Abstract
Background
Immune reconstitution inflammatory syndrome (IRIS) is a common cause of morbidity among people with human immunodeficiency virus (PWH) who initiate antiretroviral therapy (ART) with severe lymphopenia. Easily accessible tools that reliably predict emergence and elucidate pathogenesis of IRIS are needed to facilitate improved clinical management.
Methods
Plasma levels of biomarkers were measured before ART initiation in a large multinational cohort of ART-naive PWH with severe immunosuppression (CD4+ count <100 cells/mm3) in United States, Kenya, and Thailand. We performed a series of multiparametric analyses of inflammatory and clinical biomarkers and developed a composite score merging relevant biomarkers for use in a prediction model.
Results
We identified a distinct baseline inflammatory profile and changes in inflammatory networks among biomarkers in participants who subsequently developed mycobacterial or viral IRIS. We also developed a composite score incorporating biomarkers associated with IRIS (interleukin-6 [IL-6], IL-10, IL-27, sCD14, interferon-γ, tumor necrosis factor-α, hyaluronic acid, D-dimer, body mass index, and hemoglobin) that accurately predicted mycobacterial IRIS and death in this cohort.
Conclusions
Systemic inflammatory profiles in PWH with severe immunosuppression are predictive of IRIS. Composite scores for the prediction of mycobacterial IRIS and death could be useful for risk stratification in PWH and lymphopenia initiating ART.
Clinical Trials Registration
Keywords: immune reconstitution inflammatory syndrome (IRIS), HIV, tuberculosis, biomarkers
A composite score using clinical and inflammatory measurements assessed in people with HIV and severe lymphopenia prior to antiretroviral therapy was designed to predict immune reconstitution inflammatory syndrome associated with mycobacterial or viral infections in a prospective international study.
Human immunodeficiency virus (HIV) infection leads to CD4+ T-cell depletion with loss of immunity and development of opportunistic infections. Antiretroviral therapy (ART) has led to a dramatic reduction in mortality, with restoration of immune responses and subsequent improvement of opportunistic infections [1, 2]. Paradoxically, however, some patients experience a rapid clinical deterioration in response to ART, despite control of HIV viremia and no apparent drug toxicity; this condition is known as immune reconstitution inflammatory syndrome (IRIS) [3].
IRIS, an exaggerated and dysregulated immune phenomenon mediated by both innate and adaptive responses, remains a major problem in the clinical management of people with HIV (PWH), affecting anywhere from 5% up to 50% of severely immunosuppressed patients [1, 2, 4]. Risk factors of IRIS consistently identified in studies include: (1) CD4+ T-lymphocyte depletion; (2) shorter time between treatment of underlying opportunistic infections and ART initiation; and (3) underlying infection, even if subclinical, with mycobacterial disease, cryptococcal disease, and herpesviruses being most frequently associated with IRIS [2].
The diagnosis and management of IRIS pose significant challenges, especially in resource-limited settings where IRIS incidence is highest, because extensive diagnostic testing and hospitalization are often required. As such, with the current mandate to start ART early in PWH with low CD4 counts, improved understanding of pathogenesis and easily accessible tools for predicting IRIS are needed.
We have previously described results of a prospective international study of incidence and predictors of IRIS and death among PWH initiating ART with severe immunosuppression (NCT00286767) [5]. In that report, we found that low hemoglobin predicted IRIS whereas low body mass index (BMI), and high C-reactive protein (CRP) and D-dimer levels were predictors of death. In the current study, we sought to further investigate predictors of IRIS and death by expanding our analyses in our large multinational cohort of ART-naive PWH with severe immunosuppression (CD4 < 100 cells/µL). We included plasma biomarkers of systemic inflammation, coagulation, and fibrosis to investigate differences in systemic inflammatory profiles of patients who developed mycobacterial or viral IRIS during the study follow-up. Furthermore, we propose a composite score using clinical and laboratory measurements that could distinguish mycobacterial from viral IRIS risk and estimate risk of death. This type of predictive score may optimize clinical management, including closer follow-up of PWH at higher risk of IRIS and/or death.
METHODS
Study Design and Participants
The study was conducted in 3 clinical research sites in the United States, Kenya, and Thailand, and persons ≥18 years of age, documentation of HIV infection, no previous ART, CD4 count ≤100 cells/µL, and residence close to clinical sites were eligible for enrolment [5]. The protocol was approved by the ethics committees of the participating sites. All study participants signed informed consent and were followed prospectively from the initiation of ART (week 0) and at weeks 2, 4, 8, 12, 24, 36, and 48. Timing and regimen of ART was chosen according to local treatment guidelines and clinicians’ preferences. The clinical teams at study sites prospectively identified IRIS events and presented each to an endpoint review committee, as previously described [5]. Diagnosis criteria and procedures are described in the Supplementary Methods.
