospitalization or death by H2FPEF and HFA-PEFF scores among ARIC participants in late-life self-reporting dyspnea without known common causes of dyspnea, participants not reporting dyspnea, and participants with known HFpEF.
METHODS
Study population
ARIC is an prospective study designed to evaluate the natural history of atherosclerosis risk factors in 4 communities across the United States (13) that recruited 15,792 men and women, aged 45–64 years between 1987 and 1989 (visit 1). Participants returned for visit 5 between June 2011 and August 2013, during which a broad range of clinical, laboratory, and comprehensive echocardiographic data were obtained. A description of comorbidity definitions, laboratory testing, dyspnea survey, and echocardiographic measurements are provided in the Supplementary Material. The institutional review board at each participating site approved the study protocol, and informed consent was obtained at each examination. A flow diagram of the current analysis is presented in Supplementary Figure 1. From the 6,538 participants attending visit 5, we analyzed participants who completed dyspnea survey data and had available parameters needed to calculate the H2FPEF and HFA-PEFF scores (N=5,466). We excluded participants with common causes of dyspnea: 1) cardiac etiologies including LVEF<50%, >mild left-sided valvular stenosis or >moderate regurgitation, 2) pulmonary etiologies including current asthma or chronic obstructive pulmonary disease, or 3) hemoglobin <10 g/dl (consistent with an exclusion criterion in a large recent HFpEF trial) (14). Our final cohort was comprised of 4,892 participants.
Prevalent HFpEF at visit 5 was defined by preserved EF (≥50%) and a combination of previously adjudicated HF events, HF hospitalization codes, and physician or patient self-reports of HF (in combination with other requirements) (see Supplementary Material).
H2FPEF Score
The H2FPEF score is a non-invasive scoring system developed to discriminate HFpEF from noncardiac causes of dyspnea defined by invasive hemodynamic assessment (10,15). The score ranges from 1–9 and provides an estimated probability for the diagnosis of HFpEF among with unexplained dyspnea. Weighted, binary variables included in the score are obesity (body mass index [BMI] ≥ 30 kg/m2), atrial fibrillation, age >60 years, treatment with ≥2 antihypertensives, echocardiographic E/e’ ratio >9, and echocardiographic estimated pulmonary artery systolic pressure >35 mm Hg. A total score of ≥6 points has been suggested as diagnostic of HFpEF, whereas a score of 0 or 1 is suggested to exclude the diagnosis (10). Since the velocity of the tricuspid regurgitation jet cannot be adequately measured in a proportion of participants, we assumed participants with missing velocity data did not have an elevated pulmonary artery systolic pressure, mimicking a “real world” application of the score. We also performed a sensitivity analysis where we only analyzed participants with complete data (N=2,825).
ESC HFA-PEFF Score
The HFA-PEFF score is a consensus recommendation for assessing possible HFpEF (8). After an initial work-up (Step 1), echocardiographic assessment in functional and morphologic domains as well as natriuretic peptide testing (with thresholds adjusted in the presence of atrial fibrillation) is performed and categorized into major and minor criteria. (Step 2). We restricted the calculation of the score to Step 2 of the algorithm, as we examined participants with unexplained dyspnea (Step 1), and Step 3 (functional testing) is not available in the ARIC study. A total score of ≥5 points is considered diagnostic of HFpEF, whereas a score of 0 or 1 is suggested to exclude the diagnosis.
Event Ascertainment
We assessed incidence of the composite of HF hospitalization or death and its individual components post-Visit 5. Quality control and adjudication of events have been previously presented (15). Incident HF events were initially identified using ICD discharge codes and HF events were subsequently adjudicated (16). Events were ascertained during follow-up visits and through annual calls to participants, ongoing surveillance of health department certificate files, and review of local hospital-discharge lists (with outcomes determined on the basis of ICD codes). In a sensitivity analysis, we examined the risk for HFpEF specific hospitalization. Of the 233 HF hospitalizations, 85 are HFpEF, 75 are HFrEF, and the remainder are unclassified or indeterminate. Death was ascertained through linkage with the National Death Index. Follow-up is complete through December 31, 2017 and remaining participants were administratively censored after this date.
Statistical analysis
For each algorithm, we divided the cohort into 5 groups: participants free of HF and not reporting dyspnea, known (diagnosed) HFpEF, and tertiles of the diagnostic score among those with undifferentiated dyspnea. Baseline characteristics were summarized using descriptive statistics for continuous variables and counts and percentages for categorical variables. We employed Cox proportional hazard models and Harrell’s C-statistics to assess the association of group assignment with outcomes post-Visit 5. Asymptomatic participants served as the reference group.
Since each score suggests a threshold for the diagnosis of HFpEF (≥6 for H2FPEF and ≥5 for HFA-PEFF), we repeated our analyses using clinical categories for each algorithm. These four groups included asymptomatic participants, known HFpEF, those with unexplained dyspnea and diagnostic score for HFpEF, and those with unexplained dyspnea and low and intermediate risk (non-diagnostic) scores. Low and intermediate risk participants were pooled given few participants in the low risk category in each algorithm.
As the risk of HFpEF is continuous, we also assessed for linear or nonlinear association between each diagnostic score and incident HF hospitalization or death using incidence rate splines, with the optimal number of knots determined as the one that minimizes the model AIC (2–5 knots tested). These analyses demonstrated a linear association with outcomes. Analyses were performed using STATA version 14 (StataCorp LLC; College Station, TX, USA), and a two-sided p-value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
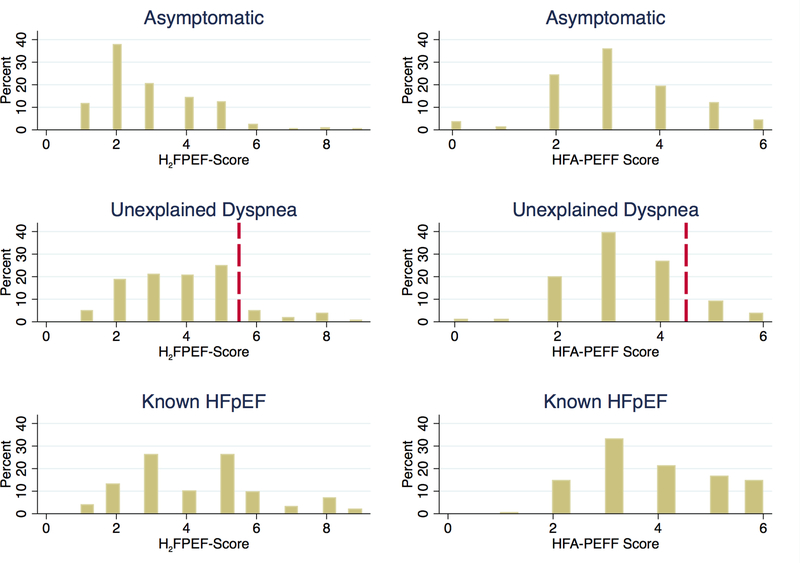
Tables 1 and 2 list the baseline characteristics of the 4,892 participants categorized into 5 study groups: dyspnea-free participants (76.6%), tertiles of diagnostic algorithm score among those with undifferentiated dyspnea (13.1%), and known HFpEF participants (10.3%). In the entire cohort, the mean age was 75±5 years, 58% were women, and 22% were black. The mean blood pressure was 130±18/66±10 mmHg, and BMI was 28.7±5.6 kg/m2. Figure 1 shows histograms of the H2FPEF and HFA-PEFF scores for dyspnea-free, symptomatic, and known HFpEF participants. The mean H2FPEF scores among these groups were 2.9±1.4, 3.9±1.6, and 4.3±1.9, respectively. Likewise, the mean HFA-PEFF scores were 3.2±1.3, 3.6±1.3, and 4.2±1.3, respectively. Using clinical cutoffs, 1.4% of all ARIC participants had unexplained dyspnea with H2FPEF score≥6, while 3.4% had unexplained dyspnea and HFA-PEFF≥5.
