Abstract
Migraine auras typically last for 5–60 min. An aura that persists for more than a week without evidence of infarction on neuroimaging is called persistent aura without infarction. Persistent migraine aura without infarction is usually described with visual auras. Herein, we are reporting a 24-year-old man who had an attack of a headache with diplopia, vertigo and tinnitus. Tinnitus and vertigo disappeared within 30 min. The headache also disappeared within 6 hours. However, diplopia and ophthalmoplegia persisted for 4 weeks. Secondary causes of bilateral ophthalmoplegia were ruled out by a proper history, clinical examinations and appropriate investigations. A trial with lamotrigine and sodium valproate led to the complete improvement in ophthalmoplegia within 2 weeks. We considered ophthalmoplegia in this patient as ‘persistent brainstem aura without infarction’. We suggest that a possibility of persistent migraine aura without infarction should be considered in all migraineurs who have unexplained and persistent neurological symptoms.
Keywords: headache (including migraines), neuro-opthalmology
Background
A migraine aura is classically defined as any neurological symptom that appears just before or during a migraine headache. Visual, sensory and speech auras are the main auras associated with migraine. However, the International Classification of Headache Disorders, third edition (ICHD-3) recognises a large number of neurological symptoms as migraine auras. Migraine auras classically persist for 5–60 min. However, it may last for months or years. The aura that persists for 1 week or longer without any evidence of infarction on neuroimaging is called persistent aura without infarction.1 Persistent migraine aura without infarction is typically described with visual auras.2 3 Herein, we are reporting a probable case of persistent aura without infarction presenting as bilateral ophthalmoplegia.
Case presentation
A 24-year-old man was referred to the department of neurology for the evaluation of persistent diplopia and external ophthalmoplegia of 4-week duration. The diplopia started suddenly. Initially, the patient noted difficulties in moving the right eye laterally, and diplopia was noted on looking to the right side. However, his ability to move both eyeballs gradually reduced; and within 1–2 hours, he noted difficulties in moving both eyes in all directions. The gaze was fixed forward and it remained as such for the next 4 weeks. He also noted mild ptosis in both eyes. However, there was no fluctuation or fatigability. There were not any bulbar symptoms, any limb weakness and any sensory complaints. He did not report any symptoms related to the bladder and bowel.
We interviewed and examined the patient 4 weeks after the onset of symptoms. There was a significant history of migraine headaches with aura since childhood. The auras were mainly visual and sensory. However, he never noted diplopia and ophthalmoplegia with headaches in the past. The frequency of headache attacks was about one to two attacks at 4-month intervals. The patient never received any preventive therapy for headaches.
On direct questioning, the patient recalled the presence of a headache, vertiginous sensation and tinnitus at the time of the onset of ophthalmoplegia. The headache was similar to previous migraine attacks and was predominantly located in the fronto-temporal region. It was throbbing, moderately severe and lasted for 6 hours. The patient noted nausea, photophobia and phonophobia with the headache. Vertiginous sensation and tinnitus were mild to moderate and disappeared within 30 min. Headaches, vertiginous sensations, and tinnitus were not severe enough and disappeared immediately, so he did not report these symptoms to the physicians. The previous physicians also did not ask about migraine headaches.
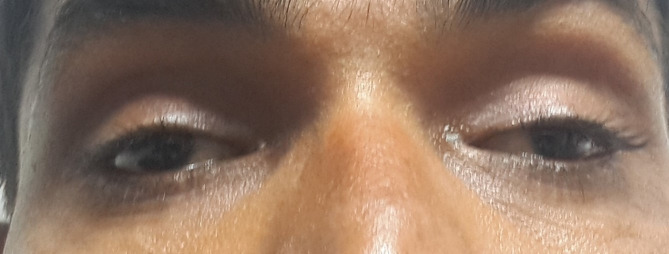
On clinical examination, we noted bilateral restriction of extraocular movements in all gazes. The gaze was fixed directly forward (figure 1). The patient did not move his eyes on command. Oculocephalic and oculovestibular reflexes were absent. There was mild bilateral ptosis. Bell’s phenomenon was absent. The pupils were 5 mm in diameter and reacting well to light. Visual acuity, colour vision and fundus were normal. Diplopia and Hess charting could not be plotted. All other physical and neurological examinations were normal (including normal deep tendon reflex, flexor plantar response, other cranial nerves and cerebellar examinations).