Biomarker Measurements
Biomarkers were batch-tested in the same laboratory from cryopreserved plasma samples collected from each participant prior to ART initiation. Plasma HIV viral load, CD4 and CD8 counts, hemoglobin, white blood cell count, platelets, and glucose were measured by each site’s clinical laboratory with approved assays. Electrochemiluminescence was used to measure CRP (MesoScale Discovery) and interleukin-27 (IL-27), IL-10, IL-2, IL-6, IL-8, interferon-γ (IFN-γ), tissue necrosis factor-α (TNF-α), C-X-C motif chemokine ligand 10 (CXCL10), and enzyme linked fluorescent assay (ELFA) for D-dimer on a VIDAS instrument (bioMerieux), and enzyme-linked immunosorbent assay (ELISA) for soluble cluster differentiation 14 (sCD14), sCD163, myeloperoxidase (MPO), and tissue factor (R&D Systems), and hyaluronic acid (Corgenix) using the manufacturer’s instructions.
Data Analysis
The median values with interquartile ranges (IQR) were used as measures of central tendency and dispersion. Cytokine levels were compared between study groups using Kruskal-Wallis test with Dunn multiple comparisons or Cochran-Armitage test for trend. P values were adjusted for multiple comparisons using the Holm-Bonferroni method. A heatmap was used to display z-score normalized data and depict the overall profile of biomarkers in the study groups. Venn diagrams were used to illustrate differentially expressed markers.
Profiles of correlations between biomarkers were examined using network analysis of the Spearman correlation matrices. In such analyses, markers that exhibited similar correlation profiles were colored based on a modularity [6], using the Fruchterman-Reingold algorithm [7].
A Cox proportional hazards regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for mycobacterial IRIS or death. Variables with univariate P value ≤ .2 were selected to the multivariate model to assess the odds ratio (OR).
A composite score was created using the variables shown to be statistically different between no IRIS, and mycobacterial and viral IRIS after P value adjustment (threshold, .002): IL-6, IL-10, IL-27, sCD14, IFN-γ, TNF-α, hyaluronic acid, D-dimer, BMI, and hemoglobin level. A score of one (+1) was attributed whenever each biomarker values were above the 75th percentile and BMI and hemoglobin levels below the 25th percentile of the entire study population. This composite score could then range between zero and 10.
Values obtained between IRIS groups were compared using the Kruskal–Wallis test with Dunn multiple comparisons. Receiver operator characteristics curves were employed to test the performance of the composite score to distinguish mycobacterial IRIS from viral IRIS or individuals who did not develop IRIS. Survival probability was calculated by multiplying probabilities for each prior score point and relative risk was calculated for indicated score ranges using Kaplan-Meyer curves.
All analyses were prespecified. Differences with 2-tailed P values < .05 were considered statistically significant.
RESULTS
Characteristics of Study Participants
The baseline clinical characteristics of enrolled participants have been previously published [5]. In brief, 506 participants were enrolled including 206 participants from the United States (157 no IRIS, 22 mycobacterial IRIS, and 18 viral IRIS); 200 from Kenya, (167 no IRIS, 17 mycobacterial IRIS, and 9 viral IRIS), and 100 from Thailand (84 no IRIS, 8 mycobacterial IRIS, and 2 viral IRIS) (Supplementary Table 1). A detailed description of IRIS events is shown in Supplementary Table 2. The age and sex of participants who developed IRIS (mycobacterial or viral) did not differ significantly from those who did not (P = .2961 and P = .9648, respectively; Supplementary Table 2).
