Abstract
Invasive group A Streptococcus (GAS) in immunocompetent individuals is largely linked to hypervirulent strains. Congenital immunodeficiencies and those acquired from chronic disease or immunosuppressant drugs also increase risk of severe illness. We recovered GAS from the blood of a patient receiving a biologic inhibitor of interleukin 6 (IL-6). Growth of this serotype M4 isolate in human blood or a murine bacteremia model was promoted by interleukin 1 or IL-6 inhibition. Hyperinvasive M1T1 GAS was unaffected by IL-6 in both models. These findings based on a natural experiment introduce IL-6 signaling deficiencies as a risk factor for invasive GAS.
Keywords: group A Streptococcus, sepsis, adverse events, IL-6, immunotherapy
In immunocompetent individuals, invasive infection by group A Streptococcus infection is linked to hypervirulent strains. We report that IL-6 immunosuppression can enhance the pathogenesis of lower virulence strains but not hypervirulent M1T1 clones, indicating that host and pathogen factors interplay in risk.
Group A Streptococcus (GAS; Streptococcus pyogenes) is a top cause of infectious mortality responsible for >500 000 annual deaths worldwide (reviewed in [1]). Necrotizing fasciitis, toxic shock syndrome, and other invasive GAS (iGAS) infections have been associated with a globally disseminated clone of serotype M1T1 that emerged in the early 1980s upon acquiring additional virulence determinants that antagonize immune signaling and innate antimicrobial defenses [2]. Host factors contributing to iGAS are largely unknown, but infections have been associated with the use of nonsteroidal anti-inflammatory drugs [3] and anakinra (recombinant human interleukin 1 [IL-1] receptor antagonist, IL-1Ra) [4]. These infectious adverse events suggest that specific proinflammatory signals are needed to coordinate an effective immune response to iGAS, leaving the possibility that other immunodeficiencies may increase the risk of infection.
The proinflammatory cytokine interleukin 6 (IL-6) is a key component of the acute phase response (reviewed in [5]). Elevated IL-6 contributes to inflammatory pathology in numerous chronic disorders including rheumatoid arthritis, Castleman disease, and juvenile idiopathic arthritis [5]. Inhibition of IL-6 signaling can be therapeutic in these diseases and may be accomplished with antibodies that block the IL-6 receptor (tocilizumab) or IL-6 itself (siltuximab). Primary adverse events observed during clinical trials were infections [6–9], and several case reports have since described necrotizing fasciitis caused by GAS in individuals taking IL-6 signaling inhibitors for the treatment of rheumatoid arthritis [10, 11]. Anticytokine drugs are often associated with infectious adverse events, for example, the association of Mycobacterium tuberculosis with tumor necrosis factor inhibitors [4], but the extent to which any particular pathogen is associated with any targeted pathway is uncertain.
This study examines an M4 iGAS isolate from a pediatric patient with systemic juvenile idiopathic arthritis who developed sepsis while under treatment with inhibitors of IL-1 and IL-6. The patient began immunotherapy with biologic IL-1 inhibitors. When IL-1 therapy failed, the patient was switched to IL-6 inhibitors, after which they developed sepsis. We show that anti–IL-6 immunotherapeutics promote iGAS, as previously observed with IL-1 inhibition. However, this effect is not extrapolated to hyperinvasive strains, which are already resistant to the effects of IL-6. Infectious adverse events are common with broad-action immunosuppressants, but highly targeted pharmaceuticals like biologics may have a more limited risk profile. Our results find these risks depend on the immune pathway targeted and intrinsic differences between bacterial strains.
METHODS
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. Blood was collected from healthy adult volunteers under informed consent and approved by the Institutional Review Board of Emory University. Animal experiments were approved by the Institutional Animal Care and Use Committees of the University of California, San Diego or Emory University.