TABLE 1.
Clinical Characteristics of the Study Sample at Visit 5 by H2FPEF Score
| Asymptomatic N=3749 | Unexplained Dyspnea, H2FPEF Score 1–2 N=150 | Unexplained Dyspnea, H2FPEF Score 3–4 N=264 | Unexplained Dyspnea, H2FPEF Score ≥5 N=227 | Known HFpEF N=502 | P-value across Unexplained Dyspnea Groups | Unexplained Dyspnea, H2FPEF Score ≥5 vs. Known HFpEF P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 75.1 ± 5.0 | 77.3 ± 5.2 | 76.8 ± 5.3 | 75.4 ± 5.0 | 76.6 ± 5.5 | < 0.001 | 0.005 |
| Women, n (%) | 2160 (57.6%) | 95 (63.3%) | 174 (65.9%) | 164 (72.2%) | 252 (50.2%) | < 0.001 | < 0.001 |
| Black race, n (%) | 678 (18.1%) | 31 (20.7%) | 91 (34.5%) | 87 (38.3%) | 177 (35.3%) | < 0.001 | 0.42 |
| Physical Exam | |||||||
| Systolic blood pressure (mm Hg) | 130 ± 18 | 129 ± 16 | 132 ± 18 | 134 ± 20 | 131 ± 20 | 0.023 | 0.16 |
| Diastolic blood pressure (mm Hg) | 67 ± 10 | 65 ± 9 | 67 ± 11 | 67 ± 11 | 64 ± 11 | < 0.001 | < 0.001 |
| Heart rate (beats/minute) | 64 ± 11 | 66 ± 10 | 67 ± 12 | 66 ± 11 | 63 ± 11 | < 0.001 | < 0.001 |
| Body mass index (kg/m2) | 27.9 ± 4.8 | 26.0 ± 2.8 | 31.6 ± 7.2 | 35.9 ± 6.3 | 30.4 ± 6.8 | < 0.001 | < 0.001 |
| Comorbidities, n (%) | |||||||
| Hypertension | 2709 (70.9%) | 93 (62.0%) | 222 (82.5%) | 220 (92.1%) | 427 (86.6%) | < 0.001 | 0.06 |
| Diabetes mellitus | 1248 (32.4%) | 57 (37.5%) | 113 (41.7%) | 149 (62.1%) | 286 (57.1%) | < 0.001 | 0.23 |
| Coronary heart disease | 385 (10.2%) | 13 (8.8 %) | 37 (14.0%) | 31 (13.1%) | 225 (45.1%) | < 0.001 | < 0.001 |
| Current smoker | 190 (5.0 %) | 13 (8.6 %) | 25 (9.3 %) | 9 (3.8 %) | 25 (5.0 %) | 0.01 | 0.39 |
| Paroxysmal or permanent atrial fibrillation or flutter | 195 (5.1 %) | 0 (0.0 %) | 2 (0.7 %) | 62 (25.8%) | 110 (22.0%) | < 0.001 | 0.22 |
| Medication Use | |||||||
| Number of anti-hypertensive medications | 1.3±1.2 | 0.7±0.7 | 1.7±1.2 | 2.5±1.1 | 2.5±1.3 | <0.001 | 0.46 |
| Aspirin (n, %) | 2494 (66.7%) | 101 (67.3%) | 189 (71.6%) | 169 (74.4%) | 378 (75.3%) | <0.001 | 0.81 |
| Statin (n, %) | 1817 (48.6%) | 69 (46.0%) | 144 (54.5%) | 139 (61.2%) | 327 (65.1%) | <0.001 | 0.31 |
| Anticoagulation (n, %) | 164 (4.4 %) | 4 (2.7 %) | 12 (4.5 %) | 36 (15.9%) | 85 (16.9%) | <0.001 | 0.72 |
| Glucose lowering medication (n, %) | 594 (15.9%) | 20 (13.3%) | 65 (24.6%) | 100 (44.1%) | 155 (30.9%) | <0.001 | < 0.001 |
| Laboratory Testing | |||||||
| Hemoglobin (g/dL) | 13.5 ± 1.3 | 13.2 ± 1.3 | 13.2 ± 2.4 | 12.7 ± 1.4 | 13.1 ± 1.4 | < 0.001 | 0.004 |
| Hemoglobin A1c (%) | 5.9 ± 0.7 | 5.8 ± 0.6 | 6.0 ± 0.9 | 6.5 ± 1.3 | 6.1 ± 0.9 | < 0.001 | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 71 ± 16 | 71 ± 15 | 69 ± 18 | 66 ± 21 | 64 ± 20 | < 0.001 | 0.44 |
| High sensitivity C-reactive protein (mg/L)* | 1.79 [0.87 , 3.75] | 1.71 [0.78 , 3.63] | 2.24 [1.01 , 5.08] | 3.38 [1.79 , 6.83] | 2.55 [1.15 , 5.01] | < 0.001 | < 0.001 |
| N-terminal pro-B-type natriuretic peptide (ng/L)* | 109 [60 , 212] | 124 [77 , 235] | 134 [75 , 270] | 183 [83 , 488] | 264 [143 , 599] | < 0.001 | < 0.001 |
| High sensitivity cardiac troponin T (ng/L)* | 10 [7 , 14] | 10 [7 , 15] | 12 [8 , 18] | 13 [9 , 21] | 14 [9 , 23] | < 0.001 | 0.22 |
| Low density lipoprotein (mg/dL) | 107 ± 34 | 110 ± 35 | 101 ± 34 | 97 ± 33 | 96 ± 38 | < 0.001 | 0.63 |
| Spirometry | |||||||
| FEV1 (% predicted) | 96 ± 21 | 87 ± 29 | 91 ± 25 | 85 ± 21 | 89 ± 30 | < 0.001 | 0.2 |
| FEV1/FVC | 98 ± 12 | 92 ± 23 | 96 ± 14 | 98 ± 15 | 96 ± 17 | < 0.001 | 0.33 |
| Electrocardiography and Echocardiography | |||||||
| QRS duration (ms) | 95 ± 19 | 95 ± 20 | 96 ± 21 | 98 ± 23 | 103 ± 26 | < 0.001 | 0.021 |
| LV end-diastolic diameter (cm) | 4.4 ± 0.5 | 4.2 ± 0.5 | 4.4 ± 0.5 | 4.5 ± 0.5 | 4.6 ± 0.6 | < 0.001 | 0.19 |
| LV end-diastolic volume (mL) | 80.5 ± 22.9 | 73.7 ± 19.1 | 78.0 ± 19.3 | 86.3 ± 22.9 | 90.1 ± 28.6 | < 0.001 | 0.09 |
| Mean LV wall thickness (cm) | 0.97 ± 0.13 | 0.98 ± 0.15 | 1.01 ± 0.13 | 1.07 ± 0.16 | 1.05 ± 0.16 | < 0.001 | 0.09 |
| LV mass/height2.7(g/m2) | 36 ± 9 | 35 ± 8 | 40 ± 11 | 45 ± 12 | 43 ± 12 | < 0.001 | 0.08 |
| LV ejection fraction (%) | 66 ± 5 | 66 ± 6 | 66 ± 6 | 65 ± 5 | 64 ± 7 | < 0.001 | 0.017 |
| Average LV global longitudinal strain (%) | −18.2 ± 2.3 | −18.4 ± 2.2 | −17.8 ± 2.3 | −17.5 ± 3.0 | −16.9 ± 2.9 | < 0.001 | 0.02 |
| Left atrial volume (mL) | 47 ± 17 | 42 ± 13 | 48 ± 15 | 59 ± 19 | 58 ± 22 | < 0.001 | 0.52 |
| Left atrial volume index (mL/m2) | 25 ± 8 | 24 ± 7 | 26 ± 8 | 30 ± 10 | 31 ± 11 | < 0.001 | 0.30 |
| Tricuspid annular peak systolic velocity (cm/s) | 11.8 ± 2.8 | 12.1 ± 3.1 | 11.7 ± 3.3 | 11.4 ± 2.9 | 10.6 ± 3.0 | < 0.001 | < 0.001 |
| Peak tricuspid regurgitation velocity (cm/s) | 2.35 ± 0.26 | 2.31 ± 0.23 | 2.42 ± 0.27 | 2.53 ± 0.36 | 2.49 ± 0.37 | < 0.001 | 0.30 |
| E wave deceleration (ms) | 206 ± 45 | 206 ± 47 | 209 ± 48 | 202 ± 47 | 207 ± 54 | 0.49 | 0.23 |
| E-a ratio | 0.86 ± 0.27 | 0.79 ± 0.23 | 0.80 ± 0.23 | 0.88 ± 0.35 | 0.90 ± 0.39 | < 0.001 | 0.49 |
| Septal e’ velocity (cm/s) | 5.79 ± 1.45 | 5.65 ± 1.49 | 5.40 ± 1.29 | 5.54 ± 1.50 | 5.39 ± 1.52 | < 0.001 | 0.21 |
| Septal E/e’ ratio | 11.9 ± 3.8 | 11.6 ± 4.1 | 12.6 ± 4.6 | 14.3 ± 4.9 | 14.6 ± 6.5 | < 0.001 | 0.65 |
HFpEF, heart failure with preserved ejection fraction; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LV, left ventricular.