Mycobacterial IRIS Patients Exhibit Distinct Expression Profiles of Inflammatory Biomarkers in Peripheral Blood
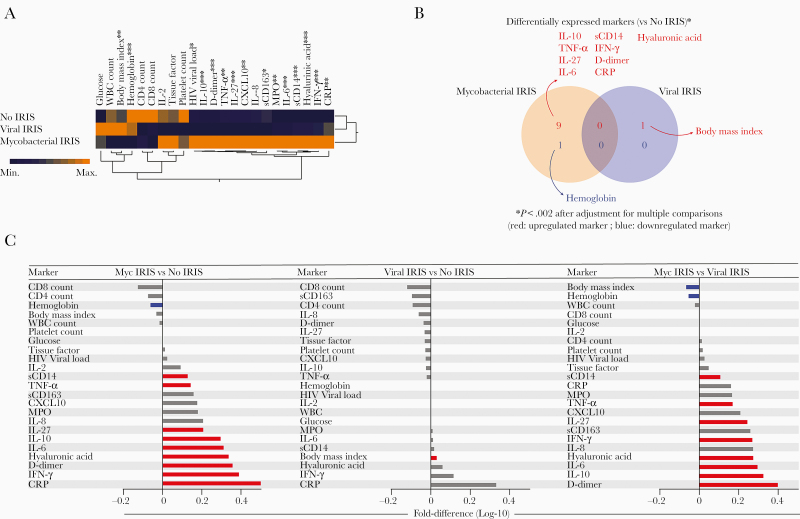
We examined the overall expression profile of 23 plasma inflammatory biomarkers and CD4+ and CD8+ T-cell counts to compare the baseline expression profiles of participants who developed IRIS (mycobacterial or viral IRIS) with those who did not (no IRIS). Unsupervised hierarchical clustering analyses of the log10-transformed mean expression values from each clinical group revealed that participants with mycobacterial IRIS had a very distinct biomarker expression profile compared to no IRIS and viral IRIS, with overall higher expression of mostly inflammatory markers, including TNF-α, IL-27, MPO, sCD163, CXCL10, sCD14, hyaluronic acid, CRP, IFN-γ, D-dimer, IL-10, and IL-6 (Supplementary Table 3). The profile of most markers was similar between no IRIS and viral IRIS, with high expression of white blood cells and BMI in viral IRIS and increased platelet count in no-IRIS patients (Figure 1A and Supplementary Table 2).
Figure 1.
Mycobacterial IRIS patients exhibit a distinct inflammatory biosignature of plasma markers compared to no IRIS and viral IRIS. Plasma samples were assessed in no-IRIS individuals (n = 409), patients with mycobacterial IRIS (n = 47), or viral IRIS (n = 29). Data were log10 transformed and z-score normalized. A, A hierarchical cluster analysis (Ward method with 100 × bootstrap) was employed to test the overall expression pattern of plasma cytokines, chemokines, and inflammatory markers in the study population. Dendrograms represent Euclidean distance. *P < .05, **P < .01, ***P < .001. B, Venn diagram demonstrates the markers for which values were statistically different between IRIS groups (mycobacterial or viral IRIS) and the no-IRIS group. C, Differences in plasma levels for each IRIS group compared to the no-IRIS group. Differences that reached statistical significance after adjustment for multiple comparisons (adjusted P < .05) are represented in colored bars. Abbreviations: CRP, C-reactive protein; CXCL10, C-X-C motif chemokine ligand 10; HIV, human immunodeficiency virus; IFN-γ, interferon-γ; IL, interleukin; IRIS, immune reconstitution inflammatory syndrome; MPO, myeloperoxidase; Myc, mycobacterial; sCD, soluble cluster differentiation; TNF-α, tumor necrosis factor-α; WBC, white blood cells.
We next designed a Venn diagram including only the markers that were differentially expressed between the IRIS groups and no-IRIS controls after adjustment for multiple measurements. This analysis revealed that BMI was the only variable that was different (higher) in the viral IRIS group compared to the no-IRIS group (Figure 1B). In the mycobacterial IRIS group, hemoglobin levels were lower whereas concentrations of D-dimer, CRP, hyaluronic acid, IFN-γ, TNF-α, IL-10, IL-27, sCD14, and IL-6 were higher (Figure 1B). These findings highlight the activation of inflammatory pathways in mycobacterial infection. Fold-differences of the circulating concentrations of each biomarker are summarized in Figure 1C. When mycobacterial was compared to viral IRIS, the profile of the fold differences was similar to that observed between mycobacterial IRIS vs no IRIS. Hence, BMI and hemoglobin levels were higher in viral IRIS whereas sCD14, TNF-α, IL-27, IFN-γ, hyaluronic acid, IL-6, and IL-10 levels were higher in mycobacterial IRIS (Figure 1C). To identify possible markers independently associated with the IRIS or death outcomes, we performed a Cox-regression model and found that higher BMI, white blood cell count, and IL-27 levels were independently associated with occurrence of IRIS in the adjusted model, whereas higher levels of hemoglobin and sCD163 were protective. Similar analysis applied to death revealed that increasing white blood cell counts as well as IL-10 and IL-8 levels were associated with mortality (Supplementary Figure 1).
Mycobacterial Infection Is Associated With Changes in Inflammatory Networks Between Markers in Peripheral Blood
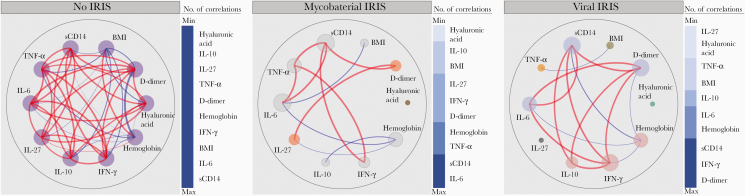
We investigated the inflammatory networks, at baseline, of participants who did not develop IRIS (controls) in comparison to those who developed mycobacterial and viral IRIS. We have previously shown that network analysis of Spearman correlation matrices can provide insights on the degree and quality of inflammation in tuberculosis-HIV as well as in other diseases [8–11].