Bacterial Characterization and Culture
Blood culture from the patient yielded colonies with β-hemolytic activity on 5% blood agar and confirmed to express the group A antigen by Streptex Rapid Latex Agglutination Test (Remel, Lenexa, Kansas). Genomic DNA was isolated with DNAzol (MRC) and the emm type identified by polymerase chain reaction amplification with primers targeting the emm hypervariable region (TATT(C/G)GCTTAGAAAATTAA and GCAAGTTCTTCAGCTTGTTT) and Sanger sequenced. The sequence was submitted to the Centers for Disease Control and Prevention Streptococci Group A Subtyping Blast Server and found to be a 100% match to the M4.0 serogroup. The strain was named M4(C20). Consistent with other M4 serotype GAS [12], M4(C20) produced no capsule when examined by hyaluronic acid enzyme-linked immunosorbent assay (Corgenix). All colonies cultured had the ability to hydrolyze azocasein, indicating that SpeB was functional and the covRS operon was not inactivated, as has been described for some isolates from invasive infections [4]. Bacteria were routinely grown in Todd–Hewitt broth with 5% yeast extract (Difco Laboratories) statically at 37°C and 5% carbon dioxide.
Animal Infection Model
Eight- to 10-week-old C57BL/6 (Jackson Laboratory) mice of both sexes were treated intravenously 4 hours preinfection with anakinra (Kineret; 50 mg/kg; inhibits both human and murine IL-1R1 [4]), or a monoclonal antibody targeting murine IL-6R (BioXcell; 50 mg/kg; tocilizumab does not inhibit murine IL-6R), or immunoglobulin G (IgG) isotype control (BioXcell; 50 mg/kg). IL6-/- mice (M. Karin) were not pretreated with any monoclonal antibodies. All mice were inoculated intravenously with 108 colony-forming units (CFUs) of either GAS strain 5448 or M4(C20). Mice were monitored for 5 days postinfection.
Ex Vivo Infection Models
Heparinized whole human blood treated with Anakinra (Kineret; 50 μg/mL), tocilizumab (Actemra; 50 μg/mL), or IgG isotype control (BioXcell; 50 μg/mL) was inoculated with 107 CFUs of clinical isolate M4(C20). Bacterial growth was monitored by dilution plating 3 hours postinfection. THP-1 macrophages were infected as detailed previously [4]. In brief, THP-1 cells (ATCC) were differentiated with 200 nM phorbol 12-myristate 13-acetate (PMA; Sigma) for 72 hours, then media exchanged with RPMI 1640 (Gibco) supplemented with 10% pooled human serum and no antibiotics 1 hour prior to infection. Recombinant human IL-6 (Invivogen) was added at this time in experiments featuring its use. Subcultured GAS grown to an optical density at 600 nm of approximately 0.4 (log-phase) were washed and resuspended in phosphate-buffered saline (PBS) and diluted for a multiplicity of infection of 4. Plates were centrifuged for 3 minutes at 160g to promote bacteria-macrophage contact. One hundred micrograms per milliliter of gentamicin was added at 90 minutes to prevent overgrowth of extracellular bacteria. THP-1 cells were washed with PBS, lysed with 0.05% Triton X-100 (Sigma), and bacteria were quantified by dilution plating onto THY agar. Supernatants were used for cytokine measurement using IL-6 reporter cells (HEK-Blue IL-6; InvivoGen) according to the manufacturer’s protocol. In brief, cells were seeded at 8 × 105 cells/mL in a 96-well plate, 20 µL of sample or dilutions of recombinant human IL-6 (Invivogen) was added, and after 24 hours, IL-6–induced secreted alkaline phosphatase was measured with QUANTI-Blue (Invivogen). Concentrations were determined relative to a standard curve of recombinant IL-6; IL-6-neutralizing antibodies abolish all activity, confirming the receptor specificity of signaling.
Statistical Analysis
Values are expressed as mean ± standard error of the mean. Differences between groups were analyzed using 1-way analysis of variance with Dunnett multiple comparisons analysis unless otherwise indicated. Differences are considered statistically significant at a P value of <.05 using GraphPad Prism version 8.4.1 software. Data include a minimum of 3 biological replicates to ensure reproducibility. All figures were generated using Prism version 8.4.1.