Presented as median [25th–75th percentile]
Table 2.
Clinical Characteristics of the Study Sample at Visit 5 by ESC HFA-PEFF Score
| Asymptomatic N=3749 | Unexplained Dyspnea, HFA PEFF Score 0–2 N=117 | Unexplained Dyspnea, HFA PEFF Score 3 N=214 | Unexplained Dyspnea, HFA PEFF Score 4–6 N=310 | Known HFpEF N=502 | P-value across Unexplained Dyspnea Groups | Unexplained Dyspnea, HFA-PEFF Score ≥4 vs. Known HFpEF P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 75.1 ± 5.0 | 75.3 ± 4.7 | 75.6 ± 5.1 | 77.4 ± 5.3 | 76.6 ± 5.5 | < 0.001 | 0.05 |
| Women, n (%) | 2160 (57.6%) | 79 (67.5%) | 143 (66.8%) | 211 (68.1%) | 252 (50.2%) | < 0.001 | < 0.001 |
| Black race, n (%) | 678 (18.1%) | 47 (40.2%) | 91 (42.5%) | 71 (22.9%) | 177 (35.3%) | < 0.001 | < 0.001 |
| Physical Exam | |||||||
| Systolic blood pressure (mm Hg) | 130 ± 18 | 129 ± 16 | 131 ± 19 | 133 ± 19 | 131 ± 20 | 0.02 | 0.11 |
| Diastolic blood pressure (mm Hg) | 67 ± 10 | 67 ± 10 | 68 ± 10 | 66 ± 11 | 64 ± 11 | < 0.001 | 0.013 |
| Heart rate (beats/minute) | 64 ± 11 | 67 ± 11 | 69 ± 11 | 64 ± 10 | 63 ± 11 | < 0.001 | 0.28 |
| Body mass index (kg/m2) | 27.9 ± 4.8 | 31.8 ± 7.0 | 32.7 ± 7.4 | 31.2 ± 6.9 | 30.4 ± 6.8 | < 0.001 | 0.11 |
| Comorbidities, n (%) | |||||||
| Hypertension | 2630 (70.6%) | 88 (75.2%) | 172 (81.5%) | 254 (82.5%) | 426 (86.2%) | < 0.001 | 0.15 |
| Diabetes mellitus | 1226 (32.7%) | 56 (47.9%) | 109 (50.9%) | 141 (45.5%) | 286 (57.0%) | < 0.001 | 0.001 |
| Coronary heart disease | 376 (10.2%) | 9 (7.8 %) | 21 (10.1%) | 49 (16.1%) | 223 (44.7%) | < 0.001 | <0.001 |
| Current smoker | 186 (5.0 %) | 6 (5.1 %) | 16 (7.5 %) | 23 (7.5 %) | 25 (5.0 %) | 0.22 | 0.15 |
| Paroxysmal or permanent atrial fibrillation or flutter | 191 (5.1 %) | 10 (8.5 %) | 20 (9.3 %) | 32 (10.3%) | 112 (22.3%) | < 0.001 | <0.001 |
| Medication Use | |||||||
| Number of anti-hypertensive medications | 1.3±1.2 | 1.6±1.3 | 1.6±1.2 | 1.9±1.3 | 2.5±1.3 | < 0.001 | <0.001 |
| Aspirin (n, %) | 2494 (66.7%) | 78 (66.7%) | 149 (69.6%) | 232 (74.8%) | 378 (75.3%) | < 0.001 | 0.88 |
| Statin (n, %) | 1817 (48.6%) | 68 (58.1%) | 115 (53.7%) | 169 (54.5%) | 327 (65.1%) | < 0.001 | 0.003 |
| Anticoagulation (n, %) | 164 (4.4 %) | 9 (7.7 %) | 14 (6.5 %) | 29 (9.4 %) | 85 (16.9%) | < 0.001 | 0.003 |
| Glucose lowering medication (n, %) | 594 (15.9%) | 33 (28.2%) | 67 (31.3%) | 85 (27.4%) | 155 (30.9%) | < 0.001 | 0.29 |
| Laboratory Testing | |||||||
| Hemoglobin (g/dL) | 13.5 ± 1.3 | 13.1 ± 1.3 | 13.3 ± 2.6 | 12.8 ± 1.3 | 13.1 ± 1.4 | < 0.001 | 0.026 |
| Hemoglobin A1c (%) | 5.9 ± 0.7 | 6.2 ± 1.0 | 6.3 ± 1.0 | 6.1 ± 1.0 | 6.1 ± 0.9 | < 0.001 | 0.55 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 71 ± 16 | 75 ± 18 | 71 ± 17 | 64 ± 18 | 64 ± 20 | < 0.001 | 0.9 |
| High sensitivity C-reactive protein (mg/L)* | 1.79 [0.87 , 3.75] | 2.21 [1.06 , 4.94] | 2.50 [1.12 , 5.78] | 2.50 [1.10 , 5.34] | 2.55 [1.15 , 5.01] | < 0.001 | 0.94 |
| N-terminal pro-B-type natriuretic peptide (ng/L)* | 109 [60 , 212] | 76 [50 , 101] | 86 [41 , 129] | 285 [183 , 538] | 264 [143 , 599] | < 0.001 | 0.035 |
| High sensitivity cardiac troponin T (ng/L)* | 10 [7 , 14] | 10 [7 , 15] | 11 [8 , 17] | 13 [9 , 21] | 14 [9 , 23] | < 0.001 | 0.19 |
| Low density lipoprotein (mg/dL) | 107 ± 34 | 102 ± 34 | 105 ± 35 | 99 ± 34 | 96 ± 38 | < 0.001 | 0.17 |
| Spirometry | |||||||
| FEV1 (% predicted) | 96 ± 21 | 90 ± 26 | 89 ± 24 | 87 ± 25 | 89 ± 30 | < 0.001 | 0.47 |
| FEV1/FVC | 98 ± 12 | 96 ± 17 | 98 ± 15 | 95 ± 18 | 96 ± 17 | 0.001 | 0.29 |
| Electrocardiography and Echocardiography | |||||||
| QRS duration (ms) | 95 ± 19 | 96 ± 21 | 95 ± 21 | 97 ± 22 | 103 ± 26 | < 0.001 | 0.002 |
| LV end-diastolic diameter (cm) | 4.4 ± 0.5 | 4.5 ± 0.4 | 4.2 ± 0.5 | 4.4 ± 0.5 | 4.6 ± 0.6 | < 0.001 | <0.001 |
| LV end-diastolic volume (mL) | 80.5 ± 22.9 | 77.8 ± 19.8 | 78.7 ± 20.3 | 81.4 ± 22.1 | 90.1 ± 28.6 | < 0.001 | <0.001 |
| Mean LV wall thickness (cm) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.2 | < 0.001 | 0.77 |
| LV mass/height2.7(g/m2) | 36 ± 9 | 37 ± 8 | 40 ± 10 | 43 ± 13 | 43 ± 12 | < 0.001 | 0.48 |
| LV ejection fraction (%) | 66 ± 5 | 65 ± 7 | 66 ± 5 | 66 ± 6 | 64 ± 7 | < 0.001 | <0.001 |
| Average LV global longitudinal strain (%) | −18.2 ± 2.3 | −18.0 ± 2.2 | −17.8 ± 2.4 | −17.8 ± 2.8 | −16.9 ± 2.9 | < 0.001 | <0.001 |