We found that controls exhibited a greater number of correlations when compared with those who developed mycobacterial or viral IRIS (Figure 2). Controls displayed several positive and some negative correlations, highlighting the negative association of D-dimer with BMI and hemoglobin levels (Figure 2, left). Furthermore, the control inflammatory networks demonstrated a strong involvement of proinflammatory mediators such as between IFN-γ and TNF-α, IL-6, IL-27, and sCD14. Intriguingly, in no-IRIS controls, all markers included in our analysis presented the same number of correlations with each other, suggesting a balanced systemic immune activation.
Figure 2.
Network analysis of the biomarker correlation matrices in the groups of study. Spearman correlation matrices of the biomarker expression levels in each study group were built and Circos plots illustrate the correlation networks. Each circle represents a different plasma biomarker. The diameter of each circle is proportional to the number and power of significant correlations prospectively. The connecting lines represent statistically significant correlations (P < .05). Red connecting lines represent positive correlations while blue lines infer negative correlations. Node analysis was used to illustrate the number of strong correlations per marker. Markers were vertically grouped according to the number of connections from minimum to maximum numbers detected. Abbreviations: BMI, body mass index; IFN-γ, interferon-γ; IL, interleukin; sCD, soluble cluster differentiation; TNF-α, tumor necrosis factor-α.
In mycobacterial inflammatory IRIS networks, we noted a decrease in the number of correlations, suggesting potential uncoupling of the immune responses (Figure 2, center). Node analysis of mycobacterial IRIS network indicated that IL-6, sCD14, and TNF-α, followed by hemoglobin, were the most highly connected markers, suggesting that these biomarkers are associated with the unbalanced inflammation that may result in IRIS events driven by mycobacteria. We also found that hemoglobin levels displayed statistically significant negative correlations with the inflammatory markers IL-6, IL-10, and IL-27, whereas BMI negatively correlated with IL-6 levels.
In viral IRIS inflammatory networks, we observed important differences. Specifically, TNF-α, that showed the largest number of correlations in the other groups, was only negatively correlated with BMI in viral IRIS. Furthermore, 3 markers exhibiting the highest numbers of significant correlations in the network were sCD14, IFN-γ, and D-dimer, and were also positively correlated with each other, likely modulating the dynamic inflammatory process in viral infection in individuals that developed IRIS. These findings suggest an uncoupling and disorganization of the immune responses during HIV infection before ART initiation in participants who subsequently developed viral or mycobacterial IRIS; these participants exhibited a distinct inflammatory profile characterized by diminished number of correlations between biomarkers.
Inflammatory Score of Plasma Markers to Predict Viral or Mycobacterial IRIS
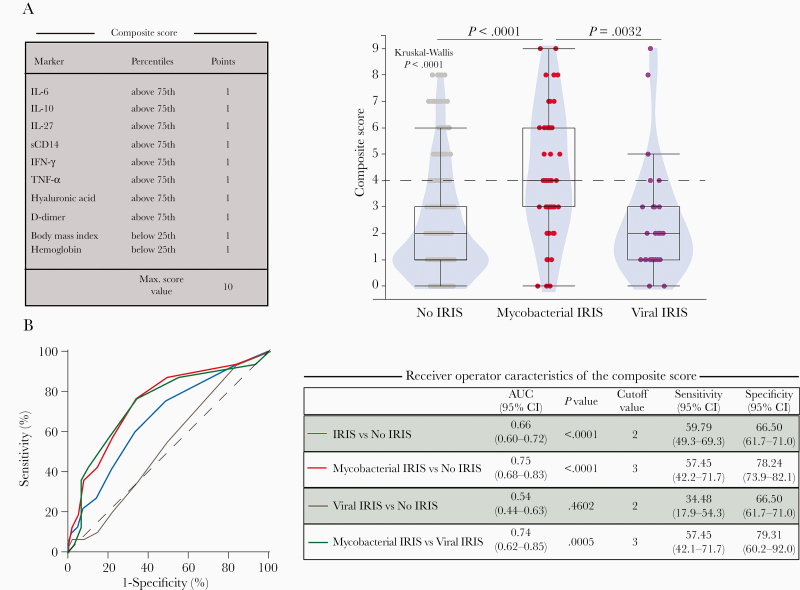
We then sought to develop a composite score composed of clinical, laboratory, and immunological parameters for the prediction of IRIS, either mycobacterial or viral, using a similar approach to the one we recently described [12]. We included baseline variables significantly associated with mycobacterial and/or viral IRIS after adjustment for multiple comparisons in univariate analysis (Figure 3A, Supplementary Table 2, and Supplementary Table 3), including IL-6, IL-10, IL-27, sCD14, IFN-γ, TNF-α, hyaluronic acid, D-dimer, BMI, and hemoglobin (Figure 3A). Mycobacterial IRIS participants had higher score values than viral IRIS or no-IRIS participants (Figure 3A). We explored the potential of this composite score to predict IRIS using receiver operating characteristic curves with C-statistics and found that composite scores have significant potential for the prediction of mycobacterial IRIS (area under the curve [AUC] = 0.75; sensitivity, 57.45 [95% CI, 42.2–71.7]; specificity, 78.24 [95% CI, 73.9–82.1]) using a cutoff value above 3 points. Of note, our composite score showed 100% specificity in scores above 8, 98.29% above 7 points, and 95.1% above 6 points, exhibiting a higher predictive value for mycobacterial IRIS prediction in levels above 6 points. In addition, albeit less robustly, our composite score displayed potential to predict all IRIS types when analyzed versus no-IRIS patients (Figure 3B).