RESULTS
IL-6 Inhibition Promotes Group A Streptococcus Replication
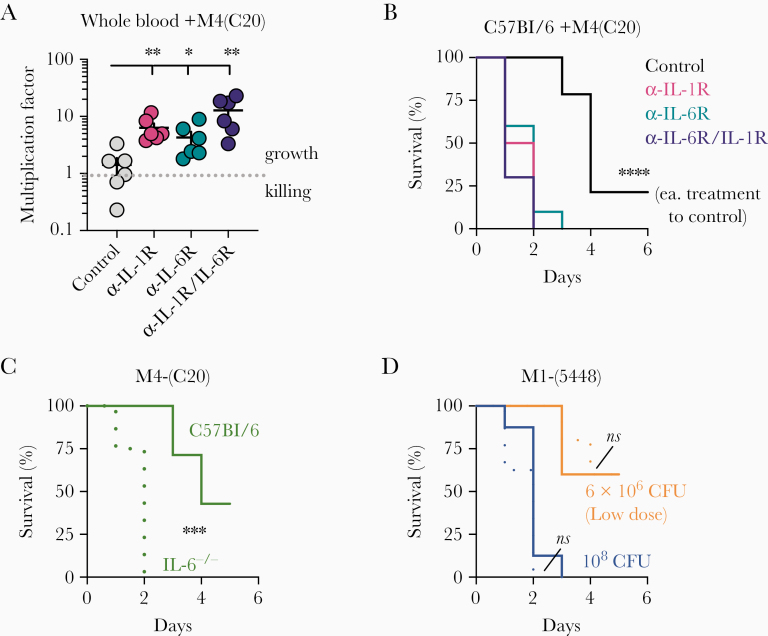
We previously reported that anakinra-treated and IL-1R-/- mice are more susceptible to iGAS [4]. Postmarketing surveillance of adverse events revealed a correlation with not only anti–IL-1 immunotherapeutics, but also anti–IL-6, with multiple reports of sepsis, necrotizing fasciitis, and toxic shock syndrome caused by GAS in individuals taking tocilizumab [4]. We therefore sought to confirm if IL-6 repression promoted host susceptibility to GAS using the patient isolate M4(C20). We examined the role of anti–IL-1 and anti–IL-6 therapeutics in promoting GAS growth using a modified Lancefield assay, wherein heparinized whole blood from healthy human donors was inoculated with a known quantity of GAS and bacterial replication monitored. Blood was treated with either Anakinra (Kineret; 50 μg/mL), tocilizumab (Actemra; 50 μg/mL), both anakinra and tocilizumab, or IgG isotype control (BioXcell; 50 μg/mL). Inhibition of signaling by either anti–IL-1 or anti–IL-6 significantly promoted GAS replication (Figure 1A).
Figure 1.
Impaired interleukin 6 (IL-6) signaling promotes growth of group A Streptococcus (GAS) M4(C20). A, Heparinized whole human blood was treated with Kineret (α-IL-1R through rIL-1RA; 50 μg/mL) and/or tocilizumab (α-IL-6R neutralizing antibody; 50 μg/mL) with isotype antibody control (50 μg/mL) was inoculated with 107 colony-forming units (CFUs) of GAS M4(C20). After 24 hours, CFUs were enumerated for each group and growth determined relative to starting inoculum (multiplication factor); values >1 denote growth. Statistical significance between groups (n = 6) was determined by 1-way analysis of variance using Dunnett multiple comparisons analysis, using the immunoglobulin G (IgG) control group as the reference. Data are representative of 3 independent donors. B, Wild-type C57BL/6 mice were treated with a neutralizing monoclonal antibody against either IL-1R (red; n = 8), IL-6 (blue; n = 8), both (purple; n = 8), or an IgG (black; n = 8) isotype, then inoculated intravenously with 108 CFUs of GAS strain M4(C20) and monitored for the given time intervals. Statistics were calculated by log-rank (Mantel–Cox) test in comparison to control mouse group. C and D, C57BL/6 wild-type (solid lines) or IL6-knockout (IL6-/-; dotted lines) mice were inoculated intravenously with 108 CFUs of GAS strain M4(C20) (clinical isolate, green), 108 CFUs M1(5448) (blue), or 6 × 106 CFUs M1(5448) (orange; lower dose to more closely match M4(C20) kinetics) and monitored for the given time intervals. Statistics were calculated by log-rank (Mantel–Cox) test. Data represent at least 2 independent experiments with 8 mice each. *P < .05; **P < .005; ***P < .0005; ****P < .00005; ns, not significant.