| Left atrial volume (mL) | 47 ± 17 | 42 ± 10 | 45 ± 13 | 57 ± 20 | 58 ± 22 | < 0.001 | 0.58 |
| Left atrial volume index (mL/m2) | 25 ± 8 | 22 ± 4 | 23 ± 6 | 30 ± 10 | 31 ± 11 | < 0.001 | 0.86 |
| Tricuspid annular peak systolic velocity (cm/s) | 11.8 ± 2.8 | 11.8 ± 2.8 | 11.7 ± 3.1 | 11.7 ± 3.2 | 10.6 ± 3.0 | < 0.001 | <0.001 |
| Peak tricuspid regurgitation velocity (cm/s) | 2.35 ± 0.26 | 2.37 ± 0.30 | 2.36 ± 0.28 | 2.49 ± 0.31 | 2.49 ± 0.37 | < 0.001 | 0.93 |
| E wave deceleration (ms) | 206 ± 45 | 202 ± 48 | 208 ± 44 | 206 ± 50 | 207 ± 54 | 0.82 | 0.88 |
| E-a ratio | 0.86 ± 0.27 | 0.81 ± 0.22 | 0.76 ± 0.19 | 0.87 ± 0.34 | 0.90 ± 0.39 | < 0.001 | 0.29 |
| Septal e’ velocity (cm/s) | 5.79 ± 1.45 | 5.66 ± 1.36 | 5.45 ± 1.46 | 5.49 ± 1.41 | 5.39 ± 1.52 | < 0.001 | 0.34 |
| Septal E/e’ ratio | 11.9 ± 3.8 | 11.8 ± 3.1 | 12.5 ± 4.1 | 13.8 ± 5.4 | 14.6 ± 6.5 | < 0.001 | 0.07 |
ESC, European Society of Cardiology; HFpEF, heart failure with preserved ejection fraction; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LV, left ventricular.
Presented as median [25th–75th percentile]
Figure 1: Histogram of Diagnostic Scores by Symptoms.
Percent of participants at each H2FPEF (left) and HFA-PEFF (right) score shown for asymptomatic participants, participants with unexplained dyspnea, and established HFpEF participants. A reference line is drawn in the histograms of participants with unexplained dyspnea to indicate the cutoff above which “high risk” for HFpEF has been suggested. HFpEF, heart failure with preserved ejection fraction.
Comparison of H2FPEF and HFA-PEFF Tertiles Among Participants with Unexplained Dyspnea
Among the 641 participants with undifferentiated dyspnea, we analyzed characteristics not included in each diagnostic score associated with higher tertile. Higher H2FPEF-score tertile was associated with decreasing age, black race, diabetes mellitus, less frequent smoking, lower hemoglobin, worse renal function, higher C-reactive protein (CRP), higher NT-proBNP, and higher hs-TnT. Higher HFA-PEFF score was associated with increasing age, non-black race, lower heart rate, more coronary heart disease, lower hemoglobin, worse renal function, and higher hs-TnT.
On echocardiographic analysis, higher H2FPEF-score tertile was associated with typical findings in HFpEF, including greater LV mass index, larger left atrial volume, worse EF and global longitudinal strain, lower right ventricular S’ velocity, and higher E/A ratio.
Comparison of H2FPEF and HFA-PEFF Tertiles to Participants with Diagnosed HFpEF
We compared characteristics of participants with unexplained dyspnea in the highest tertile of both scores to those with known HFpEF. Notably, participants with unexplained dyspnea and highest probability tertile of HFpEF were more likely to be women compared to known HFpEF (72.9 vs 49.5%, p<0.001). These participants also had higher BMI than known HFpEF at most H2FPEF scores even though the H2FPEF score includes BMI (Supplementary Figure 2). Otherwise, these participants were younger, had lower prevalence of coronary artery disease, higher hemoglobin A1c, and higher CRP concentrations compared to known HFpEF (p<0.05 for all comparisons). Although they had lower NT-proBNP levels (183 [IQR 83–488] ng/L vs 264 [143–599] ng/L, p<0.001), there was no difference after adjusting for age, sex, race, BMI, and eGFR (p=0.07).
Likewise, comparing participants in the highest tertile of HFA-PEFF score to known HFpEF, the former were more likely to be women, less often black, have higher diastolic blood pressure, less diabetes mellitus, coronary heart disease, and atrial fibrillation and lower hemoglobin (p<0.001 for all comparisons). NT-proBNP concentrations were higher in the highest HFA-PEFF tertile compared to known HFpEF (285 [183–538] ng/L vs 258 [141–556] ng/L, p=0.04), though similar after adjusting for age, sex, race, BMI, and eGFR (p=0.08). Echocardiography revealed very similar profiles between participants in the highest tertile of either score and known HFpEF, though the participants in the highest H2FPEF-tertile and HFA-PEFF tertile had better LV strain and tricuspid annular peak systolic velocity compared to known HFpEF (Table 1 and Table 2).