Figure 3.
A composite score of inflammatory markers to predict mycobacterial IRIS. A, A composite score was created using IL-6, IL-10, IL-27, sCD14, IFN-γ, TNF-α, hyaluronic acid, D-dimer, BMI, and hemoglobin values to discriminate the different types of IRIS (mycobacterial vs viral). A score of one (+1) was attributed if each of IL-6, IL-10, IL-27, sCD14, IFN-γ, TNF-α, hyaluronic acid, or D-dimer values were above the 75th percentile and BMI and hemoglobin levels below the 25th percentile of the entire study population (percentile values were: IL-6 = 5.63 pg/mL, IL-10 = 17.8 pg/mL, IL-27 = 746.1 pg/mL, sCD14 = 3.11 μg/mL, IFN-γ = 9.63 pg/mL, TNF-α = 17.42 pg/mL, hyaluronic acid = 171.1 pg/mL, D-dimer = 1.9 μg/L, BMI = 17.9 kg/m2; and hemoglobin levels = 9.25 g/dL). This composite score could then range between zero and 10. Violin plot with box and whiskers representing median values and interquartile range of the composite score per each study group is shown. Median values between the study groups were compared using the Kruskal-Wallis test with Dunn multiple comparisons post test. P values were adjusted using the Holm-Bonferroni method based on variables shown to be statistically different in the mycobacterial IRIS group. B, Receiver operator characteristic curves were employed to test the performance of the composite score to distinguish IRIS from no IRIS, and mycobacterial IRIS cases from viral IRIS or no-IRIS individuals, and viral IRIS from no IRIS. Abbreviations: AUC, area under the curve; CI, confidence interval; IFN-γ, interferon-γ; IL, interleukin; IRIS, immune reconstitution inflammatory syndrome; sCD, soluble cluster differentiation; TNF-α, tumor necrosis factor-α.
We next evaluated the relative contribution of clinical laboratory variables compared to immunological markers in the IRIS predictive power of the composite scores. AUC values using only the clinical laboratory parameters were in general inferior to those utilizing the immunological variables, especially in the prediction of mycobacterial IRIS (Supplementary Figure 2A and 2B). These findings suggest that plasma biomarkers together with clinical measurements had better accuracy in prediction of mycobacterial IRIS and could aid in clinical management of PWH at higher risk of IRIS.