C57BL/6 mice treated with anti–IL-1R, anti–IL-6R, both, or an isotype IgG control were intravenously inoculated with 1 × 108 CFUs of the clinical isolate M4(C20). Each of the neutralizing antibodies was sufficient to induce a significantly shortened survival time (Figure 1B). Recapitulating the anti–IL-6 phenotype, IL-6-/- C57BL/6 mice infected with M4(C20) also experienced a significant increase in mortality compared to wild-type C57BL/6 mice (Figure 1C). In contrast, M1(5448), a clone of the epidemic strain contributing to the resurgence in invasive infections, resulted in rapid death of both wild-type and IL6-/- mice. In order to recapitulate the slower mortality kinetics of M4(C20), we also infected mice with 6 × 106 CFUs of M1(5448). Both wild-type and IL-6-/- mice were equally susceptible to M1(5448) (Figure 1C, D). These data suggest that GAS is not universally restricted by IL-6, but the severity of infection by some strains is enhanced by its inhibition.
Group A Streptococcus Evasion of IL-6 Restriction
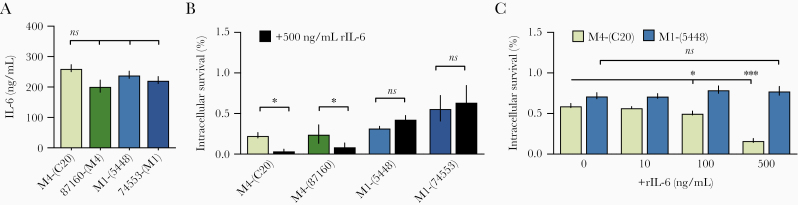
To examine whether IL-6 is differentially induced by GAS strains, THP-1 macrophages were infected with M4(C20), strain M1(5448), or other isolates collected from iGAS infection of either serotype, 87160 (M4) and 74553 (M1). IL-6 levels did not vary significantly (Figure 2A), suggesting that strains may vary in their resistance to IL-6–regulated immune effectors through a mechanism that does not involve changes in IL-6 expression. We next examined whether clinical isolate M4(C20) was more susceptible to IL-6–mediated killing than M1(5448), and if this trend was recapitulated in other M1 and M4 clinical isolates. For this, we preincubated THP-1 macrophages with 500 ng/mL of recombinant IL-6 and infected them with M4(C20), M1(5448), 87160 (M4), or 74553 (M1). IL-6 attenuated the growth of both M4 strains, but neither M1 strain (Figure 2B). The concentration required for increased restriction is <100 ng/mL (Figure 2C). Together these data show that IL-6–induced defenses have a direct role in restricting growth of some strains of GAS, and that M1 GAS may possess a mechanism to evade these effects.
Figure 2.
Interleukin 6 (IL-6)–mediated killing is strain-specific. A, Differentiated THP-1 cells were infected with M4(C20), M1(5448), or additional control M1 (74553) and M4 (87160) invasive group A Streptococcus isolates (multiplicity of infection = 4) for 2 hours and IL-6 release was measured. Statistical significance was measured by 1-way analysis of variance (ANOVA) using Dunnett multiple comparisons analysis, with M4(C20) as the reference strain. B and C, THP-1 cells were treated for 1 hour with 500 ng/mL (B) or titrations of exogenous recombinant human IL-6 (C), and colony-forming units were enumerated 2 hours postinfection. Data were analyzed by 1-way ANOVA using Dunnett multiple comparisons analysis. All data represent at least 3 independent experiments with 4 replicates. Bars show median values ± standard error of the mean. *P < .05; ****P < .00005; ns, not significant.
DISCUSSION
Immunosuppression is a risk factor for severe infections in humans and animal models of disease. How a pathogen gains virulence from any specific defect in immune signaling is not always well-defined, and this can be particularly complicated in the cytokine cascade of sepsis. IL-6 knockout mice, for example, can have an increased pathogen burden that shortens the time to death in experimental infection models [5]. In some sepsis models, the failure to control pathogen replication without IL-6 leads to earlier death, while in others, the absence of IL-6 in the cytokine storm prolongs survival [5, 13]. Here, we demonstrate that anti–IL-6 immunotherapy can promote iGAS infection. It is well documented that some strains like M1(5448) have a greater propensity for invasion and can produce iGAS in otherwise healthy individuals [2, 4]. This finding is consistent with our observation that M1(5448) is completely unaffected by IL-6 repression, suggesting it has virulence mechanisms to resist the immune effects of IL-6 signaling. However, M4(C20) shows that some strains may be more opportunistic in nature and produce disease of increased severity upon immunosuppression by drugs such as tocilizumab. Our prior identification of greater iGAS reporting risk associated with IL-1 inhibitors compared to IL-6 inhibitors [4] may reflect these observations that some strains are not restricted by IL-6. Thus, we note that infectious risks from immunomodulation are not uniform—just as immune pathways are robust but independent, bacterial pathogens can dissimilarly be impacted by these treatments, even within the same species.