Figure 1.
The patient with fixed forward gaze. Mild bilateral ptosis was also noted.
Investigations
He was extensively investigated before reporting to us. All investigations were negative and these included complete blood count, serum glucose, kidney function tests, liver function tests, thyroid profiles (including thyroid antibodies), antinuclear antibody profiles, acetylcholine receptor antibodies, anti-GQ1b IgG antibody and chest X-ray. A cerebrospinal fluid (CSF) examination revealed no abnormality. A test for antibodies to muscle-specific serum kinase was not done. MRI of the brain and orbit (plain and contrast) did not reveal any abnormality.
A nerve conduction velocity test and electromyography examination did not suggest any potential cause for ophthalmoplegia. A repetitive nerve stimulation test, performed in facial and distal hand muscles, did not show any abnormality.
Differential diagnosis
Previous working diagnoses/differential diagnoses of the patient included myasthenia gravis, occult snakebite, botulism, Miller-Fisher syndrome and brainstem lesion. The neostigmine test was negative. He received empirical antisnake venom treatment for snakebite as a possibility of occult snakebite could not be ruled out.
Diplopia and ophthalmoplegia in patients with migraine have been described as a part of ophthalmoplegic migraine.4 However, the ICHD-3 classification system rejects the concept of ophthalmoplegic migraine and suggests that such symptoms are not migrainous but rather a recurrent painful neuropathy, so they changed the name of ophthalmoplegic migraine to recurrent painful ophthalmoplegic neuropathy (RPON).1 A possibility of RPON was less likely in our patient, as RPON usually presents with unilateral ophthalmoplegia. There are just a few case reports of RPON with bilateral ophthalmoplegia.5 Complete ophthalmoplegia is also rare with RPON.4 5 Moreover, the presence of vertiginous sensation and tinnitus is less likely with ophthalmoplegic neuropathy.
We reviewed the literature for the causes of bilateral ophthalmoplegia.6 7 Miller-Fisher syndrome and Guillain-Barre syndrome are the two main causes of bilateral ophthalmoplegia. Other common causes of bilateral ophthalmoplegia are brainstem lesions, neuromuscular junction (NMJ) disorders, cavernous sinuses pathologies and infections.6 7 The possibility of these disorders was ruled out by the proper clinical history, clinical examinations and appropriate investigations. A presence of vertiginous sensation and tinnitus is less likely with the pathologies of peripheral nerves, NMJ and muscle disorders. A possibility of brainstem pathology was more likely. However, structural brainstem lesions and other cranial pathologies were ruled out by neuroimaging and CSF examinations.
Diplopia with ophthalmoplegia is the most common aura in patients with migraine with brainstem aura.8 The other two common auras are vertigo and tinnitus.8 Both features were present in the beginning in our patient. ICHD-3 suggests that a typical aura persists for 5–60 min. Both vertigo and tinnitus disappeared within 30 min. However, diplopia and ophthalmoplegia persisted for 4 weeks. In one of the clinical observations, it has been demonstrated that about 26% of patients may have an aura for more than 1 hour.9 However, auras may present for months to years. An aura persisting for more than 1 week is labelled as persistent aura without infarction.1 We considered the ophthalmoplegia of our patient as a ‘persistent aura without infarction.’
Treatment
The patient received intravenous sodium valproate (500 mg 12 hourly) and lamotrigine (50 mg two times per day). The symptoms started to improve after 24 hours. The patient got a marked response in 2–3 days. He started to move both eyeballs in all directions. Ptosis disappeared. Injectable sodium valproate was switched to oral sodium valproate (500 mg two times per day). Lamotrigine was continued with the same dose. The patient was discharged with the advice to continue lamotrigine and sodium valproate. At the time of discharge, there was no ptosis. There was a slight restriction in the lateral gaze in both eyes.
Outcome and follow-up
The patient got complete relief in 2 weeks. Sodium valproate and lamotrigine were continued for the next 3 months. Two years later, he developed another episode of diplopia and ophthalmoplegia during an attack of a migraine headache. The attack persisted for about 2–3 hours and resolved spontaneously. He did not consult any physician at that time and reported to us after 1 week. Physical examinations did not reveal any abnormality.