A Composite Score Indicates Higher Probability of Death in Patients With High Scores
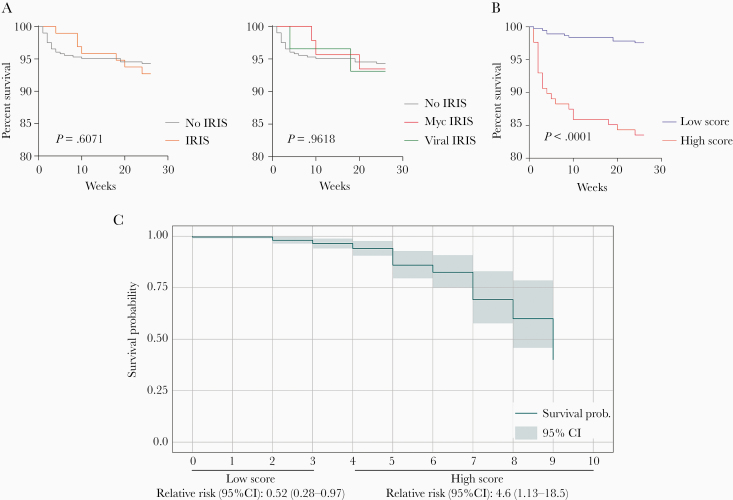
Next, we asked whether the composite score proposed here could also be used to predict death in study participants. For this, we stratified all patients according to death or survival and arbitrarily defined a low (<4 points) and high (≥4 points) score to analyze the baseline distribution in relation to the composite score (Figure 3). To extend this analysis, we used Kaplan-Meier curves to initially assess the behavior of the groups during the weeks of follow-up (Figure 4A); we found no statistically significant difference between the survival curves from participants that developed IRIS analyzed here from that of those who did not (P = .6071) and no-IRIS, mycobacterial, and viral IRIS groups (P = .9618). Extending this approach, however, to investigate mortality in patients according to high or low composite scores, we observed that patients with a high score had increased mortality during the study period (Figure 4B). Next, we applied an adapted Kaplan-Meier model to assess survival probability according to score values and observed that patients with high composite score were 4.6 times more likely to die (Figure 4C). Low scores, especially between zero and 2 points, were not associated with change in the probability of survival. Starting at 2 points we could detect a decrease in the probability curve, which fell abruptly from the fourth point in the composite score, with a greater reduction in the probability of survival between 6 and 7 points. This approach argues that patients with mycobacterial coinfection and higher composite scores are more likely to have unfavorable outcomes.
Figure 4.
An inflammatory composite score predicts death in late presenters with HIV infection. A, Kaplan-Meier curves show percentage survival over 26 weeks for participants who developed IRIS and those who did not (left panel) or in groups of patients stratified per IRIS subtype (no IRIS, mycobacterial IRIS, and viral IRIS; right panel). B, Survival curve of patients stratified according to the composite score in low (0–3 points) or high score group (4–10 points). Curves were compared using log-rank (Mantel-Cox) test. C, Survival probability according to the composite score. Relative risk was calculated in the group of individuals with low or high score values. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; Myc, mycobacterial.
DISCUSSION
In the present study we explored a panel of plasma biomarkers in a large, geographically diverse cohort of severely immunocompromised PWH [5]. Our primary results corroborate previous evidence of increased innate inflammation and expand the current knowledge by directly comparing the inflammatory profile and demonstrating unique predictive signatures of PWH who developed mycobacterial versus viral IRIS.
Coinfection with mycobacteria in PWH is associated with increased innate and adaptive immune activation [13, 14]. The pathogenesis of mycobacterial IRIS has been associated with CD4+ T-lymphocyte activation [3], and a major role of innate immunity [8, 15–17]. We have previously shown that activation of monocytes and related markers (sCD163 and sCD14) are potential predictors of tuberculosis-IRIS [8]. In addition, we have previously reported that a combination of biomarkers, including CRP, sCD14, IFN-γ, and hemoglobin, could accurately predict tuberculosis-IRIS in a cohort from Mexico and South Africa [12].
To our knowledge, very few studies have directly investigated details of immune responses and predictors in viral IRIS [2]. We have previously shown that levels of hyaluronic acid, D-dimer, IL-6, IL-8, and sCD14 and high HBV viral load were associated with death or flares in PWH coinfected with hepatitis B and/or C virus [18]. High viral antigen load has been observed in patients who further develop Kaposi sarcoma IRIS [19, 20]. In the present study, we investigated the immunological and inflammatory profile of patients who developed viral IRIS and found no statistically significant differences in biomarker concentrations compared to those who did not develop IRIS. Patients from the viral IRIS group, however, presented with higher BMI values compared to no IRIS. It has been demonstrated in mouse and nonhuman primates that bacterial pathogens lead to a more robust activation of innate immunity [21] and intracellular pathogens such as mycobacteria or fungi can cause more intense myeloid and macrophage responses. In contrast, chronic viral pathogens are frequently subclinical and typically activate inflammatory pathways related to interferon responses [22]. We hypothesize that the concurrent viral infections triggered immune responses similar to HIV, and did not result in a strong immune disturbance that would lead to a distinctive biomarker signature and may have different pathways affected without prominent myeloid and macrophage activation as in intracellular pathogens such as mycobacteria or fungi, so that viral IRIS did not result in a distinctive inflammatory response and was masked by the overall immune activation pathways triggered by HIV itself. Future studies analyzing data from larger cohorts are warranted to directly test this hypothesis.