Co-incidence of GAS and IL-6 immunotherapy is expected to be generally low; GAS carriage is primarily in the young [14] and use of immunotherapeutics is most common in the elderly. Use of tocilizumab in other populations for indications other than rheumatoid arthritis, such as juvenile idiopathic arthritis, cytokine release syndrome, or coronavirus disease 2019, may carry greater risk of iGAS due to elevated exposure to the pathogen, and likely different risks for other opportunistic bacterial infections as well. Furthermore, immunotherapeutics may act to not only impair immune function, but to mask symptoms and alter inflammatory laboratory findings, leading to delays in treatment. Additional clinical studies will be required to examine the molecular mechanisms of how immunotherapeutics may impact infection severity and incidence in different populations. There is currently no vaccine against GAS, but if specific high-risk patient groups are identified, they could benefit from screening for asymptomatic carriage and the use of antibiotic prophylaxis to preclude severe disease, which has been successful for preventing epidemics in at-risk military populations [15].
Notes
Acknowledgments. We thank the Clinical and Translational Discovery Core of Children’s Healthcare of Atlanta and Emory University for support and coordination of blood collection. IL6-/- mice were generously provided by M. Karin (University of California, San Diego).
Financial support. This work was supported by startup funds from Emory University and the National Institutes of Health (grant number K22 AI130223 to C. L.). This study was also supported in part by the Investigational Clinical Microbiology Core, which was supported by the Department of Medicine, Division of Infectious Diseases, Emory University School of Medicine.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5:685–94. [DOI] [PubMed] [Google Scholar]
- 2. Sumby P, Porcella SF, Madrigal AG, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 2005; 192:771–82. [DOI] [PubMed] [Google Scholar]
- 3. Hamilton SM, Bayer CR, Stevens DL, Bryant AE. Effects of selective and nonselective nonsteroidal anti-inflammatory drugs on antibiotic efficacy of experimental group A streptococcal myonecrosis. J Infect Dis 2014; 209:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaRock CN, Todd J, LaRock DL, et al. IL-1β is an innate immune sensor of microbial proteolysis. Sci Immunol 2016; 1:eaah3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol 2017; 13:399–409. [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Beaulieu A, Rubbert-Roth A, et al. OPTION Investigators . Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008; 371:987–97. [DOI] [PubMed] [Google Scholar]
- 7. Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004; 50:1761–9. [DOI] [PubMed] [Google Scholar]
- 8. Diao H, Kohanawa M. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infect Immun 2005; 73:3745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dileepan T, Linehan JL, Moon JJ, Pepper M, Jenkins MK, Cleary PP. Robust antigen specific Th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog 2011; 7:e1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosa-Gonçalves D, Bernardes M, Costa L. Necrotizing fasciitis in a patient receiving tocilizumab for rheumatoid arthritis. Reumatol Clínica Engl Ed 2018; 14:168–70. [DOI] [PubMed] [Google Scholar]
- 11. van de Sande MGH, van Slobbe-Bijlsma ER. Necrotizing fasciitis in a rheumatoid arthritis patient treated with tocilizumab. Rheumatol Oxf Engl 2012; 51:577–8. [DOI] [PubMed] [Google Scholar]
- 12. Beres SB, Richter EW, Nagiec MJ, et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci U S A 2006; 103 :7059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riedemann NC, Neff TA, Guo RF, et al. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol 2003; 170:503–7. [DOI] [PubMed] [Google Scholar]
- 14. Roberts AL, Connolly KL, Kirse DJ, et al. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr 2012; 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webber BJ, Kieffer JW, White BK, Hawksworth AW, Graf PCF, Yun HC. Chemoprophylaxis against group A Streptococcus during military training. Prev Med 2019; 118:142–9. [DOI] [PubMed] [Google Scholar]