Discussion
The migraine aura is defined as any neurological feature that appears just before or during the development of migraine headaches. A large number of neurological symptoms are recognised as migraine auras. Visual auras, sensory auras and auras related to speech/language are considered typical auras.
Although ICHD-3 has rejected the concept of ophthalmoplegic migraine, a few authors still debate on that.10 11 Moreover, ICHD-3 recognises diplopia in patients with migraine in relation to migraine with brainstem aura.1 Brainstem auras include dysarthria, vertigo, tinnitus, hypacusis, diplopia, ataxia and decreased level of consciousness.1 ICHD-3 recognises migraine with brainstem aura as a separate entity. However, there is a debate on whether it is a subtype of migraine with typical aura or a distinct phenotype or genotype. Kirchmann et al, in the largest observation on the basilar migraine, concluded that brainstem aura may occur at any time in patients with migraine with typical aura.12 ICHD-3 also considers a typical aura as an essential feature in patients with migraine with brainstem aura.1
Our case had a long history of migraine with typical aura. In the recent attack, he had a brainstem aura (diplopia, tinnitus and vertigo) (ICHD-3 code: 1.2.2). Tinnitus and vertigo persisted for only 30 min. However, diplopia and ophthalmoplegia persisted for 4 weeks. Our case fulfilled the ICHD-3 criteria of persistent aura without infarction (ICHD-3 code: 1.4.2). All other possible secondary causes were ruled out carefully. The response to lamotrigine and sodium valproate further reinforces the diagnosis of persistent aura without infarction. Lamotrigine is the most effective drug in patients with persistent aura without infarction.2 In a few cases, sodium valproate has also shown positive effects on the persistent visual aura without infarction.2 13
ICHD-3 mentions that ‘persistent aura symptoms are rare but well documented… and may last for months or years.’ However, the literature is mainly limited to visual auras. Thissen et al reviewed the literature and noted 47 cases with persistent aura without infarction.2 All cases are related to visual auras. There are few case reports where patients with persistent visual auras also had a persistent sensory aura (ie, two different types of persistent auras at the same time).2 13 To the best of our literature search, there is no case report of persistent aura without infarction describing the brainstem auras.
ICHD-3 recognises another group of auras, ‘a typical aura without headaches’ (ICHD code: 1.2.1.2). In this subgroup, patients will have various neurological symptoms, consistent with migraine aura but without headaches. About 20% of patients with migraine may experience various transient symptoms in the absence of any headache. It is suggested that migraine should be considered as an aetiology in any unexplained episodic symptoms.14 In the same way, we suggest that a possibility of migraine (persistent aura without infarction) should be considered in all migraineurs who have any unexplained and persistent neurological symptoms. A suspicion of persistent aura without infarction should be done only after excluding all other causes. Thissen et al rightly point out that ‘accurate history taking is the most important diagnostic tool’, as clinical examinations and investigations would be normal.2 A history targeting migraine and migraine auras in the past could be very important. In doubtful cases, a trial of lamotrigine with or without other drugs could be worthwhile.
The pathophysiology of persistent aura without infarction is in the speculative stage. An aura is explained by the mechanisms of cortical spreading depression (CSD). CSD classically starts in the occipital cortex and spreads to the other lobes and brainstem. However, it is presumed that CSD could start in any part of the brain. Depending on the involvement of various parts of the brain by CSD, the patients will have various symptoms. The abnormal excitability of various neurons has been suggested as a likely factor for the initiation of CSD. A few pathophysiological studies have been done in patients with persistent visual auras. It has been suggested that sustained cortical neuronal dysfunction could be the reason for persistent visual auras.15 16 We speculate a similar phenomenon for our patient in the brainstem neuron.
Limitation
Although all the possible causes described in the literature for bilateral ophthalmoplegia had been entertained before giving a trial of the antimigraine drugs, we cannot rule the possibility of other hidden secondary causes in this patient. There was a temporal relation between the administration of lamotrigine and sodium valproate and the improvement of the clinical features. However, it could be just a chance factor and improvement could be just because of spontaneous remission of the disease.
Conclusion
Just like the persistent visual aura, a patient may have persistent brainstem and other auras. We suggest that a possibility of persistent migraine aura without infarction should be considered in all migraineurs who have any unexplained and persistent neurological symptoms. A trial of lamotrigine and other antimigraine drugs should be taken in such patients.
Patient’s perspective.
I am surprised that it was because of migraine. Thanks to my doctor.