The observation that higher IL-27 was independently associated with occurrence of IRIS is a novel and interesting finding. IL-27 is a cytokine that critically regulates T-cell differentiation and regulatory function and represents an important checkpoint in the immune system [23]. IL-27 upregulates many inhibitory receptors, such as PD-1, and is also directly involved in immune responses and mycobacterial burden control in tuberculosis [23] and, thus, should be further studied with regard to IRIS.
Immune activation is a coordinated process that involves cross-regulation by production of several pro- and anti-inflammatory mediators [24, 25], the intricate relationship of which can be interrogated through analysis of correlation matrices [9, 26]. In the context of IRIS, particularly viral IRIS which has not been systematically studied previously, Spearman matrices help to understand the dynamic pathways involved in the immune activation that precedes the IRIS events. In the present study, we found that the presence of infection (bacterial or viral) probably leads to IRIS by uncoupling immune responses, suggested by the reduction of statistically relevant correlations. In addition, identification of the most relevant markers in the node analysis of the networks suggested a prominent role for IL-6, which has been implicated in the prediction and pathogenesis of mycobacterial IRIS in both clinical [17] and animal [27] studies. Our analysis also demonstrated a role of D-dimer in the network of viral IRIS; however, its involvement in virus-driven inflammation associated with IRIS requires further clarification. Finally, the monocyte-derived protein sCD14 was a highly connected marker in all the matrices evaluated, indicating that innate inflammation may be central in both viral and mycobacterial IRIS.
Importantly, we propose an inflammatory score composed of markers that have been widely studied in mycobacterial IRIS and which can be used to predict the occurrence of this syndrome, and death. The application of blood-based markers can facilitate a more specific approach, when the immunological status is directly linked with the clinical outcome [28]. An inflammatory score for predicting mycobacterial IRIS could potentially serve as a new tool in the clinical management of coinfected patients before ART initiation. Diagnosing IRIS is challenging, and delays for diagnostic procedures can hamper quick therapy and lead to more tissue damage [29], procedures, and hospitalizations. Our score, composed of 10 host blood-based and clinical parameters, showed great power to predict mycobacterial IRIS. Considering the current therapy strategy in IRIS management, corticosteroids have been extensive studied [30–32] and can attenuate the inflammatory manifestations [32]. Our results suggest the possibility of using the composite score to identify patients who would mostly benefit from implementation of preventive corticosteroid therapy to prevent IRIS. Additionally, our inflammatory composite score can be used to evaluate the relative risk of death between patients with IRIS, which, in turn, could be used as a guide to intensify clinical follow-up. Our findings suggest that patients with a score above 6 at pre-ART would exhibit higher risk of death and thus may benefit from closer monitoring or additional interventions. Although IRIS was independently associated with mortality in the parent study, where other types of IRIS were analyzed beyond mycobacterial and viral [5], only 6 patients with mycobacterial IRIS died, and mortality was not substantially impacted by occurrence of mycobacterial IRIS, probably due to small sample size for that specific comparison. Additional studies will be needed to validate the clinical applicability of our composite scores as tools to help decisions to initiate corticosteroid therapy and/or modify intensity of follow-up.
The limitations of our study include the small number of participants, especially in the viral IRIS group and in the death group, preventing a more detailed analysis. Regardless, the results generate the hypothesis that there is an uncoupling of the systemic immune activation, which precedes the onset of mycobacterial IRIS.
In summary, our study revealed differences in the systemic inflammatory profile between patients with severe immunosuppression who develop IRIS and demonstrated the dynamic interplay between cytokines and soluble proteins preceding this syndrome. We further characterized differences between systemic inflammation among patients who will develop mycobacterial or viral IRIS and composed a score with clinical and laboratory parameters that predicted IRIS and death. If validated by other studies in severe immunosuppressed PWH at ART initiation, this composite score could be further implemented in the clinical setting to assess the risk of IRIS and death and optimize patient care.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants and the clinical staff of all participating sites.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Financial support. The study was supported in part by the Intramural Research Program of National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH). B. B. A. was supported by the NIH (grant numbers U01AI115940 and U01AI069923). B. B. A. is a senior scientist and C. L. V. received a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil. M. B. A. received a research fellowship from the Fundação de Amparo à Pesquisa do Estado da Bahia, Brazil.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol 2012; 10:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulougoura A, Sereti I. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS 2016; 11:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gopalan N, Andrade BB, Swaminathan S. Tuberculosis-immune reconstitution inflammatory syndrome in HIV: from pathogenesis to prediction. Expert Rev Clin Immunol 2014; 10:631–45. [DOI] [PubMed] [Google Scholar]