Concordance and Discordance between H2FPEF and HFA-PEFF Scores
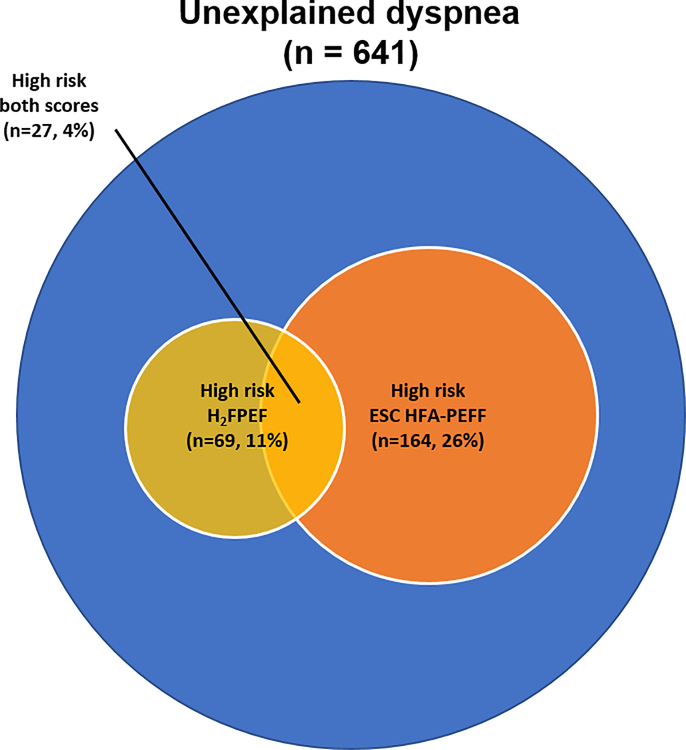
Figure 2 shows a Venn diagram demonstrating that 435 participants (68%) were considered non-high risk for both scores (H2FPEF<6 and HFA-PEFF<5), while 27 participants (4%) were considered “high risk” by both scores, and 179 participants (28%) had “discordant” findings (i.e. high risk by only one score). Participants with a “high H2FPEF, low HFA-PEFF-score” had higher heart rate and BMI, and more prevalent diabetes mellitus and atrial fibrillation. Those with “high HFA-PEFF, low H2FPEF-score” had greater NT-proBNP, LV end-diastolic diameter and E’ velocity, and lower peak tricuspid regurgitation velocity and E wave deceleration (Supplementary Table 1).
Figure 2: Venn Diagram of Participants with Unexplained Dyspnea by Diagnostic Scores.
Of the 641 participants with unexplained dyspnea, 435 participants were non-high risk by both scores (H2FPEF<6 and HFA-PEFF<5). By the H2FPEF score, 69 were “high risk” (≥6) and by the HFA-PEFF score, 164 were “high risk” (≥5). 27 participants were “high risk” by both scores.
Clinical Outcomes
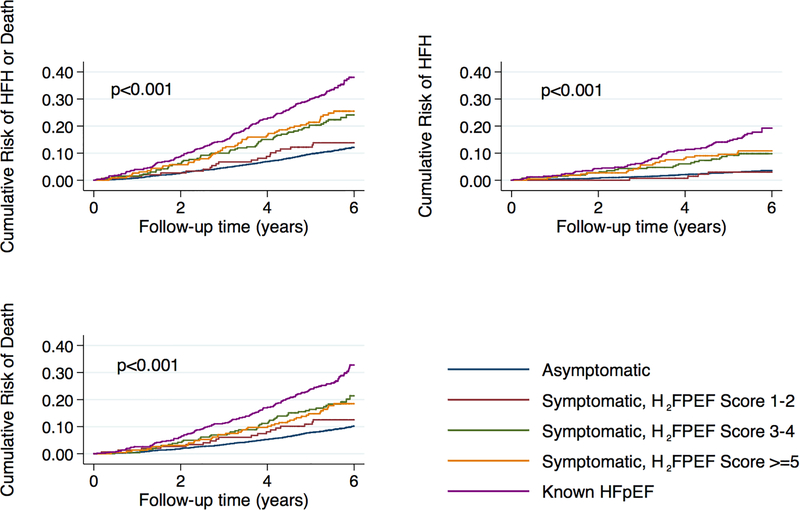
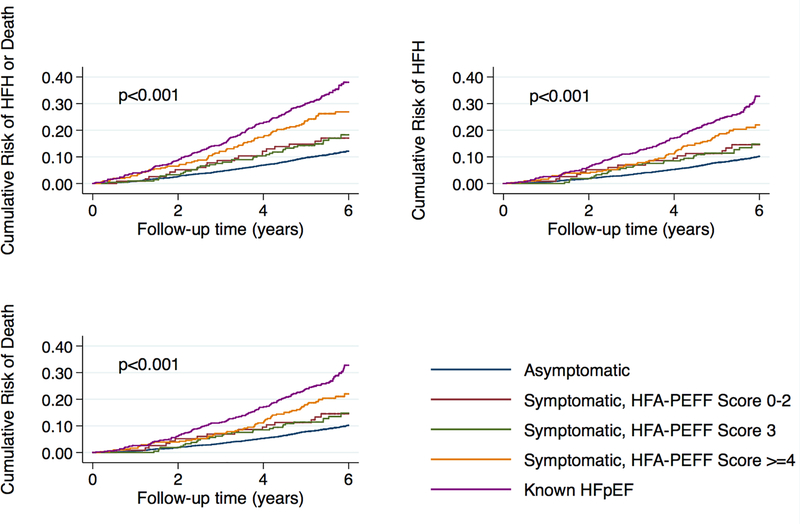
Tables 3 and 4 show incidence rates for HF hospitalization or death for the H2FPEF-score and HFA-PEFF score categories, respectively. After a mean follow-up of 5.3±1.2 years, 233 participants were admitted for HF and 596 died. Participants in both the middle and highest tertiles of H2FPEF-score demonstrated risk of HF hospitalization or death that was intermediate between dyspnea free participants and those with HFpEF (Figure 3). Risk among the lowest tertile of H2FPEF score was not significantly different from dyspnea-free participants. Sensitivity analysis excluding participants with missing pulmonary artery systolic pressure demonstrated similar findings (Supplementary Table 2). Using the HFA-PEFF score, risk was generally more graded from dyspnea free participants, to tertiles of HFA-PEFF score, to known HFpEF (Figure 4). We repeated our analyses using clinical categories as suggested by the algorithms: H2FPEF<6 and ≥6 and HFA-PEFF<5 and ≥5 (Tables 5 and 6; Supplementary Figure 3). Participants with unexplained dyspnea and H2FPEF or HFA-PEFF scores above these cutoffs had equivalent event rates (84.9 and 61.8 events per 1000 person-years, respectively) to those with known HFpEF (72.6 events per 1000 person-years) for the primary outcome (p=0.42 and 0.31, respectively). The C-statistics for incident HF for each component of the HFA-PEFF scores and H2FPEF scores are listed in Supplementary Table 3.
TABLE 3.