Learning points.
Just like the persistent visual aura, other auras may also persist for a longer period.
A patient having unexplained symptoms should be enquired about the history of migraine and auras in the past.
All migraineurs who have any unexplained and persistent neurological symptoms should receive a trial of lamotrigine and other antimigraine drugs.
Footnotes
Contributors: SP and AP were involved in the conception and design. SP and DL were involved in the acquisition of data. SP was involved in the manuscript preparation. AP and DL were involved in revising the draft for intellectual content. All authors approved the final version of this manuscript. SP was the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Olesen J. Headache classification Committee of the International headache Society (IHS) the International classification of headache disorders, 3rd edition. Cephalalgia 2018;38:1–211. 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 2.Thissen S, Vos IG, Schreuder TH, et al. Persistent migraine aura: new cases, a literature review, and ideas about pathophysiology. Headache 2014;54:1290–309. 10.1111/head.12392 [DOI] [PubMed] [Google Scholar]
- 3.San-Juan OD, Zermeño PF. Migraine with persistent aura in a Mexican patient: case report and review of the literature. Cephalalgia 2007;27:456–60. 10.1111/j.1468-2982.2007.01307.x [DOI] [PubMed] [Google Scholar]
- 4.Gelfand AA, Gelfand JM, Prabakhar P, et al. Ophthalmoplegic "migraine" or recurrent ophthalmoplegic cranial neuropathy: new cases and a systematic review. J Child Neurol 2012;27:759–66. 10.1177/0883073811426502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey R, Chakrabarty B, Saini L, et al. Bilateral ophthalmoplegia in a child with migraine. Brain Dev 2016;38:525–8. 10.1016/j.braindev.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 6.Keane JR. Acute bilateral ophthalmoplegia: 60 cases. Neurology 1986;36:279–81. 10.1212/WNL.36.2.279 [DOI] [PubMed] [Google Scholar]
- 7.Keane JR. Bilateral ocular paralysis: analysis of 31 inpatients. Arch Neurol 2007;64:178–80. 10.1001/archneur.64.2.178 [DOI] [PubMed] [Google Scholar]
- 8.Ying G, Fan W, Li N, et al. Clinical characteristics of basilar-type migraine in the neurological clinic of a university hospital. Pain Med 2014;15:1230–5. 10.1111/pme.12402 [DOI] [PubMed] [Google Scholar]
- 9.Viana M, Linde M, Sances G, et al. Migraine aura symptoms: duration, succession and temporal relationship to headache. Cephalalgia 2016;36:413–21. 10.1177/0333102415593089 [DOI] [PubMed] [Google Scholar]
- 10.Lal V, Caplan L. Are some ophthalmoplegias migrainous in origin? Neurol Clin Pract 2019;9:256–62. 10.1212/CPJ.0000000000000653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SV, Schuster NM. Relapsing painful ophthalmoplegic neuropathy: no longer a “migraine,” but still a headache. Curr Pain Headache Rep 2018;22:1–9. 10.1007/s11916-018-0705-5 [DOI] [PubMed] [Google Scholar]
- 12.Kirchmann M, Thomsen LL, Olesen J. Basilar-type migraine: clinical, epidemiologic, and genetic features. Neurology 2006;66:880–6. 10.1212/01.wnl.0000203647.48422.dd [DOI] [PubMed] [Google Scholar]
- 13.Rothrock JF. Successful treatment of persistent migraine aura with divalproex sodium. Neurology 1997;48:261–2. 10.1212/WNL.48.1.261 [DOI] [PubMed] [Google Scholar]
- 14.Prakash S, Rathore C, Makwana P, et al. Recurrent spontaneous paresthesia in the upper limb could be due to migraine: a case series. Headache 2015;55:1143–7. 10.1111/head.12568 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y-F, Fuh J-L, Chen W-T, et al. The visual aura rating scale as an outcome predictor for persistent visual aura without infarction. Cephalalgia 2008;28:1298–304. 10.1111/j.1468-2982.2008.01679.x [DOI] [PubMed] [Google Scholar]
- 16.Chen W-T, Lin Y-Y, Fuh J-L, et al. Sustained visual cortex hyperexcitability in migraine with persistent visual aura. Brain 2011;134:2387–95. 10.1093/brain/awr157 [DOI] [PubMed] [Google Scholar]