- 4. Sereti I, Rodger AJ, French MA. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Curr Opin HIV AIDS 2010; 5:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sereti I, Sheikh V, Shaffer D, et al. Prospective international study of incidence and predictors of immune reconstitution inflammatory syndrome and death in people with HIV and severe lymphopenia. Clin Infect Dis 2020; 71:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vinhaes CL, Oliveira-de-Souza D, Silveira-Mattos PS, et al. Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: a prospective cohort study. Cytokine 2019; 123:154759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andreev VP, Liu G, Zee J, Henn L, Flores GE, Merion RM. Clustering of the structures by using “snakes-&-dragons” approach, or correlation matrix as a signal. PLoS One 2019; 14:e0223267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrade BB, Singh A, Narendran G, et al. Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog 2014; 10:e1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mesquita ED, Gil-Santana L, Ramalho D, et al. ; Rede-TB Study Group . Associations between systemic inflammation, mycobacterial loads in sputum and radiological improvement after treatment initiation in pulmonary TB patients from Brazil: a prospective cohort study. BMC Infect Dis 2016; 16:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveira-de-Souza D, Vinhaes CL, Arriaga MB, et al. Molecular degree of perturbation of plasma inflammatory markers associated with tuberculosis reveals distinct disease profiles between Indian and Chinese populations. Sci Rep 2019; 9:8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinhaes CL, Arriaga MB, de Almeida BL, et al. Newborns with Zika virus-associated microcephaly exhibit marked systemic inflammatory imbalance. J Infect Dis 2020; 222:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musselwhite LW, Andrade BB, Ellenberg SS, et al. Vitamin D, D-dimer, interferon γ, and sCD14 levels are independently associated with immune reconstitution inflammatory syndrome: a prospective, international study. EBioMedicine 2016; 4:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng Q, Sayin I, Canaday DH, Mayanja-Kizza H, Baseke J, Toossi Z. Immune activation at sites of HIV/TB co-infection contributes to the pathogenesis of HIV-1 disease. PLoS One 2016; 11:e0166954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Bruyn E, Wilkinson RJ. The immune interaction between HIV-1 infection and Mycobacterium tuberculosis. Microbiol Spectr 2016; 4: doi: 10.1128/microbiolspec.TBTB2-0012-2016. [DOI] [PubMed] [Google Scholar]
- 15. Lai RPJ, Meintjes G, Wilkinson KA, et al. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun 2015; 6:8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conradie F, Foulkes AS, Ive P, et al. Natural killer cell activation distinguishes Mycobacterium tuberculosis-mediated immune reconstitution syndrome from chronic HIV and HIV/MTB coinfection. J Acquir Immune Defic Syndr 2011; 58:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8:e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrade BB, Hullsiek KH, Boulware DR, et al. ; INSIGHT Study Group . Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J Infect Dis 2013; 207:1379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letang E, Almeida JM, Miró JM, et al. Predictors of immune reconstitution inflammatory syndrome-associated with Kaposi sarcoma in Mozambique: a prospective study. J Acquir Immune Defic Syndr 2010; 53:589–97. [DOI] [PubMed] [Google Scholar]
- 20. Letang E, Lewis JJ, Bower M, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS 2013; 27:1603–13. [DOI] [PubMed] [Google Scholar]
- 21. Zinman G, Brower-Sinning R, Emeche CH, et al. Large scale comparison of innate responses to viral and bacterial pathogens in mouse and macaque. PLoS One 2011; 6:e22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 2010; 10:514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torrado E, Fountain JJ, Liao M, et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J Exp Med 2015; 212:1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kulkarni OP, Lichtnekert J, Anders HJ, Mulay SR. The immune system in tissue environments regaining homeostasis after injury: is “inflammation” always inflammation? Mediators Inflamm 2016; 2016:2856213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med 2013; 5:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cruz LAB, Moraes MOA, Queiroga-Barros MR, Fukutani KF, Barral-Netto M, Andrade BB. Chronic hepatitis B virus infection drives changes in systemic immune activation profile in patients coinfected with Plasmodium vivax malaria. PLoS Negl Trop Dis 2019; 13:e0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goovaerts O, Jennes W, Massinga-Loembé M, et al. ; TB-IRIS Study Group . LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS One 2013; 8:e81856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silveira-Mattos PS, Barreto-Duarte B, Vasconcelos B, et al. Differential expression of activation markers by Mycobacterium tuberculosis-specific CD4+ T-cell distinguishes extrapulmonary from pulmonary tuberculosis and latent infection [published online ahead of print 30 October 2019]. Clin Infect Dis doi: 10.1093/cid/ciz1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5:361–73. [DOI] [PubMed] [Google Scholar]
- 30. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis 2004; 190:869–78. [DOI] [PubMed] [Google Scholar]
- 32. Meintjes G, Skolimowska KH, Wilkinson KA, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 2012; 186:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.