Event Rates for Adverse Cardiovascular Outcomes using H2FPEF Score Categories
| Outcomes, n (%) | Asymptomatic N=3749 |
Unexplained Dyspnea, H2FPEF Score 1–2 N=152 |
Unexplained Dyspnea, H2FPEF Score 3–4 N=271 |
Unexplained Dyspnea, H2FPEF Score ≥5 N=240 |
Known HFpEF N=502 |
|---|---|---|---|---|---|
| Heart failure hospitalization or death | |||||
| • Number of events | 416 | 21 | 62 | 55 | 172 |
| • Event rate (95% CI) per 1000 person-years | 20.7 (31.6, 45.8) | 26.8 (17.4, 41.0) | 47.1 (36.7, 60.4) | 48.4 (37.2, 63.1) | 72.6 (62.6, 84.3) |
| • Hazard ratio (95% CI) | ref | 1.30 (0.84, 2.01) | 2.31 (1.77, 3.02) | 2.38 (1.80, 3.16) | 3.62 (3.03, 4.32) |
| Heart failure hospitalization | |||||
| • Number of events | 119 | 4 | 25 | 22 | 63 |
| • Event rate (95% CI) per 1000 person-years | 5.9 (5.0, 7.1) | 5.1 (1.9, 13.6) | 19.1 (12.9, 28.2) | 19.6 (12.9, 29.8) | 32.4 (25.3, 41.5) |
| • Hazard ratio (95% CI) | ref | 0.86 (0.32, 2.34) | 3.26 (2.12, 5.02) | 3.37 (2.14, 5.31) | 5.59 (4.12, 7.59) |
| Death | |||||
| • Number of events | 343 | 19 | 52 | 39 | 143 |
| • Event rate (95% CI) per 1000 person-years | 16.9 (15.2, 18.8) | 24.1 (15.4, 37.7) | 38.4 (29.3, 50.4) | 32.9 (24.0, 45.0) | 58.0 (49.2, 68.3) |
| • Hazard ratio (95% CI) | Ref | 1.43 (0.90, 2.28) | 2.32 (1.73, 3.11) | 1.98 (1.42, 2.75) | 3.55 (2.92, 4.32) |
CI, confidence interval; HFpEF, heart failure with preserved ejection fraction.
TABLE 4.
Event Rates for Adverse Cardiovascular Outcomes using ESC HFA-PEFF Score Categories
| Outcomes, n (%) | Asymptomatic N=3749 |
Unexplained Dyspnea, HFA PEFF Score 0–2 N=117 |
Unexplained Dyspnea, HFA PEFF Score 3 N=214 |
Unexplained Dyspnea, HFA PEFF Score 4–6 N=310 |
Known HFpEF N=502 |
|---|---|---|---|---|---|
| Heart failure hospitalization or death | |||||
| • Number of events | 416 | 19 | 36 | 83 | 172 |
| • Event rate (95% CI) per 1000 person-years | 20.7 (31.6, 45.8) | 31.8 (20.3, 49.9) | 32.4 (23.4, 44.9) | 54.3 (43.8, 67.3) | 72.6 (62.6, 84.3) |
| • Hazard ratio (95% CI) | Ref | 1.56 (0.98, 2.47) | 1.58 (1.12, 2.22) | 2.67 (2.11, 3.38) | 3.62 (3.03, 4.32) |
| Heart failure hospitalization | |||||
| • Number of events | 119 | 4 | 10 | 37 | 63 |
| • Event rate (95% CI) per 1000 person-years | 5.9 (5.0, 7.1) | 6.8 (2.5, 18.0) | 9.1 (4.9, 16.9) | 24.3 (17.6, 33.5) | 32.4 (25.3, 41.5) |
| • Hazard ratio (95% CI) | ref | 1.16 (0.43, 3.13) | 1.54 (0.81, 2.94) | 4.17 (2.88, 6.03) | 5.59 (4.12, 7.59) |
| Death | |||||
| • Number of events | 343 | 16 | 29 | 65 | 143 |
| • Event rate (95% CI) per 1000 person-years | 16.9 (15.2, 18.8) | 26.4 (16.2, 43.1) | 25.7 (17.8, 36.9) | 40.8 (32.0, 52.1) | 58.0 (49.2, 68.3) |
| • Hazard ratio (95% CI) | Ref | 1.59 (0.96, 2.62) | 1.54 (1.05, 2.25) | 2.46 (1.88, 3.20) | 3.55 (2.92, 4.32) |
ESC, European Society of Cardiology; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction.
Figure 3: Cumulative Incidence Curves for Adverse Events by H2FPEF Score.
Cumulative incidence curves for each of the study outcomes by symptoms (unexplained dyspnea) and H2FPEF score at Visit 5: 1) asymptomatic; 2) symptomatic and H2FPEF score 1–2; 3) symptomatic and H2FPEF score 3–4; 4) symptomatic and H2FPEF-score ≥5; and 5) known HFpEF. P-value shown for log-rank test.
Figure 4: Cumulative Incidence Curves for Adverse Events by HFA-PEFF Score.
Cumulative incidence curves for each of the study outcomes by symptoms (unexplained dyspnea) and HFA-PEFF score at Visit 5: 1) asymptomatic; 2) symptomatic and HFA-PEFF score 0–2; 3) symptomatic and HFA-PEFF score 3; 4) symptomatic and HFA-PEFF score ≥4; and 5) known HFpEF. P-value shown for log-rank test.
TABLE 5.
Event Rates for Adverse Cardiovascular Outcomes using H2FPEF Score Categories
| Outcomes, n (%) | Asymptomatic N=3749 |
Unexplained Dyspnea, H2FPEF Score <6 N=572 |
Unexplained Dyspnea, H2FPEF Score ≥6 N=69 |
Known HFpEF N=502 |
|---|---|---|---|---|
| Heart failure hospitalization or death | ||||
| • Number of events | 416 | 111 | 27 | 172 |
| • Event rate (95% CI) per 1000 person-years | 20.7 (31.6, 45.8) | 38.0 (31.6, 45.8) | 84.9 (58.2, 123.7) | 72.6 (62.6, 84.3) |
| • Hazard ratio (95% CI) | ref | 1.86 (1.51, 2.29) | 4.27 (2.89, 6.31) | 3.62 (3.03, 4.32) |
| Heart failure hospitalization | ||||
| • Number of events | 119 | 40 | 11 | 63 |
| • Event rate (95% CI) per 1000 person-years | 5.9 (5.0, 7.1) | 13.7 (10.1, 18.7) | 36.0 (20.0, 65.1) | 32.4 (25.3, 41.5) |
| • Hazard ratio (95% CI) | ref | 2.34 (1.64, 3.35) | 6.32 (3.40, 11.72) | 5.59 (4.12, 7.59) |
| Death | ||||
| • Number of events | 343 | 89 | 21 | 143 |
| • Event rate (95% CI) per 1000 person-years | 16.9 (15.2, 18.8) | 29.8 (24.2, 36.7) | 61.9 (40.4, 94.9) | 58.0 (49.2, 68.3) |
| • Hazard ratio (95% CI) | Ref | 1.78 (1.41, 2.25) | 3.80 (2.45, 5.91) | 3.55 (2.92, 4.32) |
CI, confidence interval; HFpEF, heart failure with preserved ejection fraction.
TABLE 6.
Event Rates for Adverse Cardiovascular Outcomes using ESC HFA-PEFF Score Categories
| Outcomes, n (%) | Asymptomatic N=3749 |
Unexplained Dyspnea, HFA-PEFF Score <5 N=477 |
Unexplained Dyspnea, HFA-PEFF Score ≥5 N=164 |
Known HFpEF N=502 |
|---|---|---|---|---|
| Heart failure hospitalization or death | ||||
| • Number of events | 416 | 89 | 49 | 172 |
| • Event rate (95% CI) per 1000 person-years | 20.7 (31.6, 45.8) | 36.4 (29.6, 44.8) | 61.8 (46.7, 81.8) | 72.6 (62.6, 84.3) |
| • Hazard ratio (95% CI) | ref | 1.78 (1.41, 2.23) | 3.07 (2.28, 4.12) | 3.62 (3.03, 4.32) |
| Heart failure hospitalization | ||||
| • Number of events | 119 | 28 | 23 | 63 |
| • Event rate (95% CI) per 1000 person-years | 5.9 (5.0, 7.1) | 11.5 (8.0, 16.7) | 29.2 (19.4, 44.0) | 32.4 (25.3, 41.5) |
| • Hazard ratio (95% CI) | ref | 1.96 (1.30, 2.96) | 5.05 (3.23, 7.90) | 5.59 (4.12, 7.59) |
| Death | ||||
| • Number of events | 343 | 73 | 37 | 143 |
| • Event rate (95% CI) per 1000 person-years | 16.9 (15.2, 18.8) | 29.3 (23.3, 36.9) | 44.2 (32.0, 61.0) | 58.0 (49.2, 68.3) |
| • Hazard ratio (95% CI) | Ref | 1.76 (1.36, 2.26) | 2.68 (1.91, 3.77) | 3.55 (2.92, 4.32) |
ESC, European Society of Cardiology; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction.
We examined the risk of HFpEF specific hospitalization in a subgroup of individuals with available data (Supplementary Table 4). The risks for HFpEF hospitalization among those with unexplained dyspnea in the highest diagnostic score tertiles were equivalent to those with known HFpEF (H2FPEF algorithm HR 0.95, p=0.89, and HFA-PEFF algorithm HR 1.17, p=0.61, compared to known HFpEF).
Among the 641 participants with undifferentiated dyspnea, individuals with a non-high risk score by both algorithms had a lower risk of the primary outcome compared to both those with discordant findings and those with a high score by both algorithms (Supplementary Figure 4). While risk was higher among those with a high risk score by both algorithms, this was not statistically different than the risk of both discordant groups (p>0.05 for both).
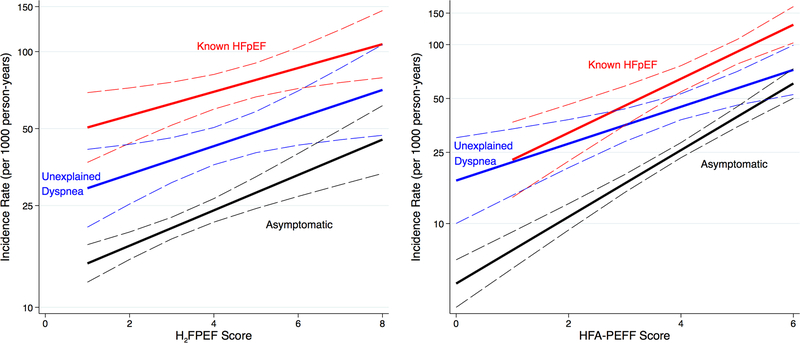
Figure 5 demonstrates the association between diagnostic score and the risk for the primary outcome for asymptomatic, symptomatic, and known HFpEF participants, showing a graded increase in all groups with higher scores. The overall relationship between H2FPEF score and the primary outcome (HR 1.25, 95%CI 1.20–1.30 per 1-unit score increase) was not modified by categorization as dyspnea free, undifferentiated dyspnea, and known HFpEF (interaction p=0.59). In contrast, the relationship between increasing HFA-PEFF score and the primary outcome was modified by this categorization, such that those with undifferentiated dyspnea had a lower hazard ratio per 1-unit increase in HFA-PEFF score (HR 1.28, 95%CI 1.12, 1.46) compared to the other two groups (HR 1.64, 95%CI 1.54 – 1.75) (interaction p=0.045).
Figure 5 (CENTRAL ILLUSTRATION). Incidence Rates for Heart Failure Hospitalization or Death by H2FPEF and HFA-PEFF Scores.
Incidence rates by H2FPEF and HFA-PEFF scores for the combined outcome for asymptomatic participants, participants with unexplained dyspnea without heart failure, and known HFpEF. HFpEF, heart failure with preserved ejection fraction.
DISCUSSION
In a large, epidemiologic study of community-dwelling older adults, we present the distribution of two recently published diagnostic algorithms for HFpEF with their associations to clinical characteristics and outcomes. Higher scores of both algorithms were associated with increased risk of incident HF hospitalization or death among those with unexplained dyspnea. Scores above both “diagnostic thresholds” identified participants with unexplained dyspnea at equivalent risk to those with known HFpEF. However, these scores associated differentially with clinical characteristics. Accordingly, 28% of participants are classified discordantly by these algorithms. Regardless, high risk designation by either algorithm was associated with elevated risk.
Participants with undifferentiated dyspnea and a high score of either algorithm were at a particularly high risk of incident HF hospitalization (and HFpEF specific hospitalization) but also all-cause death. Indeed, using clinical cutoffs for high risk designation, these participants faced equivalent risk for HF hospitalization or death compared to known HFpEF. In contrast, participants with undifferentiated dyspnea and a low score by either algorithm were at comparable risk to asymptomatic participants. The prognostic value for both scores is supported by their graded association with cTnT and NT-proBNP, and inversely with eGFR, all well-known prognostic factors in the general population (17). Still, dissociation between the diagnostic and prognostic value of the criteria existed. For example, risk did not differ substantially between those with a score of 3–4 and those with a score ≥5 in the H2FPEF algorithm, though the average H2FPEF scores in these two tertiles were similar (3.9 vs. 4.3). These data highlight that, despite their respective strengths, both algorithms are imperfect. Future algorithm development might benefit from incorporating prediction of future risk of meaningful clinical events, such as hospitalization or death.
Despite common prognostic utility, these algorithms unsurprisingly differentially associate with clinical characteristics, since the fundament for developing these algorithms are very different. The H2FPEF score was derived from characteristics modeled using invasive hemodynamic testing as the gold standard (10). In contrast, the HFA-PEFF algorithm was an Expert Consensus Recommendation (8). The HFA-PEFF algorithm is more complex than the 6-variable H2FPEF algorithm, and involves 9 echocardiographic variables (versus 2 for the H2FPEF algorithm) and NP testing. Still, both scores associate similarly with key echocardiographic measures of diastolic function and LV mass, and participants in the highest tertile of both algorithms had comparable echocardiographic findings to those with established HFpEF.
Atrial fibrillation plays a prominent role in HFpEF. The HFA-PEFF algorithm uses a higher threshold for NT-proBNP in patients with AF, while AF is heavily weighted (3 points) in the H2FPEF algorithm. Accordingly, likelihood of HFpEF in participants with AF will be downgraded by the HFA-PEFF score and upgraded by the H2FPEF score. Nearly all participants with AF and unexplained dyspnea were in the highest H2FPEF tertile with comparable prevalence to established HFpEF. In contrast, AF was evenly distributed across the HFA-PEFF score, and the highest category had lower prevalence than established HFpEF.
NP levels comprise an important component of the HFA-PEFF, but not H2FPEF algorithm, in which NP levels did not independently predict HFpEF (10). Expectedly, the HFA-PEFF score was more strongly associated with NPs compared to the H2FPEF score. As such, the participants in the highest HFA-PEFF tertile had even higher NT-proBNP concentrations than those with established HFpEF, while participants in the highest H2FPEF tertile had lower concentrations than established HFpEF (though similar when adjusted for confounding variables). There are indeed challenges in interpreting NPs in HFpEF. A subgroup with a particularly obese phenotype have been shown to have hemodynamic evidence of HFpEF despite lower NP concentrations (4). Still, many physicians rely on NP measurements in the diagnostic evaluation for HFpEF, highlighting the need for a ‘gold standard’ diagnostic reference for this disease (18).
Diagnosing HFpEF is rendered more challenging by the presence of several comorbidities that may confound the diagnosis or be alternative causes of symptoms, as well as lack of proven biomarker thresholds or echocardiographic parameters. The diagnosis can be even more difficult among outpatients without frank pulmonary edema or jugular distention, but more subtle and less specific symptoms and signs such as dyspnea on exertion and edema (19). Accordingly, classifying HFpEF in such patients varies across studies. The National Health and Nutrition Examination Survey (NHANES) determines the prevalence of HF based upon self-report, while other epidemiologic studies use hospitalization ICD codes or a version of the Framingham criteria. Although the Olmsted County Study used ICD codes from outpatient visits, in addition to hospital discharges (20), less epidemiologic data is available regarding HF diagnosed in the absence of hospitalization (5). Using the ARIC population, we found that higher score of both algorithms was associated with heightened risk for HF hospitalization or death among dyspnea-free persons, those with undifferentiated dyspnea, and those with known HFpEF. In addition, we identified important groups of individuals with dyspnea and without HFpEF diagnosis at high risk for adverse events, such as women and the obese. Importantly, our aim was not to validate these algorithms, as ARIC does not have the gold standard to diagnose HFpEF, but instead to illustrate the range and frequency of scores that would be found when applied to a community sample with undifferentiated dyspnea. Validation of the algorithms in a broader community-based sample, using patients with dyspnea where HFpEF or non-HFpEF status is determined definitively by invasive assessment, is needed prior to widespread application (11).
Study Limitations
Pulmonary artery systolic pressure was not available in roughly 40% of participants, and therefore we counted a missing value as absent pulmonary hypertension. This assumption likely underestimated H2FPEF scores. However, our approach is consistent with what clinicians would do with similar missing information when using the H2FPEF-score. In addition, we performed a complete case sensitivity analysis with similar results. Dyspnea symptoms were assessed through a validated questionnaire, though no clinical evaluation of the participants was performed nor was more comprehensive testing performed such as cardiopulmonary exercise testing. We cannot exclude that some participants with undifferentiated dyspnea may actually have an outpatient diagnosis of HF that was not reported to ARIC. However, we attempted to minimize this risk by employing an inclusive definition of prevalent HF that incorporates self-report. Next, while we used the ARIC recommended definition for prevalent HF at visit 5, this relies on self-reported historical features. Further, invasive hemodynamic characteristics of study participants in ARIC among those with HF or incident HF hospitalization are not uniformly available. Finally, we were limited by studying a predominantly older population presenting to visit 5 in ARIC. However, 80% of patients with HF are over the age of 65, similar to our population (1).
Conclusions
Among older adults in the community, higher H2FPEF- and HFA-PEFF scores predict heightened risk of incident HF hospitalization or death among patients without a clinical diagnosis of heart failure. Scores above both diagnostic thresholds identified participants with unexplained dyspnea at equivalent risk to those with known HFpEF. Participants with unexplained dyspnea and high score-based probability of HFpEF were more likely to be women and obese, but display similar cardiac structure and function abnormalities to those with previously diagnosed HFpEF. There are important differences in the clinical characteristics identified by each algorithm and a substantial fraction of participants are classified discordantly.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
In a community-based study using the H2FPEF and HFA-PEFF scores, participants with undifferentiated dyspnea and elevated scores were at heightened risk of HF hospitalization or death. Each algorithm highlighted different clinical characteristics associated with increasing score, and the two scores produced discordant findings in risk classification in 28% of participants.
Competency in Patient Care:
Each score can risk stratify participants with unexplained dyspnea, though employing both scoring systems might be synergistic. Clinicians should maintain a higher degree of suspicion in detecting HFpEF in populations with unexplained dyspnea in conjunction with these algorithms.
TRANSLATIONAL OUTLOOK.
Validation of the algorithms in a broader community-based sample, using patients with dyspnea where HFpEF or non-HFpEF status is determined definitively by invasive assessment, is needed.
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I and HHSN268201700005I). The work for this manuscript was also supported by NIH grants R01HL135008 and R01HL143224 (A.M.S.), and R01AG18915 (DWK).
Disclosures: Dr. Selvaraj is supported by the National Institutes of Health (Training Grant 5-T32HL007843-23). Dr. Myhre is supported by a postdoctoral research grant from the South-Eastern Norway Regional Health Authority and the University of Oslo, and has received consulting fees from Novartis and AmGen. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Bayer AG, and Baxter Healthcare, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. Dr. Shah reports research funding from the National Heart, Lung, and Blood Institute (R01HL135008 and R01HL143224) and Novartis, and consulting fees from Philips Ultrasound and Bellerophon. Dr. Borlaug has received research funding from the National Heart, Lung, and Blood Institute (R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262). Dr. Kitzman has received consulting fees from Novartis, Bayer, Merck, AstraZeneca, and Corvia. Dr. Solomon has received consulting fees from Novartis and Bayer and research grants from the National Heart, Lung, and Blood Institute. The other authors report no disclosures.
ABBREVIATIONS AND ACRONYMS
- ARIC
Atherosclerosis Risk in the Community
- BMI
Body mass index
- CRP
C-reactive protein
- CHARM
Candesartan in Heart failure Assessment of Reduction in Mortality and Morbidity
- CV
Coefficient of variation
- ESC
European Society of Cardiology
- HFA
Heart Failure Association
- HFpEF
Heart failure with preserved ejection fraction
- hs-cTnT
High sensitivity cardiac troponin T
- ICD-9
International Classification of Diseases-9
- LV
left ventricular
- ICD-9
Modified British Medical Research Council (mMRC)
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- NHANES
National Health and Nutrition Examination Survey
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial
- TAPSE
Tricuspid annular planar systolic excursion
Contributor Information
Senthil Selvaraj, Division of Cardiology, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA.
Peder L. Myhre, Division of Medicine, Akershus University Hospital and University of Oslo, Norway.
Muthiah Vaduganathan, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Brian L. Claggett, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Kunihiro Matsushita, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health; Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, MD.
Dalane W. Kitzman, Department of Internal Medicine, Sections on Cardiovascular Medicine and Geriatrics, Wake Forest School of Medicine, Winston-Salem, North Carolina.
Barry A. Borlaug, Division of Cardiology, Department of Medicine, Mayo Clinic, Rochester, MN.
Amil M. Shah, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Scott D. Solomon, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019:CIR0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 3.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013;10:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013. [Google Scholar]
- 7.Nagueh SF, Smiseth OA, Appleton CP et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 8.Pieske B, Tschope C, de Boer RA et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019. [DOI] [PubMed] [Google Scholar]
- 9.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepehrvand N, Alemayehu W, Dyck GJB et al. External Validation of the H2F-PEF Model in Diagnosing Patients With Heart Failure and Preserved Ejection Fraction. Circulation 2019;139:2377–2379. [DOI] [PubMed] [Google Scholar]
- 12.Aizpurua AB, Wijk SS, Rocca HB et al. Validation of the HFA-PEFF-score for the Diagnosis of Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail 2019. [DOI] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Solomon SD, Rizkala AR, Gong J et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC Heart Fail 2017;5:471–482. [DOI] [PubMed] [Google Scholar]
- 15.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–22. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chang PP, Baggett C et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nambi V, Liu X, Chambless LE et al. Troponin T and NT–Pro B-Type Natriuretic Peptide: A Biomarker Approach to Predict Heart Failure Risk—the Atherosclerosis Risk in Communities Study. Clinical chemistry 2013;59:1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myhre PL, Vaduganathan M, Greene SJ. Diagnosing heart failure with preserved ejection fraction in 2019: the search for a gold standard. Eur J Heart Fail 2019. [DOI] [PubMed] [Google Scholar]
- 19.Selvaraj S, Claggett B, Shah SJ et al. Utility of the Cardiovascular Physical Examination and Impact of Spironolactone in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2019;12:e006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber Y, Weston SA, Redfield MM et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.